Prenatal cranial bone development of Thomas's horseshoe bat (Rhinolophus thomasi): with special reference to petrosal morphology
Funding information: KAKENHI, Grant/Award Numbers: 26711023, 24000015, and 24370035 (to D.K.), and 15K1458 (to T.S.); NAFOSTED, Grant/Award Number: 106-NN.05-2016.14 (to N.T.S and V.T.T.); Swiss National Science Foundation, Grant/Award Number P300PA_164720 (to I.W)
Abstract
Cochlear morphology has been regarded as one of the key traits to understand the origin and evolution of echolocation in bats, given its functionality and performance for receiving echolocation sonar. While numerous researchers have compared adult-stage morphology, few have studied the prenatal development of the cochlea. Here, we provide the first detailed three-dimensional description of the prenatal cranial development in bats, using Rhinolophus thomasi as a model, with particular interest to the petrosal which houses the cochlea. Results revealed that among all cranial bones the onset of the ossification of the petrosal is earlier in R. thomasi when compared to other reported mammals. Generally, the cochlea reaches adult size and shape before or around birth in placental mammals including bats, but we found that its shape and size growths continue until maturity in Rhinolophus species. The relationship of cochlear size and skull size is maintained constant throughout the postnatal ontogeny to adulthood in Rhinolophus, a pattern previously reported neither in any other bats nor other mammals. The peculiar developmental pattern in Rhinolophus possibly allows them to form their characteristically large cochlea and facilitate their distinctive echolocation behavior. A recent study reported that non-echolocating Pteropodidae shares a similar prenatal cochlear size to laryngeal echolocating bats. The apparent resemblance of fetal cochlear size was proposed to be a vestigial signal of large cochlear size in the last common ancestor of bats and thus as supporting evidence for the single origin of laryngeal echolocation. However, results from the present observations suggest that limited aspects of the cochlear development were captured in this previous investigation and that the resulting interpretations may be questionable. We point out that diversity and patterns of cochlear development among bats are still not resolved, and the controversy on the origins of laryngeal echolocation is still open to discussion.
1 INTRODUCTION
Over 1300 extant bat species (Chiroptera, Mammalia) are described (Teeling et al., 2005), and most of these recognized bat species employ a sophisticated biosonar system, the echolocation (Russo & Voigt, 2016). Echolocating bats emit high-frequency sounds (pulse) and then receive reflected sounds that return from surrounding objects (echo) (Fenton, 2013). This permits bats to compare differences between pulse and echo and to form “acoustic images” of their surroundings even under total darkness (Moss & Surlykke, 2001). Molecular evidence supports that bats are phylogenetically divided into two major clades, Yinpterochiroptera and Yangochiroptera (Teeling et al., 2005). Echolocation behavior is found in Yinpterochiroptera (all members of Rhinolophoidea and some members of Pteropodidae) and all members of Yangochiroptera (Jones & Teeling, 2006). Several types of echolocation are characterized based on the emission method and its sound route. The echolocation in bats can be basically categorized into the “laryngeal echolocation”, in which pulse emerges from the larynx, and “lingual echolocation”, in which pulse is generated by tongue clicks (Kössl, Foeller, & Faulstich, 2004; Holland & Waters, 2005). Laryngeal echolocation, the more sophisticated biosonar system, is employed in Rhinolophoidea (Yinpterochiroptera) and in all bats of Yangochiroptera while lingual echolocation is found in several members of Pteropodidae (Kössl et al., 2004; Holland & Waters, 2005; Jones & Teeling, 2006). A line of molecular evidence has supported that laryngeal-echolocating bats (Rhinolophoidea and all members of Yangochiroptera) are not monophyletic (Teeling et al., 2005; Jones & Teeling, 2006; Parker et al., 2013). Thus, it has been highly disputed whether laryngeal echolocation was acquired by the common ancestor of all extant bats and was subsequently lost in Pteropodidae, or it evolved independently in Yangochiroptera and Rhinolophoidea (e.g., Li, Wang, Rossiter, Jones, & Zhang, 2007; Veselka et al., 2010; Zhang et al., 2013; Wang et al., 2017).
