Technique considerations for implementing volumetric-modulated arc therapy for total body irradiation within an Australian tertiary institution
Abstract
Over the past decade, our institution delivered conventional total body irradiation (TBI) using Elekta's Monaco treatment planning system (TPS) with an extended SSD field arrangement and 18 megavoltage (MV) energy lateral fields. In 2020, there was a transition to the Eclipse™ treatment planning system and Truebeam® linear accelerators with 6 MV and 10 MV energies. These changes meant that essential components of the existing technique were unavailable for clinical use and a new approach to the institution technique was required to ensure continuation of service. The aim was to implement a volumetric-modulated arc therapy (VMAT) TBI technique using existing infrastructure, the new planning system and treatment hardware to continue providing a TBI service for patients of all ages, including those under general anaesthetic (GA). A multidisciplinary team within the institution was created to evaluate existing literature and to implement a VMAT TBI technique that was feasible within our institution. This article will discuss the resultant technique, the practicalities faced and the radiation therapy pathway within our institution.
Introduction
Total body irradiation (TBI) is a radiotherapy technique used to provide an immunosuppression effect for patients undergoing haemopoietic stem cell transplantation. The basic premise of conventional TBI is to use large radiation fields at extended focus axis distance (FAD) to deliver a homogenous dose to the body, with a dose reduction to the sensitive organs at risk such as the lungs and kidneys1 enabled by custom lead shielding.
Previously, our institution utilised an adaptation of the conventional technique of lateral field in field (FiF).2 This required a 400 cm extended FAD, 18 MV energy and was delivered on a Clinac iX™ linear accelerator. The patient was in a lying position with a customised bed and spoiler, with 18 MV energy used because of distance requirements.
In 2020, our institution transitioned to the Eclipse™ treatment planning system and to Truebeam® linear accelerators with 6 MV and 10 MV energies. The new linear accelerators were installed in smaller bunkers that could not accommodate 400 cm FAD. With two key components of the existing technique unavailable, a new approach to TBI treatment was required.
A multidisciplinary team within the institution comprised of radiation therapists, medical physicists and radiation oncologists was created to evaluate existing literature and ultimately decide on a modern technique for implementation given our departmental considerations.
- suitable for paediatric cases under GA
- planned on Eclipse™ planning system from a planning CT scan
- compatible with Mosaiq® record and verify system
- deliverable on all TrueBeam® linacs in standard treatment bunkers (6 MV or 10 MV)
All modern techniques available in the literature were reviewed1-12 with departmental restrictions and multidisciplinary aims considered, resulting in the decision to proceed with a VMAT solution made in late 2020. VMAT technique considerations were guided by the literature available at the time of implementation,8, 10, 13 and modifications were made to meet department requirements. The department proceeded with a multi-isocentre VMAT solution that included a rotating couch frame.8, 13 Key points regarding custom equipment, simulation process, plan set-up, quality assurance and dose rate considerations are discussed in this report to assist other institutions with decision-making when considering the implementation of a modern TBI technique.
Custom equipment: rotating frame
The limitation of reaching relevant isocentre positions overall potential patient heights was considered. Calculations determined that a patient would have to be less than 100 cm to be treated entirely head first (HF) only. A custom frame was investigated and designed in house, taking inspiration from existing literature,8, 9, 13 to facilitate HF and feet-first (FF) treatment without impacting on original patient position and the ability to provide a consistent point of reference between CT scanner, planning and treatment position. The design brief was given to the medical technology and physics department within the institution for creation.
- lightweight sturdy material
- base plate and pivot plate
- lock points for standard immobilisation on top
- compatibility with the CT scanner and TrueBeam® couch
- highly reproducible positioning
Commercial manufacturing was considered, but due to global movement and costing restrictions at the time, in-house fabrication was decided to be the only feasible option. Figure 1 below displays the institutions custom-built rotating frame.
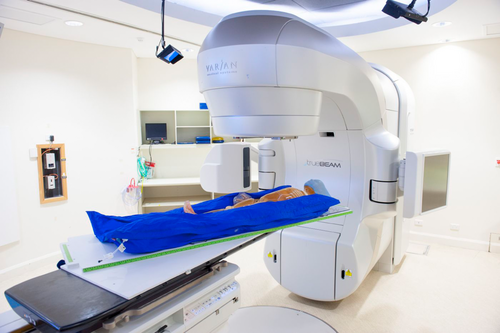
The custom frame was constructed out of Boardway-PVC Celuka Foam with the centre rotational centre made of A-Cast® cast acrylic. The length of the frame was constructed to account for CT and treatment couch limitations.
