Converging early responses to brain injury pave the road to epileptogenesis
Funding information: NIH, grant numbers: R01NS097750 and R01NS069861 and NJCBIR CBIR16IRG017, NJCBIR CBIR14RG024; and the CURE Foundation, grant number: CF 259051 (to V. S.).
Significance: Brain injury is a leading risk factor for acquired epilepsies that are refractory to treatments, emphasizing the need for better mechanistic understanding and preventive strategies. This review discusses the reinforcing associations between molecular pathways, which regulate cell loss, synaptic plasticity, and neurogenesis after brain injury, and explores the potential for modulating specific pathways early after brain injury to prevent progression to epilepsy.
Abstract
Epilepsy, characterized by recurrent seizures and abnormal electrical activity in the brain, is one of the most prevalent brain disorders. Over two million people in the United States have been diagnosed with epilepsy and 3% of the general population will be diagnosed with it at some point in their lives. While most developmental epilepsies occur due to genetic predisposition, a class of “acquired” epilepsies results from a variety of brain insults. A leading etiological factor for epilepsy that is currently on the rise is traumatic brain injury (TBI), which accounts for up to 20% of all symptomatic epilepsies. Remarkably, the presence of an identified early insult that constitutes a risk for development of epilepsy provides a therapeutic window in which the pathological processes associated with brain injury can be manipulated to limit the subsequent development of recurrent seizure activity and epilepsy. Recent studies have revealed diverse pathologies, including enhanced excitability, activated immune signaling, cell death, and enhanced neurogenesis within a week after injury, suggesting a period of heightened adaptive and maladaptive plasticity. An integrated understanding of these processes and their cellular and molecular underpinnings could lead to novel targets to arrest epileptogenesis after trauma. This review attempts to highlight and relate the diverse early changes after trauma and their role in development of epilepsy and suggests potential strategies to limit neurological complications in the injured brain.
Traumatic brain injury (TBI) is a growing silent epidemic and a major contributor to increases in the long-term risk for neurological dysfunction, including drug-resistant acquired temporal lobe epilepsies (Annegers & Coan, 2000; Gupta et al., 2014; Kharatishvili & Pitkanen, 2010b; Lowenstein, 2009). A recent report by the Centers for Disease Control and Prevention (CDC, 2015) estimates that in the US, TBI accounts for over 2.5 million civilian emergency room visits and more than 200,000 hospitalizations annually, while those in service have a 4% risk of sustaining TBI. Brain injuries ranging from mild trauma (e.g., falls in elderly) to severe penetrating injuries (e.g., motor vehicle accidents and explosive devices), heighten the risk for epilepsy (Chen, Ruff, Eavey, & Wasterlain, 2009; Kovacs, Leonessa, & Ling, 2014). There appears to be a strong correlation between injury severity and the risk for developing spontaneous seizures, which double following mild TBI, and (depending on the extent of injury) increases 7 to 14 times with severe TBI (Annegers & Coan, 2000; Christensen et al., 2009; Ferguson et al., 2010; Yeh, Chen, Hu, Chiu, & Liao, 2013). A variety of experimental paradigms have been used to model physically diverse human brain injuries, but the most widely used models are the fluid percussion injury (FPI) and cortical contusion injury (CCI; Johnson, Meaney, Cullen, & Smith, 2015). FPI models closed-head concussive brain trauma by delivering a brief fluid pulse, generated by the impact of a pendulum on one end of a