The cochlea which is part of the membranous labyrinth of the inner ear housed in the bony labyrinth of the petrosal bone has attracted much interest by chiropterologists as the functionality and performance of receiving echolocation sonar among bats appears to be reflected in its morphology and have been regarded as the key features to understand the evolution of echolocation in bats (Davies, Maryanto, & Rossiter, 2013; Kössl et al., 2004; Pye, 1966; Russell, Drexl, Foeller, Vater, & Kössl, 2003; Simmons, Seymour, Habersetzer, & Gunnell, 2008). In particular, the specialized morphology of the secondary spiral lamina of the basal turn is pointed out to improve resonance properties (Vater, 2000). Sensitivity to high-frequency sound can be predicted by the relative size of the cochlear canal in the skull at adulthood among bats (Habersetzer & Storch, 1992; Simmons & Geisler, 1998; Simmons et al., 2008). Pteropodidae, which is not capable of echolocation or just employ the lingual echolocation, exhibit the smallest cochlear canal size relative to the skull size among bats whereas Rhinolophidae, the group which is capable of using the highest duty cycle pulse and Doppler shift compensation, exhibits the largest cochlear canal size relative to the skull among bats (Habersetzer & Storch, 1992; Simmons et al., 2008). Recently, Wang et al. (2017) compared the ontogeny of the cochlear canal in several mammals and proposed that relative size of the cochlear canal in the fetal period is similar among members of non-laryngeal echolocating Pteropodidae and laryngeal echolocating Rhinolophoidea and Yangochiroptera. According to their interpretations, the relative size of the fetal cochlear canal of the former is of similar size as in the latter two, but its cochlear growth rate decreases postnatally while the growth rate of the whole skull is maintained, and subsequently its relative size becomes comparable to those of other non-echolocating mammals. Wang et al. (2017) interpreted the resemblance of fetal cochlear canal size among Pteropodidae, Rhinolophoidea, and Yangochiroptera as a vestigial signal of large cochlear size in the last common ancestor of extant bats, leading from the assumption that “ontogeny recapitulates phylogeny” (Haeckel, 1866). This led them to conclude that the common ancestor of extant bats was possibly a sophisticated echolocator and that laryngeal echolocation emerged just once. Given the evidence that prenatal development of skeletons is evolutionary labile and heterochronies are highly common among mammals (Kuhn & Zeller, 1987; Koyabu et al., 2011, 2012, 2014; Koyabu & Son, 2014; Koyabu, 2017; Werneburg et al., 2013; Zeller, 1987), whether the quantitative traits of a common ancestor can simply be inferred from fetuses of extant species is questionable. Meanwhile, regardless of the applicability of Haeckel's law of recapitulation, at the very least, Wang et al.'s (2017) interpretation that fetal growth is similar between non-laryngeal echolocators and laryngeal echolocators cannot be drawn from their dataset since only a limited number of fetal stages were collected and only a few aspects of cochlear growth were compared in their study. In particular, only a limited number of species of laryngeal echolocating Rhinolophoidea were sampled, and growth patterns were estimated from just sampling a couple of fetuses at similar developmental stages for Rhinolophus pusillus (n = 2, basicranial width 7.12 and 7.20 mm) and Rhinolophus sinicus (n = 3, basicranial width 8.37, 8.50, and 8.75 mm). Even if Haeckel's law were applicable to infer ancestral patterns, Rhinolophoidea would have been a crucial taxon to be investigated in detail, and shared developmental patterns of auditory traits between laryngeal Rhinolophoidea and non-laryngeal Pteropodidae should have been demonstrated to support their proposition, given the sister-group relationship of these two taxa within Yinpterochiroptera.