The frame was assessed by the occupational health and safety (OHS) team to ensure safe operation for both staff and patients. Guidelines for use were published for the department to ensure the OHS guidelines were upheld. These include patient weight less than or equal to 100 kg and ensuring sufficient trained staff is available at the time of rotation. Documentation and video of correct operating procedures of equipment were made available for all staff. Patients could be treated without the frame if over 100 kg, but this has not been encountered clinically to date and was outside the scope of commissioning at the time.
Mechanical commissioning was completed by the institution's physics team. The frame rotational set-up accuracy was found to be <0.5° and positional set-up accuracy to be <1.5 mm. This was deemed acceptable for sufficient reproducibility and clinical accuracy for TBI treatments.
The frame was designed with a custom scale to enable common referencing between the CT scanner bed, localising positions on the TPS and ensuring correct position on the treatment couch. Standardised positions were determined for the frame on the treatment couch and the longitudinal limit was determined.
Simulation and concatenation
Patients taller than 100 cm were unable to be scanned completely in one direction due to simulation and treatment couch longitudinal limits. A simulation protocol was developed to scan HF and FF with a 5.0 cm scan overlap to prepare for concatenation (stitching) of CT scans using Velocity™ software to allow for planning on one CT scan.
Both scans were referenced to the same physical point in either direction (CT0,0). Further referencing was completed in relation to the custom frame positioning scale to assist with localising the planning scan, standard anatomical landmarks and the treatment couch.
The frame was fixed to the bed in predefined positions. Patient immobilisation devices, including a vacuum bag, custom head support and mask, were made for the patient in a neutral position as possible, with arms placed alongside the torso and legs together. An interlocking smaller vacuum bag was used to mould feet position closely if the patient required it (patient length dependent). The key decisions in relation to patient positioning were ensuring a comfortable position for the patient due to extended treatment times.
Custom frame reference scale considerations
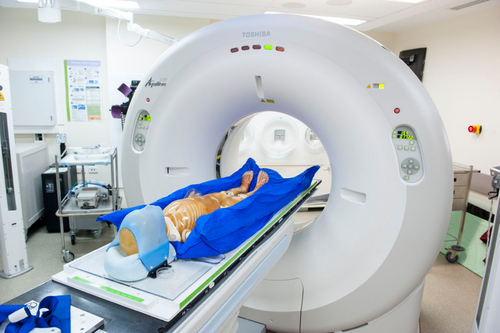
Given the frames' predefined and mandatory position on the treatment and CT couch, a scale was implemented on the frame that could be utilised to verify position and check for isocentre feasibility during the planning stages. CT0,0 positioning was required to be included in both head-first and feet-first scans and make concatenation viable. This point needed to be documented at the time of CT to ensure physical ability to reach isocentre positions on the treatment couch. A radiopaque marker was placed on the CT couch to indicate the treatment couch longitudinal limit on the planning CT scan as a secondary check for isocentre placement. Figure 2 displays the institutions set-up and positioning for the planning scan and treatment position.
Contouring and plan set-up
The TPS used for this technique was Eclipse (Varian, Palo Alto), version 15.6.
The planning target volume was defined as the anatomical body with 3 mm clipped from the skin and 3 mm into the lungs to account for breathing motion and ensure rib coverage. Skin clipping was determined clinically suitable to minimise skin reactions and side effects whilst still achieving required skin dose for TBI (verified by in vivo dosimetry). Anatomical lungs, kidneys and spinal cord were outlined. All contractions as described here were made using ‘Margin for Structure’ tool, with manual editing to extremities to ensure coverage within the target volume. The skin edge was defined using the lung window/level.
The HF plan consisted of several isocentres longitudinally placed down the body with multiple VMAT arcs per isocentres. A second plan was generated to treat the lower limbs in a feet-first direction using intensity-modulated radiation therapy (IMRT) anterior–posterior beams. The entire body plan can be visualised via a plan sum where the determination of adequate junctions is performed. The overlap required was set to be 4.0 cm per junction in a balance of physical robustness of dose uncertainty and realistic considerations of keeping isocentres to as minimum as possible. Figure 3 displays a typical plan set-up.
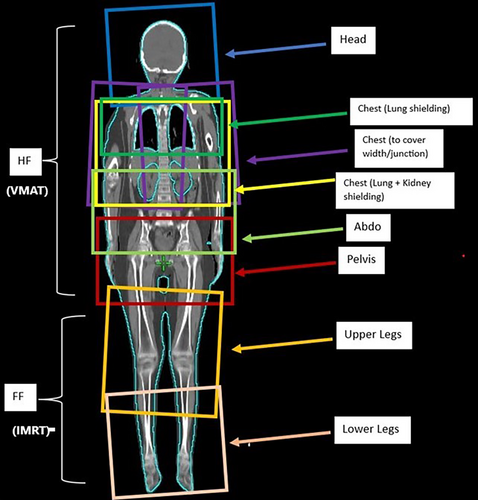
Isocentre considerations
The lung isocentre is placed first to enable the best field coverage and multi-leaf collimator (MLC) capabilities for lung sparing. Field sizes are maximised to physical limits (X = 30.0 cm and Y = 40.0 cm) with a combination of 90°, 2° and 358° collimator angles. Specifically, a 90° field is used to perform lung shielding analogous to traditional lung block shaping. Further isocentres are added to cover the body longitudinally, with a minimum of 4.0 cm field overlap with adjacent isocentres. The vertical and lateral isocentre values ideally remain constant per plan for ease of moving to the various isocentres during treatment. For patients with larger widths (generally >38.0 cm at the broadest point across shoulders), laterally shifted isocentres may need to be considered to ensure dose coverage at extremities. For each isocentre position, consideration of the longitudinal limit of the treatment couch must take place to ensure that isocentres are not placed in a physically unachievable location.