fluid filled piston, to the exposed (but intact) dura (Dixon et al., 1987; Toth, Hollrigel, Gorcs, & Soltesz, 1997). CCI simulates impact or penetrating injuries by means of controlled impact of a piston driven at a certain speed to a defined depth (Dixon, Clifton, Lighthall, Yaghmai, & Hayes, 1991; Hunt et al., 2009). Other methods employed to mimic traumatic brain injury include, weight drop models of impact injury, primary blast wave TBI, rotational TBI, and denervation injury from cortical undercut (Adelson, Dixon, Robichaud, & Kochanek, 1997; Cullen et al., 2016; Prince et al., 2009; Sundaramurthy et al., 2012). Both FPI and CCI lead to spontaneous epileptic seizures and reduce the threshold for chemically evoked seizures (Bolkvadze & Pitkanen, 2012; D'Ambrosio et al., 2005). While differences in injury modality, severity, and rodent strains make it difficult to directly compare epileptogenesis across different injury models, in comparative studies in adult C57BL/6S mice CCI resulted in greater incidence of spontaneous seizures than FPI (Bolkvadze & Pitkanen, 2012). Long term EEG monitoring of rats after FPI has revealed 50% incidence of limbic epilepsy after a prolonged latency of seven weeks to one year, while cortical epilepsies are observed as early as two weeks after injury (Curia et al., 2011; Kharatishvili, Nissinen, McIntosh, & Pitkanen, 2006). In comparison, about 20–36% of rats and mice develop behavioral epileptic seizures over 9–10 weeks after penetrating CCI (Hunt et al., 2009; Kelly, Miller, Lepsveridze, Kharlamov, & McHedlishvili, 2015). Both clinical and experimental data suggest that, like adults, the developing brain is also susceptible to post-traumatic epileptogenesis (Keret et al., 2017; Semple et al., 2017). The link between injury severity, histopathology, and epilepsy is particularly well characterized after FPI (Kharatishvili & Pitkanen, 2010a). Curiously, the risk for epilepsy after blast wave exposure is still unknown (Kovacs et al., 2014). Rising incidences of civilian and military TBI highlight a pressing need to identify the mechanisms of post-traumatic epileptogenesis. The progressive increase in risk for epilepsy with injury severity and long latency to seizures suggest that convergence of early pathological responses to trauma culminate in epilepsy (Kharatishvili & Pitkanen, 2010a; Lowenstein, 2009). In this review, we focus on the period within a week after injury to identify potentially epileptogenic alterations and shared mechanisms that may be targeted to prevent post-traumatic epilepsy.
1 CELLULAR AND CIRCUIT MECHANISMS OF POST-TRAUMATIC EPILEPSY
TBI has been shown to contribute to the development of neocortical and limbic epileptic foci (Curia et al., 2011). Among neocortical circuits, principal neurons in layer 2/3 of the prefrontal cortex exhibit increased intrinsic excitability and glutamatergic synaptic drive and a reduction in inhibitory synaptic inputs within a week after FPI (Smith, Xiong, Elkind, Putnam, & Cohen, 2015). Moreover, studies conducted 8–10 weeks after FPI have identified that the cortical tissue around the site of injury exhibits enhanced excitability indicating lasting perturbations that could underlie the development of cortical epileptic foci after TBI (D'Ambrosio et al., 2004). Interestingly, while reports that CCI augments epileptogenesis following amygdala kindling are consistent with the reduction in inhibition and an increase in cholinergic excitation after CCI (Eslami et al., 2016), amygdala excitability appears to be reduced after FPI, suggesting that injury modality may dictate specific circuit changes after TBI (Almeida-Suhett et al., 2014; Palmer, Metheny, Elkind, & Cohen, 2016). In contrast, both FPI and CCI have been consistently shown to augment excitability of the hippocampal dentate gyrus.