Further detailed investigation on the inner ear of Rhinolophoidea may help understandig the origins of echolocation among bats. Therefore, we collected various fetal stages of Rhinolophus thomasi, a member of Rhinolophoidea, and described the prenatal development of the skull anatomically and morphometrically with special reference to the inner ear. Rhinolophus is a predominantly insectivorous taxon of Old World bats consisting of about 80 species, distributed in temperate and tropical habitats today (Csorba, Ujhelyi, & Thomas, 2003; Simmons, 2005). It possesses intricately shaped protuberances from the nasal, the noseleaves, which allow differential processing of subbands of the pulse in the acoustic domain (Zhuang & Müller, 2006). Rhinolophus is characterized by emitting the longest pulses and having the highest duty cycles among bats (Jones, 1999), and its long echolocation calls are emitted with very pronounced constant frequency (CF) segments (Schnitzler & Ostwald, 1983). Rhinolophus is capable of compensating for Doppler shifts by adjusting their call frequency and maintaining the echo frequency within the narrow frequency range that is optimal for the acoustic sensing (Hiryu, Katsura, Lin, Riquimaroux, & Watanabe, 2005). Hipposideros, another member of Rhinolophoidea, also uses CF calls with Doppler shift compensation, but its emitted pulses are shorter and duty cycles are lower than those of Rhinolophus (Jones, 1999). R. thomasi is a small horseshoe bat with forearm length ranging from 41.7 to 45.4 mm, distributed across Laos, Myanmar, Thailand, and Vietnam (Csorba et al., 2003; Kruskop, 2013). This species is commonly found in both primary and secondary forests (Kruskop, 2013). Prenatal postcranial ossification patterns in this species have been previously reported by Koyabu & Son (2014). Complete mitochondrial genome sequencing suggests its phylogenetic affinity to R. sinicus (Stoffberg, Jacobs, Mackie, & Matthee, 2010; Xing & Mao, 2016).
Previous cranial developmental studies of bats primarily focused on the tympanic roof and tympanic floor or the whole cranium, and studies on the inner ear development are still few. Among Rhinolophoidea, the otic region was described for two Rhinolophus rouxii embryos with a skull length of 15 mm (Sitt, 1943). Although the otic capsule was cartilaginous in these specimens, the remainder of the skulls already showed much ossification. A histological study on Myotis myotis (27 specimens) and Myotis capacinii (two specimens) fetuses (Yangochiroptera, Vespertilionidae) was performed by Frick (1954), which is still the most detailed developmental study on chiropteran fetal cranial anatomy to date. The ear capsule chondrifies from just one coherent blastematous mesenchymal condensation (Myotis stage 1), although much earlier stages, which could perhaps show separate mesenchymal aggregations, still need to be studied (Frick, 1954). Chondrification starts ventrally in the petrous portion of the petrosal that houses the cochlea and seems to be a rapid process. Soon after the onset of chondrification, the whole otic capsule is chondrified (Myotis stage 2), and the cartilaginous stage seems to persist for some time (Myotis stages 2–4). Ossification is described to appear by different centers of ossification with septum metacochleare, prominentia utriculo ampullaris superior, septum metacochleare (in particular its region near the cochlear basis), and the basis of processus recessus (at the ventral edge of cochlear) ossifying first (Myotis stage 5), followed by the semicircular canals (which already show hypertrophied chondrocytes in Myotis stage 5) and cavum cochleare, forming the bony border of membrana tympani secundaria (fenestra rotunda) (Myotis stage 6). The author reported that further progress of ossification occurs after that, in parallel to the increase of cochlear capsule size (Myotis stage 7). Later, Wible and Davis (2000) reviewed the developmental differences of the tympanic floor among Megaderma (Davis, 1998), Myotis (Frick, 1954; Wible, 1984; Wible & Martin, 1993), Rousettus (Van der Klaauw, 1922), and Pteropus (Van der Klaauw, 1922; Wible, 1992), and pointed out that the relative contributions of the ectotympanic and entotympanic to the auditory bulla differ between “microbats (Megaderma and Myotis)” and “megabats (Rousettus and Pteropus)”, with the ectotympanic being more substantial in the former and the entotympanic more substantial in the latter. Pedersen (2000) compared the cranial developmental differences between nasal emitters and oral emitters by cephalometry. He reported that the ontogenetic changes of the lateral semicircular canal orientation relative to the basicranial axis differ between oral emitter Myotis and nasal emitter Nycteris. Studies on the postnatal cochlear development of bats were conducted by Vater (2000) and Carter and Adams (2015). They reported that the cochlear traits particularly essential for echolocation (basilar membrane and secondary spiral lamina) are formed already at birth in bats (Vater, 2000; Carter & Adams, 2015). However, how the development of the inner ear proceeds before birth in bats and the variation of inner ear development among bats have been largely unknown.
