Reduced social investigation and increased injurious behavior in transgenic 5xFAD mice
Abstract
Social withdrawal and agitation/aggression are common behavioral and psychological symptoms of dementia presented by Alzheimer's disease (AD) patients, with males exhibiting more aggressive behaviors than females. Some transgenic mouse models of AD also exhibit social withdrawal and aggression, but many of these models only recapitulate the early stages of the disease. By comparison, the 5xFAD mouse model of AD exhibits rapid, progressive neurodegeneration, and is suitable for modeling cognitive and behavioral deficits at early, mid-, and late-stage disease progression. Anecdotal reports suggest that transgenic 5xFAD males exhibit high levels of aggression compared to wild-type controls, but to date, indirect genetic effects in this strain have not been studied. We measured home-cage behaviors in 5xFAD males housed in three different group-housing conditions (transgenic-only, wild-type only, and mixed-genotype) and social approach behaviors when exposed to a novel free-roaming or restrained, wild-type or transgenic conspecific. Transgenic-only home cages required earlier separation due to injuries arising from aggression compared to wild-type-only or mixed-genotype cages, despite no obvious increase in the frequency of aggressive behaviors. Transgenic 5xFAD males and females also spent less time investigating free-roaming conspecifics compared to wild-type controls, but they showed normal investigation of restrained conspecifics; the genotype of the conspecific did not affect approach behavior, and there was no aggression observed in transgenic males. These findings provide evidence in an animal model that amyloid pathology ultimately leads to avoidance of novel social stimuli, and that frequent interactions between individuals exhibiting an AD phenotype further exacerbates aggressive behaviors.
Significance
The present study characterizes aggression and social investigation in the 5xFAD transgenic mouse model of Alzheimer's disease (AD). We found that more injuries occur when male 5xFAD transgenic mice are housed with other transgenics, rather than with wild-type mice. Male and female 5xFAD transgenic mice also spend less time investigating a free-roaming same-sex novel conspecific. These findings suggest that altered social behaviors occur as a result of AD-related neuropathology. Our findings also highlight the importance of social environment for maintaining a healthy colony of 5xFAD mice, thereby aiding researchers in selecting housing conditions that maximize the longevity of their colonies.
1 INTRODUCTION
Alzheimer's disease (AD) is a leading cause of dementia, defined by an accumulation of amyloid-beta (Aβ) plaques and neurofibrillary (tau) tangles concomitant with progressive memory loss and cognitive impairments (Jarvik & Greenson, 1987; LaFerla & Green, 2012). Patients also present a number of behavioral and psychological symptoms of dementia (BPSD), including apathy, anxiety, agitation/aggression, and depression (Cummings, 1997; Cummings et al., 1994). Approximately 80% of community-dwelling dementia patients exhibit at least one BPSD following the onset of cognitive symptoms, with approximately 75% of all patients exhibiting at least one BPSD in the month prior to testing, compared to 16% of the general population (Lyketsos et al., 2002). Apathy, agitation/aggression, anxiety, and depression are among the most common BPSD, and patients with mild AD exhibit a significantly higher prevalence of those compared to healthy controls (Zhang, Wang, Li, & Yu, 2012; Zhao et al., 2016). These symptoms can have a profound impact on the lives of patients, their families, and caregivers, with many patients exhibiting social withdrawal even prior to diagnosis (Chow et al., 2002; Chung & Cummings, 2000; Frisoni et al., 1999; Jost & Grossberg, 1996; Steffens, Maytan, Helms, & Plassman, 2005). The frequency and severity of BPSD also affect caregiver distress (Chow et al., 2002; Craig, Mirakhur, Hart, McIlroy, & Passmore, 2005), with behavioral disturbances (such as agitation/aggression) being a leading factor in institutionalization (Chenoweth & Spencer, 1986; Cohen et al., 1993; Steele, Rovner, Chase, & Folstein, 1990). As a result, patients in care facilities, who frequently interact with other AD patients, exhibit a higher prevalence of behavioral disturbances compared to age-matched community-dwelling individuals (Steele et al., 1990; Wood et al., 2000), which can lead to a further reduction in meaningful social interactions.
Transgenic mouse strains have proved to be useful in examining cognitive and behavioral deficits associated with AD-related neurodegeneration, and several of these strains present behaviors akin to BPSD in AD patients. Here, we focus on BPSD related to social function. Six-month-old transgenic APP/PS1 males (Filali, Lalonde, & Rivest, 2011) and 18-month-old transgenic 3xTg-AD females (Bories et al., 2012) exhibit decreased sociability compared to wild-type littermates. Increased aggression has also been reported in a number of transgenic strains, including 7-month-old Tg2576 males, 6- to 12-month-old APP23 males, and 10-month-old APP/PS1 males (Alexander et al., 2011; Pugh, Richardson, Bate, Upton, & Sunter, 2007; Vloeberghs, Dam, Coen, Staufenbiel, & Deyn, 2006), mimicking the increased aggression more often seen in male human AD patients (Lövheim, Sandman, Karlsson, & Gustafson, 2009); increased aggression was not reported in male and female 3xTg-AD mice at 12 or 18 months of age (Bories et al., 2012). The varying age of onset presented in these models may be, in part, due to the differences in the progression of the pathology. Tg2576, APP23, and APP/PS1 strains exhibit a relatively slow rate of Aβ accumulation and only recapitulate the early stages of AD (Bilkei-Gorzo, 2014). In contrast, the 3xTg-AD strain exhibits neurofibrillary tangles in conjunction with Aβ, but does not exhibit the same level of neurodegeneration characteristic of AD in humans (Bilkei-Gorzo, 2014; Janelsins et al., 2008; Manaye et al., 2013). By comparison, the 5xFAD strain exhibits rapid accumulation of Aβ, with whole-brain levels at ~9–21 ng/mg of protein by 2 months of age, compared to ~10 ng/mg of protein in the Tg2576 strain at 16 months of age (Oakley et al., 2006). As a result of this accelerated Aβ pathology, transgenic 5xFAD mice have deficits in axon myelination, amyloid plaques and gliosis, and a reduction in whole-brain synaptophysin between 1 and 4 months of age (Gu et al., 2018; Oakley et al., 2006). This progresses to neurodegeneration in noradrenergic and cholinergic neurons, as well as pyramidal neurons in Layer 5 of the cerebral cortex, synaptic loss in the ventral horn of the spinal cord, and reduced basal synaptic transmission levels in the somatosensory cortex and hippocampus by 6 months of age; by 9 months of age there is a 25% reduction in whole-brain synaptophysin levels, as well as reduced syntaxin and PSD-95 (synaptic membrane-associated proteins), indicating significant synaptic degeneration and impaired signaling (Crouzin et al., 2013; Crowe & Ellis-Davies, 2014; Devi & Ohno, 2010; Eimer & Vassar, 2013; Jawhar, Trawicka, Jenneckens, Bayer, & Wirths, 2012; Li et al., 2013; Oakley et al., 2006). As a result, the 5xFAD strain provides a useful model for examining the effects of early, mid-, and late-stages of AD-related neuropathology.