Brain injury leads to a cascade of early cellular and circuit changes in the hippocampus (Bolkvadze & Pitkanen, 2012; Hunt, Boychuk, & Smith, 2013; Lowenstein, 2009). The dentate gyrus, a functional gate to the hippocampal trisynaptic circuit (Heinemann et al., 1992; Lothman, Bertram, & Stringer, 1991) and the focus of circuit reorganizations in temporal lobe epilepsy, is particularly sensitive to TBI (Bruton 1988; Lowenstein, Thomas, Smith, & McIntosh, 1992). Dentate hilar and hippocampal neuronal degeneration characterize both experimental TBI and epilepsy (Kelly et al., 2015; Lowenstein et al., 1992; Saatman, Feeko, Pape, & Raghupathi, 2006; Toth et al., 1997). However, the tightly packed dentate granule cells (GCs) are generally preserved after brain injury and, paradoxically, show transient increases in numbers with enhanced generation of newborn granule cells from the subgranular neurogenic niche 3–7 days after FPI, as is reported after seizures (Jessberger et al., 2007; Neuberger et al., 2017). Although GCs survive the trauma, they show atrophy of dendrites and spines as early as 72 hours after trauma (Gao, Deng, Xu, & Chen, 2011). GC axons, known as mossy fibers, show evidence of abnormal sprouting of molecular layer collaterals targeting GC dendrites within a week after injury, replicating a pathological hallmark of temporal lobe epilepsy (Hunt et al., 2009; Jeub, Lie, Blumcke, Elger, & Beck, 1999; Kharatishvili et al., 2006; Santhakumar, Ratzliff, Jeng, Toth, & Soltesz, 2001). This is significant because, although sprouting may not be necessary for epileptogenesis (Buckmaster & Lew, 2011), modeling data indicate that sprouting is sufficient to promote seizure-like activity (Santhakumar, Aradi, & Soltesz, 2005). Neuronal injury after FPI occurs almost instantaneously due to impact. A majority of hilar neurons undergo subsequent degeneration over the course of a few days to a week due to secondary injury processes (Hunt et al., 2013; Toth et al., 1997). The focal impact of CCI generates more extensive loss of hippocampal pyramidal neurons than concussive injuries (Peterson, Maass, Anderson, Anderson, & Hoane, 2015). In contrast, the limited data on blast TBI suggests little acute cell loss within days after trauma (Kovacs et al., 2014). Using a programmable FPI device, we found that rapidly rising injury waveforms result in less neuronal injury at 24 hours, followed by more progressive cell loss in the ensuing week, than slower injuries (Neuberger et al., 2014). These findings suggest that the evolution of cell loss following rapid blast TBI may differ from that of slower impact injuries.
At a global level, brain injury impairs hippocampal rhythms, reducing the power of theta (4–8 Hz) oscillations (Paterno, Metheny, Xiong, Elkind, & Cohen, 2016) and leading to the emergence of pathological high frequency oscillations (pHFO) in the cortex within weeks after FPI (Bragin et al., 2016; Reid, Bragin, Giza, Staba, & Engel, 2016). Emergence of pHFO correlates with injury severity and results in progression to spontaneous seizures, indicating that pHFO could serve as an EEG biomarker for development of epilepsy after brain injury (Bragin et al., 2016). At the level of hippocampal circuits, the dentate consistently shows enhanced afferent evoked excitability within two hours to one week following brain injury, while CA1 appears less excitable (Gupta, Elgammal, Proddutur, Shah, & Santhakumar, 2012; Hunt et al., 2009; Reeves, Lyeth, & Povlishock, 1995; Schwarzbach, Bonislawski, Xiong, & Cohen, 2006; Titus, Furones, Kang, & Atkins, 2013; Witgen et al., 2005; Wolf et al., 2017). Increases in dentate excitability result from the convergence of a more excitable glutamatergic circuit and the loss of GABAergic hilar neurons that mediate feedback inhibition (Hunt