In this study, we demonstrate that Rhinolophus bats exhibit a distinctive cochlear development not reported in other bat groups, which allows them to form a relatively large cochlea at maturity and possibly facilitates their distinctive echolocation behavior, and we contribute to the discussion on evolutionary origins of echolocation.
2 MATERIALS AND METHODS
2.1 Data acquisition
We studied the skulls of ten fetuses and one adult specimen of Rhinolophus thomasi (Table 1). All specimens were obtained from surveys in Vietnam by N.T.S., V.T.T, and D.K. and are stored at the University Museum, University of Tokyo. Animals were euthanized by cervical dislocation, approved by the animal welfare guideline of the affiliated institution of D.K. Collected fetuses were stored in 70% ethanol. Then we acquired grayscale images of the fetuses by a microfocal X-ray CT system at the University Museum, University of Tokyo (ScanXmate B100TSS110, Comscantecno Co., Ltd.) with 70 kV source voltage and µ114 A source currents. Voxel size was 20µm for the smallest specimen (NH31) and 36 µm for rest of the specimens. Each of the reconstructed images was in the form of 512 × 512 matrices of 12-bit grayscale values. Manual segmentation and analysis of grayscale images were conducted in Amira 5.2 (Visage Imaging, Berlin, Germany). Thresholding between the CT-values of bone and non-bone regions by half maximum height method (HMH) (Spoor, Zonneveld, & Macho, 1993), we segmented the bony boundaries in the skull with slice-by-slice manual adjustments using Segmentation Editor Tool in Amira 5.2. HMH method is commonly employed as the optimum way to place the interface between two different substances (Kubo et al., 2008). In short, the position of the boundary is placed at half the height of the maximum drop of the CT-value profile between two substances. Finally, polygon surface data was generated from the manually segmented parts using SurfaceGen Tool.
| Skull stage | Cochlearstage | ID | CRL | GM | SL | SH | BW | CW | CW/BW | PA | PV |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | – | NH31 | 7.59 | – | – | – | – | – | – | – | – |
| 2 | – | PQ109 | 13.77 | – | – | – | 5.10 | – | – | – | – |
| 3 | 2 | PQ116 | 15.15 | 5.25 | 8.55 | 3.90 | 4.33 | – | – | 1.26 | 0.06 |
| 4 | 1 | VC50 | 13.14 | 5.97 | 9.43 | 3.77 | 5.99 | – | – | 1.37 | 0.10 |
| 5 | 4 | PQ97 | 13.82 | 6.02 | 9.19 | 4.06 | 5.84 | 2.58 | 0.44 | 12.63 | 0.96 |
| 6 | 3 | VC40 | 14.99 | 6.59 | 9.91 | 4.15 | 6.97 | 2.92 | 0.42 | 11.72 | 0.89 |
| 7 | 5 | PQ105 | 15.63 | 6.67 | 9.84 | 4.61 | 6.53 | 2.75 | 0.42 | 27.67 | 2.62 |
| 8 | 6 | VC68 | 16.24 | 7.13 | 10.75 | 4.54 | 7.43 | 2.97 | 0.40 | 32.84 | 2.84 |
| 9 | 7 | VC62 | 20.58 | 7.80 | 12.27 | 5.09 | 7.59 | 2.95 | 0.39 | 53.16 | 5.50 |
| 10 | 8 | VN11_0230 | – | 10.14 | 20.37 | 5.37 | 9.53 | 3.65 | 0.38 | 58.91 | 24.68 |
- CRL: crown-rump length; GM: geometric mean of the skull; SL: skull length; SH: skull height; BW: basicranial width; CW: cochlear width; CW/BW: ratio of cochlear width to basicranial width; PV: petrosal volume; PA: petrosal surface area.