Despite the suitability of the 5xFAD mouse model for examining cognitive and behavioral deficits associated with later stages of AD (Bilkei-Gorzo, 2014), relatively little is known about the BPSD-like behaviors in this strain. Transgenic 5xFAD males at 9 months of age, and males and females at 12 months of age, exhibit deficits in nest-building, a behavior previously shown to be associated with affiliative behaviors (Devi & Ohno, 2015; Schneider, Baldauf, Wetzel, & Reymann, 2014). However, transgenic 5xFAD males and females also engage in increased home-cage social behaviors at ~11 months of age and impaired social recognition memory at 9 months of age (Flanigan, Xue, Rao, Dhanushkodi, & McDonald, 2014). More recently, we found that transgenic 5xFAD females have less interest in social odors, as well as an age-related decrease in social interest in the free social interaction task (Kosel, Torres Munoz, Yang, Wong, & Franklin, 2019). However, we were unable to test males in that study because high levels of home-cage aggression and injury in male transgenic-only cages necessitated the separation of animals and prevented us from suitably maintaining group-housing conditions.
Previous research has indicated that the 5xFAD background strain (C57B6/SJL) exhibits high levels of home-cage aggression starting at approximately 8 weeks of age, with serious injuries and/or death occurring in group-housed males between 4 and 6 months of age (Kuby, 1997, p. 27; Lyon et al., 1996, p. 1562). However, we observed that high levels of home-cage aggression occurred predominantly in cages housing transgenic 5xFAD males together, whereas cages containing only male wild-type C57B6/SJL littermates exhibited minimal injurious behavior up to 12 months of age. Moreover, our personal observations suggested that injurious behavior typically occurred with minimal warning, often progressing from normal home-cage aggression to injury within 24–48 hr. This pattern of rapid escalation between transgenic cagemates suggested the possibility of indirect genetic effects (also termed social genetic effects) wherein an individual's phenotype is affected by the genotype of a social partner (Baud et al., 2017). However, these data were largely anecdotal, and to date, there are no comprehensive studies examining aggression in this strain. Additionally, while our previous study indicated an age-related decrease in social interest by transgenic 5xFAD females, it is unclear whether similar effects are observed in males, and whether the use of mixed-genotype versus same-genotype dyads affects social approach behavior.
Given the evidence for reduced social investigation and increased aggression in APP-overexpressing strains, as well as our personal observations of home-cage aggression in 5xFAD mice, the present study examined the influence of indirect genetic effects on home-cage behaviors, including aggression, in male 5xFAD mice. We also examined the social investigation of novel same-sex mice within novel environments, and asked whether the genotype of the stimulus mouse affects the exploration of that stimulus by wild-type and transgenic, male and female 5xFAD mice. These questions were examined over two distinct experiments: (a) home-cage observations were used to determine whether indirect genetic effects under different housing conditions (transgenic-only, wild-type only, or mixed-genotype) affected aggressive and affiliative behavior toward cagemates in male 5xFAD mice; and (b) the three-chamber sociability test and free social interaction task were used to examine the social behaviors in response to wild-type and transgenic stimulus mice in both male and female 5xFAD mice. Based on the reports of aggression in transgenic 5xFAD males and observations in our own laboratory, we expected higher levels of home-cage aggression in cages containing only transgenic subjects, as well as increased aggression in transgenic males during the free social interaction task, particularly in response to transgenic stimulus animals. Additionally, based on our previous results in females, we predicted decreased affiliative and social investigative behaviors in transgenic males and females in the three-chamber sociability test and free social interaction task, and reduced home-cage affiliative behaviors in transgenic male 5xFAD mice.
2 METHODS
2.1 Animals
Male and female transgenic 5xFAD mice and wild-type litter-mate controls were used in this study. Mice were the progeny of male hemizygous C57BL/6J × SJL/J FN 5xFAD (B6SJL-Tg (APPSwFILon, PSEN1*M146L*L286V) 6799Vas/Mmjax) and female wild-type C57BL/6J × SJL/J F1 hybrid mice (B6SJLF1/J, JAX stock # 100012), and were bred in our colony. Male breeders were selected from existing in-house animals, while female breeders were obtained from Jackson Laboratories (Bar Harbor, ME, USA); female F1 hybrids were used to maintain the colony on a common background based on recommended breeding practices from the Jackson Laboratory. Mice were ear-punched for identification and assigned Mouse ID numbers between P14 and P18, then weaned between P23 and P26.
Tissue samples from ear punches were used to genotype subjects for the PSEN transgene and the phosphodiesterase-6b retinal degeneration-1 (Pde6brd1) allele using standard PCR procedures and primers recommended by Jackson Laboratories (Bar Harbor, ME, USA). Mice based on the C57B6/SJL strain exhibit a recessive Pde6brd1 allele associated with retinal degeneration and vision loss (Brown & Wong, 2007; Yassine et al., 2013). 5xFAD mice are also available on a congenic C57BL/6J background that does not exhibit this allele (MMRRC stock # 34848), but these mice were not used because they exhibit a delayed Aβ pathology, with a trend toward reduced Aβ pathology at 2, 4, and 6 months of age, and significantly lower levels of pathogenic Aβ at 9 months of age, relative to transgenic 5xFAD mice on the C57B6/SJL background used in the present study (Valenzuela, 2012).