Customised spreadsheet
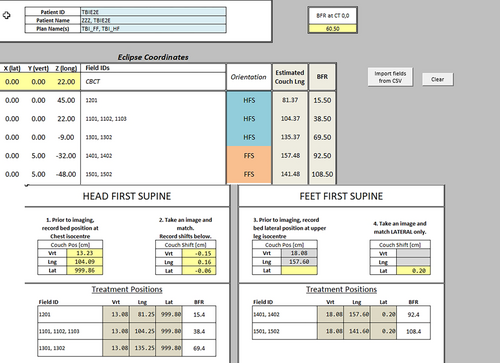
An institution spreadsheet and underlying calculation algorithm were designed to link the frame reference and physical location of isocentres in the treatment room to aid in the placement of isocentres and confirm the feasibility of positioning on the linear accelerator.
Using CT 0,0 frame reference, the isocentre coordinates are taken from the TPS through a customised script and applied to the spreadsheet. This assists in determining the HF and FF limit of isocentre coordinates in physical reality and translates to a treatment tool to verify the isocentre position with image verification. Figure 4 presents a standard field set-up ensuring HF and FF ability to reach the required isocentre position and the first fraction spreadsheet example for treatment.
Plan optimisation and review
After plan set-up, plans are optimised to meet the goals in Table 1 presented below – shown for a prescribed dose of 12 Gray (Gy) in 6 treatment fractions (treatment is delivered bi-daily with a minimum of 6 h interval). Plans are calculated with Acuros 13.5 as this is the standard algorithm used clinically for all treatment techniques within the institution.
| Structure | Dose constraints |
|---|---|
| 1PTV_12Gy | D90% ≥ 12 Gy (100%) D95% ≥ 11.4 Gy (95%) D100% ≥ 10.8 Gy (90%) Max Dose <15 Gy |
| Lungs10mm | Mean = 8 Gy |
| Lungs3mm | Mean = 9 Gy |
| Kidneys | Mean Aim ~ 12 Gy Homogenous as possible |
| Bones | Homogenous as possible Clinical Evaluation of Hot/Cold Spots |
| Spinal Cord | Homogenous as possible |
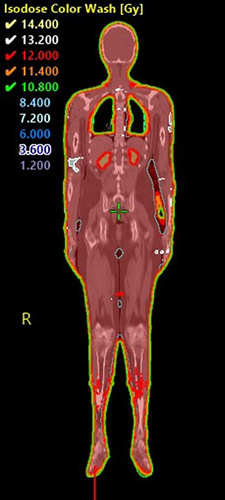
The dose is reviewed through the plan sum to ensure plan overlap is dosimetrically acceptable. Figure 5 shows a typical resultant dosimetry across the whole treatment volume.
Dose verification quality assurance
Due to the large treatment fields, portal dosimetry as a primary method was chosen as no other method was found to be suitable to measure the full dose of the individual treatment fields.
For the individual VMAT arc fields and the IMRT leg fields, portal dosimetry and ArcCHECK cylindrical detector array measurements were made with ≥95% 3%/3 mm Gamma pass criteria.
During commissioning, extensive dosimetric verification testing for the junction regions was performed. The CIRS IMRT Thorax Phantom was used for junction region point dose measurements with cc04 ionisation chamber and EBT3 gafchromic film measurements in the transversal plane. Solid water phantom was used for gafchromic film junction field measurements in the coronal plane. ArcCHECK cylindrical detector array was used to measure the field junctions between overlapping adjacent fields with ≥95% 4%/3 mm Gamma pass criteria.
For end-to-end testing, a PBU-70 life-size full-body paediatric anthropomorphic phantom was used with TLDs and gafchromic films used for in vivo dosimetry.
Based on the commissioning measurements, a customised patient-specific dose delivery verification system was put in place which included portal dosimetry measurements of the individual VMAT arcs and IMRT leg fields with ≥95% 3%/3 mm Gamma pass criteria, ArcCHECK measurements of the junction areas with ≥95% 4%/3 mm Gamma pass criteria and in vivo dosimetry measurements using gafchromic film (±10% dose compared to TPS).