et al., 2009; Lowenstein et al., 1992; Pavlov et al., 2011; Santhakumar et al., 2000; Toth et al., 1997; Witgen et al., 2005). Although GC intrinsic firing properties are largely preserved after trauma, their synaptic inputs undergo significant reorganization (Hunt et al., 2009; Santhakumar et al., 2000). In studies conducted 5–9 days after FPI, afferent activation evokes enhanced polysynaptic glutamatergic currents in GCs, with increases occurring exclusively from changes in AMPA and not NMDA currents (Li et al., 2015; Santhakumar et al., 2000). Consistent with these data, in vitro stretch injury models have identified a potential role for enhanced calcium permeable AMPA currents in excitotoxic neuronal loss after trauma (Spaethling, Klein, Singh, & Meaney, 2008). GCs also show enhanced spontaneous and evoked bursting activity as well as functional GC synaptic interconnections mediated by sprouted mossy fiber collaterals after CCI (Hunt et al., 2009). Unlike excitability, changes in inhibition vary with injury modality and severity. Action potential independent miniature inhibitory postsynaptic currents (mIPSCs) in GCs are consistently decreased across animal models, reflecting the loss of hilar GABAergic neurons (Gupta et al., 2012; Hunt, Scheff, & Smith, 2011; Pavlov et al., 2011; Toth et al., 1997; Witgen et al., 2005). However, spontaneous IPSC frequency increases within a week after mild-to-moderate FPI, reflecting a move towards homeostatic plasticity (Gupta et al., 2012; Santhakumar et al., 2001), but it remains depressed following severe and penetrating injuries (Hunt et al., 2011; Pavlov et al., 2011). Similarly, extrasynaptic, tonic GABA currents in GCs are enhanced a week after FPI but not at later time points and are persistently reduced after CCI (Boychuk, Butler, Halmos, & Smith, 2016; Gupta et al., 2012; Pavlov et al., 2011). In addition, there is a depolarizing shift in GC GABA reversal potential one week after brain injury (Bonislawski, Schwarzbach, & Cohen, 2007), which could further compromise the efficacy of both synaptic and tonic inhibition and impact brain rhythms (Proddutur & Santhakumar, 2015).
There is limited data on the effect of trauma on functional properties of neurons other than GCs. Hilar glutamatergic mossy cells, which have expansive septo-temporal projections to GCs, are lost in significant numbers after trauma and in epilepsy. Although their intrinsic excitability is unchanged, surviving mossy cells show altered intrinsic currents, depolarized membrane potential, and enhanced spontaneous and evoked glutamatergic inputs one week after FPI (Howard, Neu, Morgan, Echegoyen, & Soltesz, 2007; Li et al., 2015; Santhakumar et al., 2000). Semilunar granule cells, a morphologically and functionally distinct excitatory cell type with CA3 projections, show reduced synaptic and tonic GABAergic inhibition and are the only excitatory neuron with increased excitability after FPI (Gupta et al., 2012). Like mossy cells, the intrinsic physiology of CA1 Pyramidal cells is largely unchanged after brain injury (Cao, Hasuo, Ooba, Akasu, & Zhang, 2006). However, CA1 Pyramidal cells show reduced glutamate currents and long-term potentiation and enhanced inhibition after TBI (Johnson et al., 2014; Schwarzbach et al., 2006; Titus et al., 2013; Witgen et al., 2005). A subset of inhibitory neurons in the granule cell layer survive the initial injury, are depolarized, and fire at higher rates for hours-to-days after moderate FPI (Ross & Soltesz, 2000). Similarly, hilar GABAergic neurons receive more glutamatergic synaptic inputs and have increased spontaneous firing a week after CCI (Hunt et al., 2011). Together, the early cellular and synaptic changes after TBI likely drive increases in dentate excitability and promote epileptogenesis (Figure 1A).