- CRL, SL, SH, SH, BW, CW are in mm, PA is in mm2, and PV is in mm3.
2.2 Measurements
The crown-rump length (CRL) was taken as the distance from the cranial tip of the head to the caudal tip of the rump using sliding calipers. All other measurements were conducted on generated surfaces using 3D Length Tool in Amira 5.2. The skull length (SL) was measured as the distance from the rostral tip of the premaxilla to the ventral end of the interparietal in the sagittal plane. The skull height (SH) was taken as the distance from the midpoint of the cranial tip of the left and right parietals to the rostral tip of the basioccipital.
Previous studies quantifying the bat inner ear such as Habersetzer & Storch (1992) and Wang et al. (2017) used the wording “cochlea” in their studies, but the cochlea is in principle a soft tissue structure that is part of the membranous labyrinth of the inner ear, which is housed in the cochlear canal of the bony labyrinth of the petrosal bone. The trait that was actually measured in these studies was the cochlear canal of the bony labyrinth rather than the soft-tissue cochlea itself, but we follow their wording for consistency. Following Wang et al. (2017), which was based on the measurements by Habersetzer & Storch (1992), the basicranial width (BW) was measured as the distance between the lateral tip of the left-side cochlear canal and the lateral tip of the right-side cochlear canal. The cochlear width (CW) was measured from the end of the first half turn of the cochlear canal to the end of the second half turn at the clear outline of the cochlear canal visible in CT-images as in Wang et al. (2017). We confirmed by personal communication with one of the authors from Wang et al. (2017) that our measurement definitions were in accordance with their study. It has been shown that laryngeal-echolocating bats show relatively larger CW/BW ratio compared to non-laryngeal-echolocating bats such as Pteropodidae, and this ratio has been suggested to reflect sensitivity to high-frequency pulses (Habersetzer & Storch, 1992). Petrosal volume (PV) and petrosal surface area (PA) were also measured from the generated surfaces. All measurements were taken from the left-side petrosal on generated surfaces.
The BW, CW, and CW/BW values of R. thomasi were compared to other mammalian species reported by Wang et al. (2017) (R. pusillus, R. sinicus, Miniopterus schreibersii, Hipposideros armiger, Myotis ricketti, Cynopterus sphinx, Rosettus leschenaulti, Felis catus, Mus musculus, Erinaceus amurensis, Oryctolagus cuniculus, Rattus norvegicus). We note that their indices (BW/CW) are the inverse of ours (CW/BW), although interpretations are not affected. The first study by Habersetzer & Storch (1992) that examined the cochlear size and skull size and a subsequent study by Simmons et al. (2008) assigned BW as the independent variable and CW as the dependent variable, but Wang et al. (2017) used BW/CW values rather than CW/BW. We adopted to use CW/BW because BW which reflects skull size is more appropriate as the denominator and this allows consistency with Habersetzer & Storch (1992) and Simmons et al. (2008).

2.3 Statistical analyses
To compare relative petrosal size between specimens of different cranium size, we conducted linear regression analyses with PAST3. In this analysis, PV was log10-transformed and regressed against log10-transformed GM using reduced major axis (RMA) regression analysis. Here, GM was considered to be the implicit size variable (Mosimann & James, 1979). Using reduced major axis analysis is more appropriate than other linear regression such as the ordinary least squares method, or the major axis method, because the RMA provides the best-estimated regression between every populations from which the sample is drawn when the error variance is unknown and is not affected by the correlation coefficient of every sample (Rayner, 1985; Aiello, Freedman, & Wu, 1992).
2.4 Staging and anatomical descriptions
Because an embryonic staging system is not yet available for Rhinolophus, fetuses were ordered according to the GM of the skull of each individual. However, as an embryonic staging system for Hipposideros armiger, another member of Rhinolophoidea, is available, we additionally referred to Wang et al.'s (2010) staging system of this species to allow tentative staging. The presence/absence of each bone was recorded and compared against the GM. We adopted the anatomical nomenclature from Frick (1954), Wible and Davis (2000), and Ekdale (2013).