Two separate cohorts of mice were used to measure home-cage behaviors (Cohort 1) and social investigation in novel environments (Cohort 2). Cohort 1 included only male 5xFAD mice because this experiment was designed to primarily assess the aggression and injurious behaviors that are not observed in females. Cohort 2 included both male and female 5xFAD mice. See following sections for details on Cohorts 1 and 2.
All mice were housed on an inverted 12:12 light:dark cycle, with lights off from 09:30 to 21:30. Mice were group-housed in same-sex, same-litter cages of 2–4 animals, with standard housing conditions consisting of standard polysulfone mouse cages (30 × 19 × 13 cm; model PC7115HT; Allentown Caging Inc., Allentown, NJ, USA) containing wood-chip bedding (FreshBed, Shaw Resources, Shubenacadie, NS, Canada), two black, opaque, polymer enrichment tubes (4 cm diameter, approx. 8 cm long), and a metal cage top containing ad libitum food (Laboratory Rodent Diet #5001; Purina LabDiet, St. Louis, MO, USA) and filtered tap water. Cages were topped with a micro-isolator filter to reduce the spread of airborne contaminants and diseases. Cages were changed weekly, just prior to the end of the light cycle. Note that cages used to assess home-cage behaviors differed from the standard cages indicated above; additional housing details for home-cage observations are provided in Section 2.2.2.
Subjects homozygous for the recessive Pde6brd1 allele were not used to ensure that visual impairments did not affect results. Singly housed subjects and those exhibiting stereotypic behavior (e.g., continuously running in circles, doing backflips, jumping in one corner of the cage) were excluded from testing. Due to the possibility of home-cage aggression, subjects were monitored regularly for general health and to check for injuries; animals exhibiting signs of injury were immediately separated from cagemates and removed from testing, although data collected up to that point were included in the final analysis. All experimental procedures were performed in accordance with the guidelines published by the Canadian Committee on Animal Care and were approved by the Dalhousie University Committee on Laboratory Animals (Protocols #16-021 and #18-102).
2.2 Cohort 1: Home-cage behaviors in male 5xFAD mice
2.2.1 Subjects
Forty-eight male subjects were pair-housed in either single-genotype transgenic (containing two transgenic males), single-genotype wild-type (containing two wild-type males), or mixed-genotype (containing one transgenic and one wild-type male) cages; a total of nine transgenic-only cages, eight wild-type only cages, and seven mixed-genotype cages were used. Home-cage observations for Cohort 1 were performed between 8 weeks (2 months) and 23 weeks (5.25 months) of age; observations were stopped at 23 weeks of age due to low numbers of remaining transgenic-only cages.
2.2.2 Recording conditions, apparatus, and schedule
At approximately 7.5 weeks of age, subjects were transferred into recording cages and moved into their own colony room, specifically used for home-cage recordings (3 × 3.5 m). This room contained two metal cage racks (1.2 × 0.5 × 1.5 m tall) against two adjacent walls, each with four shelves plus the top panel. Cages were placed with the broad side facing the front of the rack, with up to three cages per shelf. Video camcorders (Canon Vixia HF R800; Canon USA Inc, Melville, NY, USA) on tripods were used for recordings; these cameras were located 1.2 m from the rack and slightly above the cages. The room was lit by two ceiling-mounted white fluorescent lights, as well as clamp-mounted desk lamps containing red bulbs at the ends of each shelf. Red lights were on a 12:12 light:dark schedule, offset from the white light schedule by −6 hr (lights on at 15:30, lights off at 03:30), and were used to facilitate video recordings during the dark period; these lights were aimed toward the wall to avoid direct light and heat into the cages.
Recording cages consisted of a standard rat cage (47 × 26 × 21 cm; Allentown Caging Inc., Allentown, NJ, USA) containing wood-chip bedding (FreshBed), two black, opaque, polymer enrichment tubes (4 cm diameter, approx. 8 cm long), and two flat-bottomed ceramic bowls (approx. 10 cm diameter × 4 cm high) for food (Laboratory Rodent Diet #5001) and filtered tap water; food and water were provided ad libitum. Enrichment tubes were held together in parallel via nylon cable ties to reduce the likelihood of tubes shifting and obscuring activity. In order to maximize visibility, metal cage tops and micro-isolator filter lids were not used, and cages were covered with clear acrylic tops containing ventilation holes.
Recordings were made every second week starting at 8 weeks (2 months) of age and ending at 23 weeks (5.25 months) of age. Subjects were recorded for 3 days per week, every second week; recordings were taken on the day of the cage change (Day 1), two days after a cage change (Day 3), and the day before a cage change (Day 7). All recordings began approximately 4.5 hr prior to the end of the dark phase, and ended approximately 30 min prior to the light phase, for a total of 4 hr per day. During non-recording weeks, subjects were placed in clean cages identical to the recording cages to reduce the stress associated with changing housing conditions. Clean food and water bowls were also provided with cage changes, while enrichment tubes and cage covers were washed with Fisherbrand Sparkleen 1 (Fisher Scientific, Pittsburgh, PA, USA) and room-temperature water, rinsed thoroughly with hot water, and dried with paper towels.