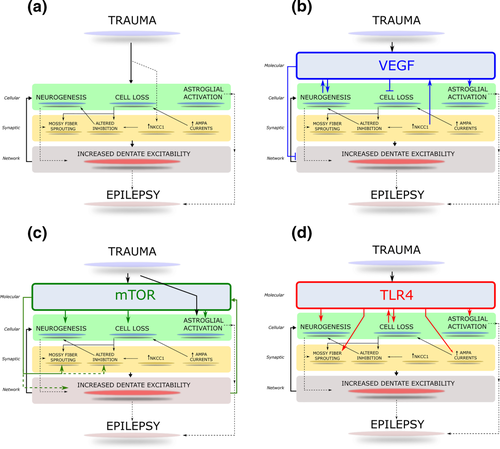
Schematic of converging molecular and cellular processes initiated early after TBI. (A) The schematic illustrates the major cellular and synaptic alterations in the dentate gyrus initiated within days to weeks after brain injury. The convergence of the processes on early dentate excitability and long term epileptogenesis is indicated. (B) Activation and influence of VEGF receptor signaling on early post-traumatic pathology. Illustration of proposed roles for VEGF signaling in modifying cellular pathology and excitability after brain injury. (C) The schematic indicates how the cellular and signaling processes illustrated in panel A could be influenced by activation of mTOR signaling after brain injury. (D) Illustration of known roles for TLR4 signaling in modifying cellular pathology and excitability after brain injury. Established pathways are indicated as solid arrows and potential interactions are denoted with dashed lines.
2 NEUROGENESIS AFTER BRAIN INJURY
The dentate gyrus is a niche region for neural stem cells, which support constitutive birth of granule cells throughout postnatal life. Similar to epilepsy, dentate neurogenesis is enhanced in the first week following TBI (Dash, Mach, & Moore, 2001; Kernie & Parent, 2010; Yu, Zhang, Liebl, & Kernie, 2008). GCs born after injury survive and mature into adult granule cells and have been proposed to contribute to long-term recovery (Sun et al., 2007; Villasana, Kim, Westbrook, & Schnell, 2015). Although the basic intrinsic properties of GCs born early after CCI are not different from controls, they do exhibit enhanced dendritic growth and branching and outward migration of somata 2–4 weeks later, which could compromise their role in the network (Villasana et al., 2015). In recordings from granule cells born after injury and examined four weeks after trauma, miniature excitatory and inhibitory synaptic events in GCs were found to be comparable to controls (Villasana et al., 2015). This is surprising, because these GCs born after injury exist alongside GCs with trauma-induced alterations in mIPSC and mEPSC frequency and in a fundamentally altered dentate network, with mossy fiber sprouting, hilar interneuron, and mossy cell loss (Hunt et al., 2013). Indeed, while physiological increases in neurogenesis can improve cognitive performance and the addition of new neurons may be beneficial, abnormal functional integration of GCs born after experimental seizures has been proposed to promote epileptogenesis (Danzer, 2012; Kernie & Parent, 2010). Consistent with this proposal, we recently demonstrated that limiting early post-traumatic neurogenesis to control levels reverses long term increases in seizure susceptibility and exhaustion of neuroproliferative capacity after FPI (Neuberger et al., 2017). Similarly, rapamycin treatment has been shown to reduce both neurogenesis and epileptogenesis after CCI (Butler, Boychuk, & Smith, 2015).
How do transient increases in neurogenesis after brain injuries contribute to epileptogenicity? While neurogenesis in the normal physiological range is reparative, it is possible that additional increases after TBI enhance excitability. Indeed, restoring post-injury neurogenesis to control levels reduced dentate hyperexcitability observed early after FPI (Neuberger et al., 2017). Concurrently, studies have also reported that ablating post-injury neurogenesis impairs recovery of cognitive function after TBI (Blaiss et al., 2011), demonstrating that basal neurogenesis is essential for recovery. Immature GCs are more excitable than mature GCs and show accelerated network integration after seizures (Dieni et al., 2016; Pugh et al., 2011). Thus, an increase in highly excitable and plastic newborn GCs, with accelerated network integration, could contribute to the early dentate hyperexcitability after TBI. It should be noted that hilar inhibitory neurons provide early and robust inhibitory innervation of newborn GCs (Markwardt, Wadiche, & Overstreet-Wadiche, 2009) and may be subject to post-traumatic loss, exacerbating the excitability of the GCs born after injury. Additionally, whether GCs born after injury show abnormal basal dendrites and axonal sprouting, as observed in some epilepsy models, remains to be tested (Kron, Zhang, & Parent, 2010). While there are several parallels between post-injury and post-seizure neurogenesis, seizures lead to ectopic hilar localization of some newborn GCs (McCloskey, Hintz, Pierce, & Scharfman, 2006), but hilar ectopics are rarely reported after brain injury (Neuberger et al., 2017; Yu et al., 2008). It is notable that the post-traumatic increase in neurogenesis is transient and leads to an accelerated decline in neurogenesis within a month (Neuberger et al., 2017). Thus, transient excesses in neurogenesis after TBI likely converge on and reinforce post-traumatic alterations to the mature circuit and augment excitability (Figure 1A).