2.5 Anatomical abbreviations
| ali | alisphenoid |
| an | angular process |
| ar | articular process |
| asca | anterior semicircular canal ampulla |
| bcc | basicochlear commissure |
| bcf | basicochlear fissure |
| bo | basioccipital |
| bs | basisphenoid |
| c | canine |
| cc | common crus |
| cop | coronoid process |
| crp | crista parotica |
| den | dentary |
| e | ectotympanic |
| er | elliptical recess of the vestibule |
| exo | exoccipital |
| fai | foramen acusticum inferior |
| fc | fenestra cochleae |
| fm | foramen magnum |
| fo | foramen ovale |
| fro | frontal |
| g | goniale |
| ha | hamulus |
| hc | hypoglossal canal (of cranial nerve XII) |
| if | infraorbital foramen |
| ip | interparietal |
| jug | jugal |
| max | maxilla |
| nas | nasal |
| ors | orbitosphenoid |
| p1 | first premolar |
| p2 | second premolar |
| p3 | third premolar |
| pal | palatine |
| par | parietal |
| pl | primary bony lamina |
| pet | petrosal |
| pmx | premaxilla |
| pp | premaxillary palatal branch |
| pro | promontorium |
| prs | presphenoid |
| psc | posterior semicircular canal |
| pt | pterygoid |
| sc | suprafacial commissure |
| sf | superior orbital fissure |
| sl | secondary bony lamina |
| so | supraoccipital |
| st | stylohyal |
| sq | squamosal |
| vo | vomer |
| zm | zygomatic process of maxilla |
| zs | zygomatic process of squamosal |
3 RESULTS
A detailed depiction of bony skull formation in all specimens is illustrated in Figures 1 and 2. All measurements are summarized in Table 1.
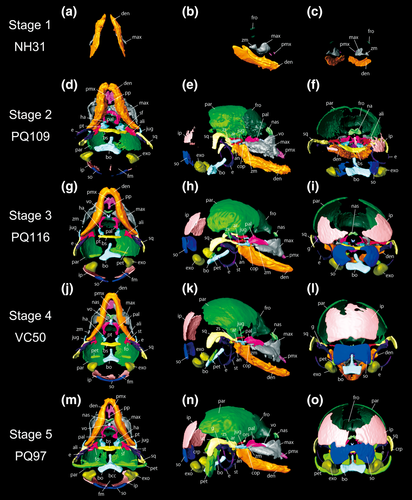
Rhinolophus thomasi, reconstructed skull model of the fetuses of skull stage 1 to 5, obtained from CT-image segmentation. (a–c) ventral, lateral, and caudal views of stage 1. (d–f) ventral, lateral, and caudal views of stage 2. (g–i) ventral, lateral, and caudal views of stage 3. (j–l) ventral, lateral, and caudal views of stage 4. (m–o) ventral, lateral, and caudal views of stage 5. See text for abbreviations
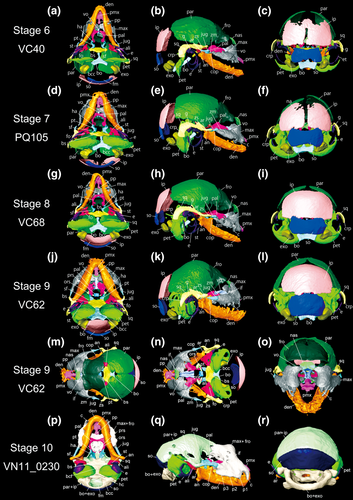
Rhinolophus thomasi, reconstructed skull model of the fetuses of skull stage 6 to 10, obtained from CT-image segmentation. (a–c) ventral, lateral, and caudal views of skull stage 6. (d–f) ventral, lateral, and caudal views of stage 7. (g–i) ventral, lateral, and caudal views of skull stage 8. (j–l) ventral, lateral, and caudal views of skull stage 9. (m–o) dorsal, dorsal (frontal and parietal bones removed), lateral, and rostral views of skull stage 9. (p–r) ventral, lateral, and caudal views of skull stage 10. See text for abbreviations
3.1 Anatomical descriptions
3.1.1 Skull stage 1 (NH31)
This sample is the earliest-stage specimen in this study. CRL is 7.59 mm. This specimen is similar to “stage 16” of H. armiger in Wang, Han, Racey, Ru, and He (2010). The frontal, maxilla, premaxilla, and dentary have started their ossification in this specimen (Figure 1a–c). Small ossification centers of the frontal are present above the maxilla. The frontal is notably smaller than the maxilla and dentary, denoting that the frontal started its ossification after the maxilla and dentary. The triangle-shaped zygomatic process of the maxilla is formed caudally to the maxilla. The ossification centers of the premaxilla started their ossification rostrally to the maxilla. The premaxilla is similar in size to the frontal. The dentary is relatively more developed than the frontal, maxilla, and premaxilla. The characteristic shape of the dentary is already obvious, indicating that the dentary is presumably the first element to start its ossification within the cranium. Left and right ossification centers of the frontal, maxilla, premaxilla, and dentary are not yet at the sagittal plane and are separated from one another.