2.2.3 Scoring of behaviors
Videos were manually scored by experimenters blind to treatment on a per cage basis. Cages were assigned ID numbers based on Mouse ID numbers; as Mouse ID numbers were assigned prior to genotyping, it was not possible to identify cage composition by ID number alone. Videos were scored for the number of instances of aggressive encounters, including attack (initiating biting and/or rolling), chase (rapid locomotion toward another animal that is rapidly moving away), mount (mounting another animal from behind and thrusting the pelvis), defensive posture (standing upright on the hind limbs with the forelimbs raised up, and the head directed away from the other animal), and induced flee (rapid locomotion away from another animal that is chasing it). Videos were also scored for allogrooming (all licks given by one animal to another), aggressive grooming (one animal holds the other down and forcibly grooms using its teeth), huddling (the bodies of both animals touching for at least 10 s consecutively), and being alone (both animals having at least one body length apart from each other for at least 10 s consecutively). Due to aggressive encounters often resulting in several instances of the same behaviors and the complementary nature of certain behaviors (such as chase and induced flee), scoring each behavior individually could artificially inflate the number of aggressive encounters. In order to prevent this, scoring of aggressive encounters based on a hierarchical scale was adapted from Williamson, Lee, and Curley (2016), using the following rankings: attack > chase>mount > defensive posture > induced flee. Aggressive encounters were scored by recording the highest-level behavior observed during a single encounter; for example, if one animal attacked the other, resulting in a flee and chase, this was scored as an attack only. Each encounter was considered as the period of time when the aggressor or target exhibited one of these ranked behaviors, until the aggressor moved away to perform a different activity (e.g., feeding or self-grooming). Due to the hierarchical scoring of aggressive behaviors, total aggressive (attack, chase, mount) and defensive (defensive posture, induced flee) behaviors were analyzed, as well as the total number of all aggressive encounters (including both aggressive and defensive behaviors), in order to assess whether the overall number of aggressive encounters differed across cage compositions. Scoring of behaviors was performed by time-sampling, as previously discussed by Arakawa, Blanchard, and Blanchard (2007); briefly, instances of the above behaviors were scored for a 30-s period every 10 min for the duration of the recording periods.
In addition to frequencies of behaviors, separation of cagemates was also tracked for survival data. As aggression is expected in the formation of dominance hierarchies (Miczek, Maxson, Fish, & Faccidomo, 2001; Williamson et al., 2016), observation of aggressive behaviors during routine checks was not considered sufficient for separation. These attacks are considered to be a typical social behavior and are not intended to break the skin (Grant & Mackintosh, 1963), so the presence of injuries was indicative of excessive or abnormal home-cage aggression, and animals were separated immediately after any injuries were observed.
2.3 Cohort 2: Social investigation within novel environments in 5xFAD mice
2.3.1 Subjects
Twenty-seven male (12 transgenic, 15 wild-type) and 21 female (9 transgenic, 12 wild-type) subjects were used to assess social approach behaviors in the three-chamber sociability test and free social interaction task. Mice were housed in mixed-genotype cages of 2–4, and were tested at 26 weeks (6 months) of age. Based on the results from the home-cage observations in Cohort 1, mixed-genotype housing was used to avoid the aggression between males and to maintain consistent housing conditions between males and females.
2.3.2 Testing conditions and schedule
Social approach behavior and aggression in a novel environment were measured using the three-chamber sociability apparatus and free social interaction task. Mice were tested in a quiet 2.4 × 2.4 m room containing a desk with a computer, and a table for the testing apparatus. Tasks were performed under red light illumination, and were recorded using the Biobserve Viewer 3.0 program (Biobserve GmbH, Bonn, Germany). Subjects and stimulus animals were transferred into clean, separate holding cages in the colony room, and were moved to the testing room at the time of testing. After testing, animals were returned to the colony room, and were returned to their home-cages once all cagemates had completed testing.
Mice were tested over 11 days, with testing sessions taking place in the latter half of the dark phase. Research has indicated that male AD-model mice exhibit a circadian rhythm of aggression (Todd et al., 2018), so all testing was performed during the same period. Each subject performed a single testing session on a given day; these were as follows: Day 1, social approach session 1; Day 4, social approach session 2; Day 8, free social interaction session 1; Day 11, free social interaction session 2. Each subject underwent two testing sessions for each task in order to test approach behavior to both wild-type and transgenic stimulus conspecifics; the presentation of each stimulus genotype was counterbalanced across sessions and subjects. Subjects were tested in order of Subject ID number.
2.3.3 Three-chamber sociability test
Apparatus
The apparatus was a three-chamber box, the same as described previously (Kosel et al., 2019). In brief, this apparatus consisted of a clear, acrylic box (69 × 20 × 20 cm), divided into three equal-sized compartments by two clear, acrylic walls. Floor-level pass-throughs in the middle of the dividing walls allowed access between these chambers, and could be blocked by removable barriers. The floor was covered in the same wood-chip bedding as used in home-cages. Stimuli were contained in two round wire cages (Galaxy Cup; Spectrum Diversified Designs Inc., Streetsboro, OH, USA), with one Galaxy Cup in each of the two outer chambers; Galaxy Cups were topped with white, opaque, 500 ml HDPE bottles (Nalgene Nunc International Corporation, Rochester, NY, USA) filled with water to prevent subjects from climbing on top of cages. Stimuli included novel same-sex transgenic 5xFAD mice and wild-type controls.
Procedure
Testing was performed on two separate days. Subjects were habituated to the testing room and the testing arena simultaneously. Each day consisted of a 5-min habituation trial, followed by a 10-min testing trial, with a 1-min intertrial interval. Subjects were placed into the central chamber, and both barriers were removed simultaneously to allow access to all three chambers. Subjects were allowed to freely explore for 5 min, at which point they were returned to the central chamber and the barriers were replaced. Subjects remained in the central chamber during the 1-min intertrial interval while a novel stimulus animal was placed under the Galaxy Cup in one of the outer chambers; the Galaxy Cup in the opposite chamber remained empty. Following the intertrial interval, the barriers separating the chambers were again removed, and subjects were allowed to explore freely for 10 min. Habituation was performed with no stimuli under either of the Galaxy Cups, while testing consisted of a single stimulus animal contained within a Galaxy Cup in either the left or right chamber; the genotype and location (left or right chamber) of the stimulus animal was counterbalanced across subjects and testing sessions. The apparatus was cleaned between each subject and session using Fisherbrand Sparkleen 1 (Fisher Scientific) and room temperature water, and then dried thoroughly using paper towels.