3 MOLECULAR REGULATORS OF POST-TRAUMATIC NEUROGENESIS
Understanding the mechanisms underlying regulation of neurogenesis after TBI could provide ways to support recovery while preventing epilepsy. Neurovascular interactions mediated by vascular endothelial growth factor (VEGF), derived from both the vascular endothelium and neural precursors, appear to be key regulators of post-traumatic neurogenesis (Jin et al., 2002; Kirby, Kuwahara, Messer, & Wyss-Coray, 2015; Licht et al., 2016; Lu et al., 2011). In the adult hippocampus, neurogenesis and angiogenesis are closely linked processes (Fournier, Lee, Banasr, Elsayed, & Duman, 2012; Licht et al., 2016). Neuronal precursors proliferate in clusters around dividing vascular endothelial cells that release factors, which can induce neural precursor differentiation, defining a neurovascular niche (Palmer, Willhoite, & Gage, 2000). VEGF-A, a hypoxia-induced angiogenic protein, has been shown to promote the survival and integration of newborn neurons by stimulating production of endothelial cells, which release neurotropic factors (Louissaint, Rao, Leventhal, & Goldman, 2002). Although angiogenesis may be required to sustain enhancements in neurogenesis (Licht et al., 2016), VEGF augments neurogenesis in vitro (Jin et al., 2002), suggesting that VEGF may acutely enhance neurogenesis by direct effects on neural precursor cells even in the absence of angiogenesis. Among the multiple VEGF receptor subtypes, VEGF receptor type 2 (VEGFR2) is exclusively expressed in neural stem and progenitor cells and could mediate the direct effects of VEGF on neurogenesis (Kirby et al., 2015). Additionally, expression of the chloride transporter NKCC1 is upregulated within a week after brain injury and contributes to brain edema. This has been shown to trigger VEGF expression through the Raf-MEK-ERK signaling pathway and increase neurogenesis (Fournier et al., 2012). Consistent with the NKCC1-VEGFR2 regulation of neurogenesis, Bumetanide, (NKCC1 antagonist) SU1498 (a VEGFR2 antagonist), and MAPK inhibitors all reduced increases in neurogenesis when administered immediately after TBI (Fournier et al., 2012; Lu et al., 2011; Neuberger et al., 2017). Post-traumatic increases in NKCC1, coupled with decreases in KCC2 (a chloride extruder) could contribute to the depolarized chloride/GABA current reversal potential reported one week after TBI (Bonislawski et al., 2007; Fournier et al., 2012). In this regard, GABA currents have also been shown to modulate neural stem cell proliferation (Song et al., 2012). Thus, post-injury changes in GABA currents and chloride transporters could simultaneously compromise inhibition and enhance generation of excitable newborn GCs. Interestingly, VEGF acutely reduces neuronal excitability (Ma et al., 2009), and prolonged antagonism of VEGFR2 promotes excitotoxic hilar neuronal loss after TBI (Lee & Agoston, 2009). In contrast, brief SU1498 treatment after FPI reduces both neurogenesis and dentate hyperexcitability without altering excitability in uninjured controls (Neuberger et al., 2017). Additionally, VEGF supports neuroinflammation by promoting blood brain barrier permeability (Abdul-Muneer, Chandra, & Haorah, 2015). Taken together, VEGF signaling has complex effects on the cellular and physiological processes (Figure 1B) which, acting together, influence dentate excitability. Temporally regulated early antagonism of VEGFR2 signaling after TBI offers the unique ability to suppress neurogenesis, network excitability, and inflammation and thereby preempt epileptogenesis.