3.1.2 Skull stage 2 (PQ109)
CRL is 13.77 mm. This specimen is similar to “stage 19” of Hipposideros armiger in Wang et al. (2010). The ossification centers of the parietal, interparietal, supraoccipital, basioccipital, exoccipital, nasal, jugal, basisphenoid, alisphenoid, pterygoid, palatine, vomer, ectotympanic, and squamosal are present (Figure 1d–f). Left and right frontal elements have started to fuse with each other in the sagittal plane. The caudal part of the frontal is curved ventrally. The caudal part of the parietal is still not ossified, denoting that the ossification of the parietal advanced from the rostral to the caudal. The parietal is slightly fused to the frontal. Left and right bony elements of the parietal are not fused with each other, leaving a rounded gap at the top of the cranium. The interparietal is present and clearly separated into three parts: left, right, and center independent elements. The supraoccipital is separated into left, right, and center independent elements like the interparietal. The parietal, interparietal, and supraoccipital are not fused with each other. The Y-shaped basioccipital is formed rostrally to the supraoccipital. The exoccipital is formed bilaterally to the basioccipital. The shape of the foramen magnum is formed by the ossification of the basioccipital and exoccipital. The nasal is formed between the frontal and maxilla. The zygomatic process of the maxilla has developed more caudally compared to stage S1 (NH31). The rostral tip of the premaxilla is branched, and the premaxillary palatal branch is already obvious, extending to the vomer. Left and right dentary bones attach to each other at the sagittal plane and the suture is obscure. The coronoid process, articular process, and angular process are formed in the caudal region of the dentary. As in the maxilla, dentition has formed in the dentary. The jugal is formed caudally to the zygomatic process of the maxilla. The fusion between the jugal and zygomatic process of the maxilla has not yet formed. The basisphenoid has formed rostrally to the basioccipital. The basioccipital and basisphenoid do not contact with each other. Left and right pterygoids are formed rostrally to the basisphenoid. A pair of the hamulus of the pterygoid is formed on the ventral part of the pterygoid. The alisphenoid is formed on each side of the basisphenoid. The superior orbital fissure becomes evident by the ossification of the pterygoid and alisphenoid. The palatine attaches to the caudal part of the maxilla, but the fusion has not formed yet. The vomer is now ossified and attaches to the maxilla. The squamosal has started its ossification and shows slight attachment to the parietal. The zygomatic process of the squamosal is extended to the maxilla. The ectotympanic started its ossification. The shape of the right side ectotympanic is crescent-shaped, but only the dorsal tip is ossified in the left ectotympanic.
3.1.3 Skull stage 3 (PQ116)
CRL is 15.15 mm. This specimen is similar to “stage 20” of Hipposideros armiger in Wang et al. (2010). GM is measurable from this developmental stage, so GM was computed for the specimens hereafter. The parietal ossification is more advanced, the caudal region of the bone is formed (Figure 1g–i). The parietal and interparietal are nearly attached to each other but are not fused. Both the interparietal and supraoccipital ossification are more advanced in the caudal region. The inferior part of the supraoccipital is concave. The zygomatic process of the maxilla is developed more caudally, nearly attaching to the jugal. The angular process of the dentary is more extended. The symphyseal suture between left and right dentaries becomes more obscure. Now the palatine has become arch-shaped but is still separated into left and right ossification centers. The squamosal shows contact to the parietal, alisphenoid, jugal, and ectotympanic. Both left and right ectotympanics are present. Now the stylohyal is formed, fusing ventrally to the ectotympanic. The goniale has ossified and well attaches to the ectotympanic. Left and right cochlear canals within the petrosal are ossified bilaterally to the basioccipital.