Scoring
As Subject ID numbers were assigned prior to genotyping and subjects were tested in order of Subject ID number, it was not possible to determine the genotype of an animal by ID number alone. All videos were only identified by Subject ID and session number (1 or 2) to ensure that experimenters were blind to the genotypes of the subject and the stimulus animals during both testing and manual scoring of videos. Videos were manually scored for the duration (in seconds) of time spent in each chamber, as well as time spent investigating the cages. Subjects were considered to have entered a chamber once their head and forelimbs had passed the threshold. Investigation of cages was defined as orientation of the snout toward the Galaxy Cups, with the snout 1 cm or less away from the Galaxy Cup. Investigation duration was used to calculate a preference ratio for the cage containing the stimulus animal over the empty cage; this was calculated by dividing the time spent investigating the social cage over the total investigation time of both cages. To ensure that preference ratios were not affected by overall differences in exploration, total investigation time in both chambers was also calculated. As investigation time is a better indicator of sociability than time spent in chamber (Fairless, Shah, Guthrie, Li, & Brodkin, 2011), all analyses were performed using investigation time.
2.3.4 Free social interaction
Apparatus
The apparatus and procedure were the same as used previously (Kosel et al., 2019). Briefly, the apparatus consisted of a 38 × 38 × 40 cm arena, with the floor raised 5 cm from the base. Three of the walls were made of plywood and painted beige, with the fourth wall being made of clear acrylic to allow for recording from the side. The floor was made of clear acrylic to facilitate cleaning between animals, and a black, opaque sheet was placed immediately below to make it appear solid. Stimulus conspecifics included novel same-sex transgenic 5xFAD mice and wild-type controls.
Procedure
The free social interaction task was performed after the three-chamber sociability test. Testing was performed on two separate days. Subjects were habituated to both the testing room and the testing arena simultaneously. Each day consisted of a 5-min habituation period, followed immediately by a 10-min trial period. Subjects were placed in the arena at the start of habituation and were allowed to freely explore for 5 min; at the end of the habituation phase, subjects remained in the arena while a novel stimulus animal was introduced into the arena, and both animals were then allowed to freely explore for 10 min. The genotype of the stimulus animal was counterbalanced across subjects and testing sessions. The apparatus was cleaned between each subject and session using Fisherbrand Sparkleen 1 (Fisher Scientific) and room temperature water, and then dried thoroughly using paper towels. Due to the possibility for aggression during this task, the researcher remained in the room during the entire session. Animals were physically separated if fighting was observed continuously for more than 30 s, and the test was ended if fighting re-occurred after separation, or if 5 attacks were made by either animal.
Scoring
Experimenters were blind to the genotypes of the subject and stimulus conspecific, as described for the three-chamber sociability test. Videos were scored manually for the duration (in seconds) of investigative (follow; orofacial sniffing; anogenital sniffing), aggressive (chase; mount; attack), defensive (induced flee; defensive posture), and self-grooming behaviors. Aggressive and defensive behaviors (as described above), and investigative and self-grooming behaviors, as previously described (Kosel et al., 2019) were quantified. All behaviors were adapted from Arakawa et al. (2007) and Grant and Mackintosh (1963).
2.4 Data analyses
Data analyses were performed using multilevel modeling in R version 3.6.1 (R Foundation for Statistical Computing) and RStudio version 1.2.1335 “Action of the Toes.” For home-cage observations, analyses were performed on each behavior separately, using cages as the subjects; cage composition (transgenic-only, wild-type-only, or mixed-genotype) was used as the between-subjects factor, while week was used as the within-subjects factor. Cage survival was examined using bootstrapped 95% confidence intervals. For social approach and free social interaction, subject genotype and sex were used as the between-subjects factors, while stimulus genotype was used as the within-subjects factors. Analyses were performed separately for each behavior in free social interaction. As females in this study, and our previous research (Kosel et al., 2019), exhibit almost no aggressive behaviors, genotype effects for aggressive and defensive behaviors were examined for males only in order to prevent female data from skewing the results. Two cages of male subjects (n = 2 transgenic, 2 wild-type) were separated after the three-chamber sociability test, but prior to free social interaction testing, due to injuries arising from home-cage aggression. Three free social interaction sessions with male subjects were ended early due to aggressive behaviors; two of these involved the same wild-type subject (once with a wild-type stimulus conspecific, once with a transgenic stimulus conspecific) and the third involved a wild-type subject and a wild-type stimulus conspecific. For these sessions, the length of the session was calculated as a percentage of the total session duration, and the duration of observed behaviors was extrapolated for the remaining time. With the exception of cage survival, all significant effects were examined using 95% confidence intervals of Cohen's d.
3 RESULTS
3.1 Home-cage observations
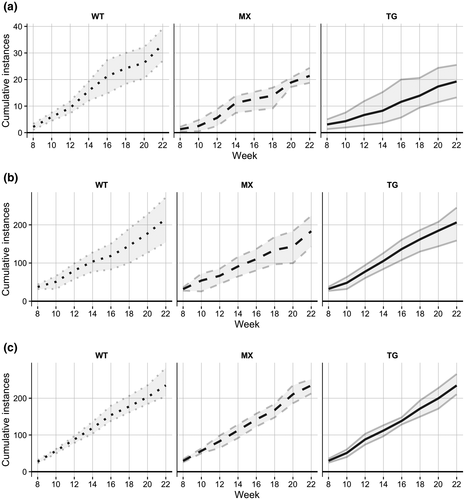
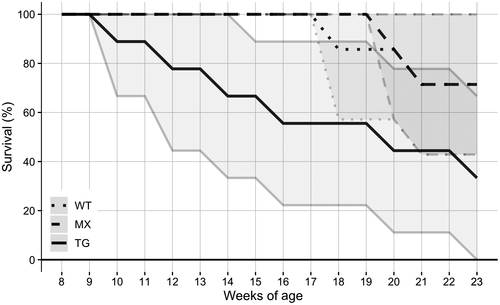
Analyses of home-cage behaviors in males from 8 to 23 weeks (2–5.25 months) of age indicated no main effects of cage composition (genotype) or age, and no interaction, for any behaviors (see Figure 1 for the most prevalent home-cage behaviors). For cage survival, a median of 67% (95% CI [33.33%, 88.89%]) of transgenic-only cages survived together until 15 weeks of age, whereas wild-type-only cages did not see any separation until 18 weeks of age (median 85.71%, 95% CI [57.14%, 100.00%] for survival at 18 weeks of age; see Figure 2). Mixed-genotype cages did not see separation until 20 weeks of age (median 85.71%, 95% CI [57.14%, 100.00%] for survival at 20 weeks of age). By 23 weeks of age, only 33% (95% CI [0.00%, 66.67%]) of transgenic-only cages remained together, whereas 71% (95% CI [42.86%, 100.00%]) of wild-type-only and mixed-genotype cages remained together.