Another pathway at the crossroads of trauma, excitability, neurogenesis, and epileptogenesis is the mammalian target of rapamycin (mTOR) pathway (Chen et al., 2007; Park et al., 2012; van Vliet et al., 2012). mTOR is an evolutionarily conserved serine threonine protein kinase which forms the catalytic core of two functionally distinct complexes, mTORC1 and mTORC2. At the cellular level, mTOR complexes control several aspects of metabolism, survival, proliferation, and differentiation and play a crucial role in a number of diseases (Bhaskar & Hay, 2007). mTOR is activated primarily through PI3K/Akt pathways, but can also be modulated through the highly interconnected Raf/MEK/ERK pathway (Saini et al., 2013). Activating mTOR signaling enhances neurogenesis and reverses age-related decline in neurogenesis, although overstimulation reduces proliferative capacity (Tee, Sampson, Pal, & Bateman, 2016). mTOR phosphorylation is transiently enhanced within minutes after TBI and recovers to control levels within three days (Chen et al., 2007; Nikolaeva, Crowell, Valenziano, Meaney, & D'Arcangelo, 2016). Suppressing mTOR signaling after TBI reduces cell death, astrogliosis, mossy fiber sprouting, and neurogenesis (Butler et al., 2015; Nikolaeva et al., 2016). mTOR inhibitors also limit post-TBI changes in synaptic and tonic GABA currents (Butler, Boychuk, & Smith, 2016). However, whether mTOR drives or is regulated by network excitability remains to be resolved (Santos et al., 2017; Shima et al., 2015), mTOR signaling does converge on structural and functional plasticity in experimental epilepsy. Interaction of the mTOR pathway with multiple aspects of early post-traumatic alterations (Figure 1C) makes it a promising target to prevent epileptogenesis.
4 NEUROIMMUNE INTERACTIONS IN BRAIN INJURY
Brain injury, regardless of injury type, leads to an increase in permeability of the blood brain barrier and early inflammatory responses (Abdul-Muneer et al., 2013; Schmidt & Grady, 1993). The resident microglia are typically the first to respond within minutes following a focal brain injury and are recruited to the injury site where they undergo morphological and functional transformation and contribute to release of cytokines (reviewed in Jassam, Izzy, Whalen, McGavern, & El Khoury, 2017). There is also an early increase in reactive astrocytes within 24 hours after injury and recruitment of peripheral immune cells (Chiu et al., 2016; Li et al., 2015). The cellular inflammatory response is accompanied by activation of innate immune receptors in response to damage-associated molecular patterns (DAMPs) released by injured and dying cells. HMGB1 (a prototypic DAMP) is increased after seizures and contributes to epileptogenesis through activation of the innate immune receptor, Toll-like receptor 4 (TLR4; Maroso et al., 2010). HMGB1-TLR4 signaling is increased after TBI and augments production of pro-inflammatory cytokines (Ahmad et al., 2013; Laird et al., 2014). We recently demonstrated that the transient increase in TLR4 in the dentate gyrus, occurring four hours to three days after FPI, is neuronal and contributes to enhanced GC AMPA currents (Li et al., 2015). Notably, TLR4 antagonists reversed the early post-traumatic dentate hyperexcitability in ex vivo slices, suggesting a role for TLR4 signaling in excitability after brain injury (Li et al., 2015). TLR4 is also expressed in neural progenitor cells and regulates neurogenesis (Rolls et al., 2007). Recent studies have identified a strong temporal correlation between TLR4 expression and enhanced neurogenesis after CCI (Ye et al., 2014). Additionally, TLR4 suppression decreases mossy fiber sprouting in experimental models of epilepsy (Sedaghat et al., 2017). Thus, TLR4 signaling converges on neuroinflammation, neuronal excitability, neuroproliferation and mossy fiber sprouting after TBI (Figure 1D) and is a potential target to prevent epileptogenesis.