Dose rate considerations
The dose rate is lowered for field treatment through the lungs and kidneys. This was a clinical decision to align with dose rates utilised for conventional TBI treatments.1 The full dose rate (600 MU/min) is utilised for remaining fields that do not pass through the lungs or kidneys.
Results and Patient Characteristics
The study received approval from the Sir Charles Gairdner Osborne Park Health Care Group as Quality Improvement Activity (NSQHSS/EQuIP) (GEKO 49289) to collect data for VMAT TBI patients with the view to audit patients treated with this technique. These results will become benchmark results for future improvements to the technique. Previous lateral technique results are not compared in this report as data migration was occurring during the time of implementation, VMAT TBI literature available at the time was utilised to ensure appropriate organ at risk and target coverage was achieved.8, 9 Patient characteristics, lung dose and PTV coverage are provided to assist other departments in implementing and comparing VMAT TBI implementation results.
Since clinical implementation in early 2021 until the end of 2023, the institution has treated four patients with the following results as presented in Table 2.
| Height (cm) | Weight (kg) | Isocentres | Lung 1.0 cm dose (Gy) | PTV volume (cm3) | PTV coverage (%) | Max dose of PTV (Gy) | |||
|---|---|---|---|---|---|---|---|---|---|
| V10.8Gy | V11.4Gy | V12Gy | |||||||
| 1 | 181 | 81 | 13 | 6.583 | 76,843.1 | 99.8 | 99.6 | 98 | 14.08 |
| 2 | 146 | 37 | 5 | 6.494 | 28,808.7 | 99.96 | 99.7 | 97 | 14.592 |
| 3 | 142 | 37 | 5 | 6.414 | 30,287 | 99.95 | 99.6 | 94.2 | 15.121 |
| 4 | 158 | 65 | 9 | 7.3 | 57,317.1 | 99.6 | 98.6 | 91.4 | 14.762 |
Summary
This report has summarised key procedural aspects of the implemented VMAT TBI technique with a customised frame. The technique was required to be feasible for paediatric patients under general anaesthetic, utilise 6 MV or 10 MV energy, deliverable in smaller bunker rooms and align the treatment method with other standardised techniques within the institution. The VMAT technique has suitably met the project aims to provide a modern TBI technique for the institution.
Considerable effort is required from a multidisciplinary team to implement such a technique and given the small and unpredictable patient numbers that are seen through our institution, clear documentation of procedures is paramount.
- Multi-disciplinary efforts are required for clinical implementation of a technique of this nature. Given the heterogeneity of evidence-based recommendations that currently exist, this is key to successful implementation.
- Existing literature and data are very siloed, a need for more robust data to inform techniques and standardise guidelines for treatment into the future is required.
- Staff training is long and complex and ensuring appropriate team available can be challenging. It is recommended a core team with sufficient experience is trained in line with technique implementation.
Conclusion
TBI has traditionally been delivered within our institutions with an extended SSD conventional technique. The institution purchased a new treatment planning system in 2020 and upgraded linear accelerators which led to the need of changing the TBI technique. A multi-disciplinary team was brought together, and a thorough literature search performed in order to identify which approach to delivering TBI would meet institutional needs and be clinically viable. VMAT was identified as the best option for institutional requirements and end to end protocols were established and commissioned to use this technique clinically.
Dosimetric results and patient characteristics have been documented and provided as a benchmark for future adaptation to the technique within our institution in the future. Key learnings from our experience in implementing this technique have been provided to assist further implementation in departments changing their TBI technique.
Future Considerations
Since implementation, literature in this area continues rapidly, with more centres developing individual techniques.3, 14 However, outcomes from national and international bodies,14, 15 with overall recommendations, are currently heterogeneous and centre specific. Work towards homogeneity and collaborative work, within Australia and worldwide is critically needed in this specialised technique.
Increasing the number of organs that are contoured will be beneficial for dose reporting in the future. Similarly, the capacity for further reduction of dose to organs at risk should be explored, with careful clinical consideration of maintaining sufficient dose to provide the desired outcomes of TBI treatment.
Integration of motion management during treatment needs to be investigated further with implementation of 4DCT planning scans and density considerations of lung volume variations during the standard breathing cycle.
Total marrow irradiation (TMI) may be considered in the future, with the current technique lending itself into future technical considerations when treating TMI.
Conflict of Interest
The authors declare no conflicts of interest.
Ethics Statement
An exemption from institutional ethics was granted (GEKO 49289) to collect data for VMAT TBI patients with the view to audit patients treated with this technique and was registered as a Quality Improvement activity with the Safety, Quality and Performance Department within the hospital.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