3.2 Three-chamber sociability test
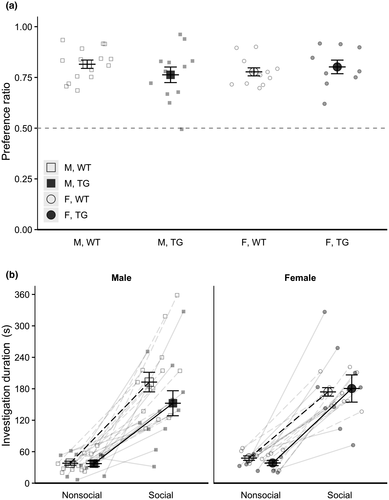
Analyses of social approach behavior in the three-chamber sociability test performed by males and females at 26 weeks (6 months) of age indicated no main effects or interactions of subject genotype, sex, or stimulus genotype for preference ratio or total investigation time of both social and non-social stimuli (see Figure 3). Analysis of investigation duration of each stimulus type (social or non-social) indicated a main effect of stimulus (χ2(1) = 292.32, p < .001), with subjects exhibiting longer investigation durations for social over non-social stimuli; there were no other main effects or interactions of subject genotype, sex, stimulus genotype, or stimulus type for investigation duration.
3.3 Free social interaction
A total of four male subjects (n = 2 transgenic, 2 wild-type) were removed from the study prior to testing free social interaction due to injuries sustained from home-cage aggression. Additionally, as female mice in this study and our previous research (Kosel et al., 2019) exhibit almost no aggressive behaviors, genotype effects for aggressive and defensive behaviors are provided for males only.
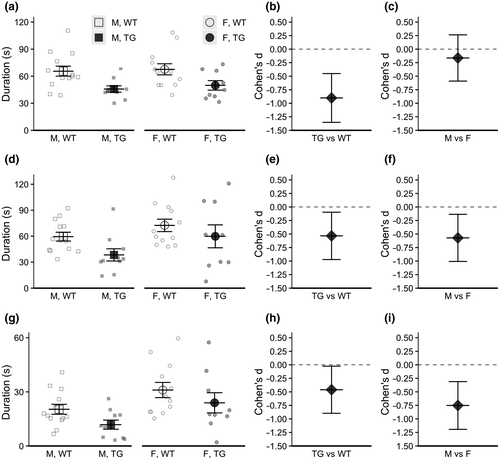
Analyses of investigative and self-grooming behaviors during free social interaction in males and females at 26 weeks (6 months) of age indicated a main effect of subject genotype for orofacial sniffing (χ2(1) = 12.86, p < .001), anogenital sniffing (χ2(1) = 4.77, p = .029), and following (χ2(1) = 4.34, p = .037), with transgenic 5xFAD mice exhibiting reduced durations of these behaviors compared to wild-type controls (see Figure 4). There was also a main effect of sex for anogenital sniffing (χ2(1) = 4.94, p = .026) and following (χ2(1) = 10.45, p = .001), with males exhibiting reduced durations compared to females. There were no other main effects or interactions of subject genotype, sex, or stimulus genotype for investigative or self-grooming behaviors.
Analyses of aggressive behaviors during free social interaction in male 5xFAD mice at 26 weeks (6 months) of age indicated no main effects or interactions of subject genotype or stimulus genotype for aggressive (attack, chase, mount) or defensive (defensive posture, flee) behaviors (data not shown).
4 DISCUSSION
Anecdotal reports in our laboratory and others have suggested that housing conditions (specifically, the use of single- or mixed-genotype group housing) can affect home-cage aggression in male 5xFAD mice. As a result, we examined how the development of affiliative and aggressive home-cage behaviors is affected by cage composition in these mice. Contrary to our hypotheses, we found that single-genotype cages housing only transgenic males do not exhibit differences in the number of aggressive or affiliative home-cage behaviors compared to male wild-type-only or mixed-genotype cages. However, male transgenic-only cages do result in more injurious behavior, with these cages requiring earlier separation due to injuries compared to male wild-type-only or mixed-genotype cages, consistent with previous observations.
In addition, we measured social investigation by male and female 5xFAD mice, and determined whether genotype of a stimulus conspecific plays a role in these behaviors during novel social interactions, expanding upon our previous research in female mice (Kosel et al., 2019). While transgenic 5xFAD males show normal affiliative behaviors in the home-cage, transgenic males and females exhibited reduced social investigation of a novel social stimulus in the free social interaction task, with males exhibiting overall lower durations of anogenital sniffing and following relative to females. This decrease in social investigation was not dependent on the genotype of the social stimulus, and was also not observed in the three-chamber social approach task, when the social stimulus is placed behind a barrier.