TLR4, VEGF, and mTOR are far from independent signaling pathways. mTOR signaling has been shown to enhance TLR4 expression (Yu, Kang, Xue, & Yin, 2011; Yu, Bo, Villani, Spencer, & Fu, 2016), and TLR4 was found to activate mTOR through Akt phosphorylation (Fang et al., 2017). VEGF, acting through VEGFR2, converges on the PI3K/Akt pathway to increase mTOR signaling, and mTOR activation enhances VEGF transcription (Liu, Chen, Cheng, Chen, & Qian, 2017). Moreover, studies in non-neuronal tissues have reported a causal association between TLR4 activation and increases in VEGF expression (Botero, Shelburne, Holland, Hanks, & Nor, 2006; Cho, Kang, Han, Um, & Lee, 2013). These studies highlight the highly interconnected molecular signaling between VEGF, mTOR, and TLR4 pathways and their convergence on various aspects of post-traumatic cellular and synaptic responses.
5 INTERACTING MECHANISMS REINFORCE PATHOLOGICAL PLASTICITY AND OFFER THERAPEUTIC TARGETS
We have focused on a subset of cellular, synaptic, and signaling mechanisms activated within hours to a week after brain injury, which we propose, set the stage for development of spontaneous epileptic activity. Increase in inhibition of glutamatergic neurons (Gupta et al., 2012; Howard et al., 2007) and excitation of GABAergic neurons (Hunt et al., 2011) may represent attempts to restore homeostasis. However, mutually reinforcing maladaptive alterations after TBI could augment dentate excitability (Figure 1). The molecular pathways activated after injury regulate critical cellular and synaptic responses to injury and many of these processes enhance excitotoxicity, cell death, neurogenesis, and sprouting, augmenting dentate excitability. The intertwined molecular pathways offer novel targets to limit early cellular changes and dentate hyperexcitability and could prevent development of epilepsy. In this regard, it is promising that suppressing either mTOR or VEGF signaling is sufficient to reverse the lowered seizure threshold after brain injury (Butler et al., 2015; Neuberger et al., 2017). Whether TLR4 antagonism or combinational therapy transiently suppressing mTOR, VEGF, and TLR4 signaling within days after brain injury could reduce the reinforcing maladaptive molecular and cellular interactions and prevent epileptogenesis remains to be determined.
6 CONCLUSION
The studies reviewed here suggest a strong reinforcing association between the molecular pathways, which regulate cell loss, synaptic plasticity, and neurogenesis. The converging influence of these pathways on cellular and synaptic processes contribute to early increases in dentate excitability following TBI and lead to the development of epilepsy. While mechanistic studies generally focus on a specific cellular or molecular pathway, the genetic and pharmacological manipulations adopted in experimental analyses inevitably modulate multiple interacting pathways, which act in concert to promote or limit the pathologies associated with TBI. The newfound convergence of multiple molecular pathways contributing to early TBI-induced pathologies may present an opportunity to target a combination of mechanisms to prevent post-injury epileptogenesis.
AUTHOR ROLES
Conceptualization, E.J.N., A.G., and V.S.; Data Curation, E.J.N., A.G., D.S., and A.A.K.; Writing – Original Draft, E.J.N., A.G., and A.A.K.; Writing – Review & Editing, E.J.N., A.G., D.S., A.A.K., and V.S.; Supervision, V.S.
Author Contributions
E.J.N., A.G., A.K. authored sections of the manuscript and reviewed relevant literature; D.S. prepared figures; E.J.N., A.G., D.S., A.K., and V.S. edited and approved the manuscript. V.S.: conception and scope of review.