4.1 Transgenic 5xFAD males require earlier home-cage separation due to injuries, but do not exhibit more aggressive encounters than wild-type controls
Overall, transgenic 5xFAD males did not initiate more aggressive encounters than wild-type controls. There were no differences in the number of aggressive encounters observed within transgenic-only cages compared to wild-type-only or mixed-genotype cages (Figure 1a), and there were no genotype effects in the number of aggressive behaviors exhibited during the free social interaction task. However, males housed in transgenic-only cages required earlier separation from cage-mates due to injuries sustained during home-cage aggression. This occurred despite no obvious increase in aggression during routine checks prior to injuries arising, indicating that the progression from normal aggression to injurious behavior occurred within 24–48 hr. Additionally, our findings show that being housed with a wild-type mouse mitigates the injurious behavior observed in transgenic-only cages, suggesting that the behavior of both the aggressor and target lead to these injuries. Overall, the involvement of two transgenic 5xFAD males and the rapid development indicates that indirect genetic effects are likely responsible for the rapid escalation of aggressive behaviors.
These results confirm previous anecdotal observations in our laboratory indicating that transgenic 5xFAD males cannot be reliably housed together, particularly in single-genotype cages. While characterizing the progression of social deficits in 5xFAD mice from 3 to 12 months of age, we attempted to house subjects in single-genotype cages (as previously reported in Kosel et al., 2019); however, we found that transgenic males could not be housed together past 6 months of age, with the majority of cages being separated between 3 and 5 months of age due to injuries sustained in the home cage. Interestingly, while the results of the present study indicate that 5xFAD males housed in transgenic-only cages require early separation due to home-cage aggression, they did not exhibit an overt increase in overall aggressive encounters. Similarly, transgenic 5xFAD males did not exhibit increased aggressive behaviors toward novel stimulus males in a novel environment during the free social interaction task, suggesting that the injuries observed in the home cage may be due to increased intensity of aggressive encounters, rather than an increased frequency.
The heightened home-cage aggression observed in transgenic 5xFAD mice may also be contributed to by their background strain, as high levels of home-cage aggression have been reported in the C57/B6SJL strain (Kuby, 1997, p. 27; Lyon et al., 1996, p. 1562). However, high levels of male–male and male–female home-cage aggression have also been reported for transgenic animals from three human APP-overexpressing lines (wild-type, London, and Swedish mutations) on the FVB/N background strain (Moechars, Gilis, Kuipéri, Laenen, & Leuven, 1998), and wild-type 5xFAD littermates did not show the same level of injurious behavior, suggesting that amyloid pathology—rather than background strain—may be the critical factor in increased aggression in these animals. The onset of injurious behaviors also correlates with the approximate presentation of neurodegeneration in transgenic 5xFAD mice, with a reduction in whole-brain synaptophysin evident by 4 months of age, and neurodegeneration evident in cholinergic neurons, as well as pyramidal neurons in Layer V of the cerebral cortex, by 6 months of age (Crowe & Ellis-Davies, 2014; Devi & Ohno, 2010; Oakley et al., 2006).
Further evidence for increased aggression in transgenic AD-model mice has been found in the resident-intruder paradigm; increased aggression was observed in 7-month-old transgenic Tg2576 males (Alexander et al., 2011), 6- and 12-month-old APP23 transgenic males (Vloeberghs et al., 2006), and 2.5- to 3-month-old males from APP-overexpressing lines (wild-type, London, and Swedish; Moechars et al., 1998). While the present results do not fully recapitulate the increase in aggressive behaviors observed in other strains, it is important to note that methodological differences may play a key role. Aggression in mice is commonly seen between two unfamiliar conspecifics, and typically serves two purposes (Brain & Parmigiani, 1990; Miczek et al., 2001; Williamson et al., 2016): (a) to establish a dominance ranking between two conspecifics; and (b) to drive an intruder out of an owned territory. The resident-intruder paradigm is designed to create the second situation, wherein an unfamiliar male (the intruder) is introduced into the owned (familiar) territory (the home cage) of the resident. Additionally, the social isolation housing required for the resident-intruder paradigm creates a chronic stress condition that itself increases aggression, in addition to a number of behavioral and physiological changes (Goldsmith, Brain, & Benton, 1976; Koike et al., 2009; Matsumoto, Pinna, Puia, Guidotti, & Costa, 2005). By comparison, the present study examined aggression between familiar conspecifics in a familiar environment (home-cage observations) and novel conspecifics in a novel environment (free social interaction), and used group-housed animals. Under similar novel conditions, Bories et al. (2012) examined social interactions using unfamiliar 3xTg-AD mixed-genotype dyads in a novel environment, and reported no aggressive behaviors in males or females at 12 or 18 months of age.
Overall, our findings suggest that indirect genetic effects—phenotypic changes arising from the genotype of a social partner—are responsible for the development of injurious home-cage behavior in transgenic 5xFAD males. The rapid escalation and mitigating effects of wild-type cagemates suggest that this behavior is due to environmental factors driven by their transgenic cage mates, and is not the result of increased aggression per se arising from AD-related pathology. This is further supported by the finding that transgenic 5xFAD males do not exhibit increased aggression in novel environments relative to wild-type controls. However, because transgenic 5xFAD single-genotype cages require separation due to injury earlier than wild-type-only or mixed-genotype cages, laboratories using single-genotype housing conditions for 5xFAD mice must be aware of a possible selection bias that leads to surviving cages being only a particular subgroup of transgenic 5xFAD mice.
4.2 Transgenic 5xFAD mice exhibit reduced investigation of novel free-roaming, but not restrained, conspecifics
Compared to wild-type controls, 6-month-old transgenic 5xFAD males and females exhibited decreased investigation time of novel conspecifics in the free social interaction task, exhibiting shorter durations of orofacial sniffing, anogenital sniffing, and following (Figure 4). However, this deficit was a specific response to a freely roaming social stimulus, as transgenic males and females exhibited no differences in preference ratio in the three-chamber sociability task when the social stimulus is contained (Figure 3b). Because both the three-chamber sociability test and the free social interaction test use novel conspecifics in a novel environment, we propose that the differing effects between the two tasks is due to the presence of free-roaming versus restrained conspecifics, which likely impact the arousal levels of the experimental animal. Additionally, the genotype of the stimulus mouse had no effect on investigative behavior, further suggesting that the behavior of the stimulus mouse has little effect on the behavior of the experimental mouse in either task, and those differences in arousal levels of the stimulus mouse are not a contributing factor.
Results presented here mirror those previously observed in 5xFAD females, where transgenic females at 9 months of age exhibit reduced orofacial sniffing, anogenital sniffing, and following behaviors in the free social interaction task, but no difference in preference ratio or social investigation time in the three-chamber apparatus (Kosel et al., 2019). Moreover, previous findings in transgenic females also exhibited a strong trend toward reduced investigative behaviors during the free social interaction task at 6 months of age, similar to the effects observed in the present study, suggesting that these behaviors can be observed even earlier than previously reported. The previous study (Kosel et al., 2019) used same-genotype housing conditions, rather than the mixed-genotype housing conditions used in the present study; similar results from 5xFAD females in either housing conditions suggest that indirect genetic effects related to home-cage social environments do not play a role in social approach behavior to a novel conspecific in a novel environment.
The contribution of altered olfaction to the social deficits observed in transgenic 5xFAD mice here cannot be entirely ruled out because transgenic 5xFAD males exhibit decreased glucose metabolism in the olfactory cortex by 3 months of age and increased latency to locate a buried food pellet by 6 months of age (Xiao et al., 2015). However, transgenic 5xFAD males and females exhibit no differences in olfactory sensitivity and no impairments during an olfactory delayed matching-to-sample task relative to wild-type controls (Roddick, Roberts, Schellinck, & Brown, 2016; Roddick, Schellinck, & Brown, 2014). The differing results of these two studies may have arisen due to variations in reward motivation and the tasks used (i.e. food restriction for 3 days in Xiao et al. 2015 and water restriction for 10 days in Roddick et al. [2014, 2016]). Additionally, while our previous study indicated reduced investigation of social odors by transgenic 5xFAD females relative to wild-type controls, transgenic females were able to detect non-social odors similar to wild-type mice, and were also able to appropriately increase their scent-marking in response to either social or non-social odor cues similar to wild-type controls (Kosel et al., 2019). Overall, the evidence suggests that transgenic 5xFAD males and females do not exhibit olfactory deficits, and that reduced social investigation in transgenic females is not due to an inability to identify social odors.
Despite evidence of altered social interaction in other transgenic mouse models of AD (Bories et al., 2012; Faizi et al., 2012; Filali et al., 2011), no consistent trend of social investigation across transgenic mouse models of AD has been indicated, and it is unclear how AD pathology specifically contributes to these behaviors. While this is consistent with the varying presentation of social withdrawal in human AD patients (Lyketsos et al., 2002; Zhang et al., 2012; Zhao et al., 2016), it is important to note that transgenic mouse models of AD recapitulate differing aspects of AD pathology (Bilkei-Gorzo, 2014; LaFerla & Green, 2012), although transgenic Tg2576 females similarly exhibit reduced social investigation during free social interaction but normal behavior in the three-chamber apparatus at 3 months of age (Pietropaolo, Delage, Lebreton, Crusio, & Cho, 2012). Together with the present study, these findings further highlight the differing responses in these two behavioral tests, and suggest that the free social interaction task may be more sensitive to differences in social interest than the three-chamber sociability test. We suggest that transgenic 5xFAD mice are able to recognize the relative safety of investigating a mouse behind a barrier but are unwilling to cross a certain arousal threshold in order to investigate a freely roaming mouse. Further work is required to identify the contribution of specific AD neuropathology to these deficits, including pinpointing the onset and progression of these deficits relative to AD pathology, and if they expand to include abnormal behaviors in the three-chamber sociability at more advanced stages, as previously shown in transgenic Tg2576 mice (Pietropaolo et al., 2012).
5 CONCLUSIONS
This study indicates that cages containing only transgenic 5xFAD males exhibit injuries arising from home-cage aggression at younger ages than wild-type-only or mixed-genotype cages, pointing to a significant contribution of indirect genetic effects to this behavior and highlighting the need for careful consideration of housing conditions for this strain. The presence of a wild-type conspecific delays the onset of injurious behavior, suggesting that during transgenic–transgenic aggressive encounters both the aggressor and target mouse exhibit altered behaviors, thereby leading to an escalation that results in injury. This has important implications for researchers working with mouse models that demonstrate social deficits, as housing conditions and perhaps the social stimulus presented can have significant impact on the animal's behavior (Kalbassi, Bachmann, Cross, Roberton, & Baudouin, 2017). Our findings also show that 6-month-old transgenic 5xFAD males and females are less motivated to explore a social stimulus, reminiscent of social withdrawal presented by AD patients. This is an earlier time point than previously reported in transgenic 5xFAD females (Kosel et al., 2019). Furthermore, the reduced social exploration observed in transgenic mice is context-specific, and is limited to high arousal situations with a free-roaming social stimulus, suggesting that transgenic 5xFAD mice may exhibit particular avoidance to perceived threats from conspecifics. However, the genotype of a novel social stimulus does not appear to mediate social exploration within a novel environment, suggesting that the reduced social exploration is due to intrinsic factors rather than as a response to the behaviors of the stimulus mouse.
DECLARATION OF TRANSPARENCY
The authors, reviewers, and editors affirm that in accordance with the policies set by the Journal of Neuroscience Research, this manuscript presents an accurate and transparent account of the study being reported and that all critical details describing the methods and results are present.
ACKNOWLEDGMENTS
The authors thank J. Renee Yang and Aimee A. Wong for their technical support and R.E. Brown for the use of his behavioral apparatus.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
AUTHOR CONTRIBUTIONS
All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Conceptualization, F.K. and T.B.F.; Methodology, F.K., J.H., and T.B.F.; Investigation, F.K., J.H., S.H., and V.G.; Formal Analysis, F.K.; Resources, F.K. and T.B.F.; Writing – Original Draft, F.K.; Writing – Review & Editing, F.K. and T.B.F., Visualization, F.K.; Supervision, T.B.F. and F.K.; Funding Acquisition, T.B.F.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in Federated Research Data Repository at http://doi.org/10.20383/101.0171.