3.1.4 Skull stage 4 (VC50)
CRL is 13.14 mm. This specimen is similar to “stage 20” of Hipposideros armiger in Wang et al. (2010). While CRL is smaller than in stage S1 (NH31), stage S2 (PQ109), and stage S3 (PQ116), both SL and GM are greater than in these three specimens. The ossification of the parietal is more advanced than in the above three specimens, left and right elements approaching each other (Figure 1j–l). The left and right interparietal elements become fused with each other, forming one median element. The left and right ossification centers of the supraoccipital are also fused with each other but are still concave inferiorly. The caudal region of the zygomatic process of the maxilla is more advanced than in the earlier staged specimens. A larger number of teeth are observed in the maxilla when compared to stage S3 (PQ116). The ossification of the dentary is more advanced, but the fusion between the left and right dentaries is not yet completed. The coronoid process, articular process, and angular process are further extended. The pterygoid is still separated from the palatine. The alisphenoid is not in contact with the basisphenoid. The foramen ovale has formed caudally to the alisphenoid. The left and right palatine elements are not fused with each other but are attached to the maxilla. The development of the cochlear canal is less advanced compared to stage S3 (PQ116), having only one ossification center.
3.1.5 Skull stage 5 (PQ97)
CRL is 13.82 mm. This specimen is similar to “stage 22” of Hipposideros armiger in Wang et al. (2010). The parietal has extended more caudally, nearly contacting the interparietal (Figure 1m–o). While the ossification of other bones is more advanced than in VC50, interparietal and supraoccipital are less developed. The interparietal attaches to the supraoccipital, but the left and right interparietal elements are not fused with each other. The cavity observed in the inferior region of the supraoccipital in stage S4 (VC50) is more obvious at this stage and was U-shaped. The rostral process of the basioccipital is more extended towards the basisphenoid. The caudal region of the zygomatic process of the maxilla has further developed and is now in contact with the jugal. The suture between the left and right dentaries is more obscure and more closed than in stage S4 (VC50). The jugal is fused to the squamosal. The medial part of the alisphenoid contacts the basisphenoid and the lateral part contacts the squamosal. The pterygoid attaches to the basisphenoid. The development of the hamulus is more advanced than in stage 4 (VC50), it is more extended towards the ventral side, and its shape has become sharper. The palatine attaches to the medial part of the maxilla. Left and right bony elements of the palatine are fused with each other, and the suture in the median line disappeared. The ossification centers of the orbitosphenoid are now formed. The caudal edge of the squamosal, the crista parotica, is curved to the ventral region, laterally covering the cochlear canal. The ectotympanic is thicker than in stage S4 (VC50) and shows contact to the squamosal. The development of the cochlear canal is much more advanced, forming a contact with the basioccipital. By this contact, the basicochlear commissure between the petrosal and basioccipital has formed. The crista parotica of the squamosal starts to laterally cover the petrosal.
3.1.6 Skull stage 6 (VC40)
CRL is 14.99 mm. This specimen is similar to “stage 22” of Hipposideros armiger in Wang et al. (2010). The frontal and parietal are more fused with each other. The parietal is more developed caudally, approaching the interparietal (Figure 2a–c). The superior parts of the left and right interparietal elements are fused with each other while leaving the remainder of the suture yet unfused. The cavity in the inferior part of the supraoccipital has disappeared, and the supraoccipital is no longer U-shaped. The interparietal and supraoccipital are independent from each other. The growth of the basioccipital and basisphenoid proceeded further, and nearly contact each other. The interpremaxillary suture has closed. The premaxillary palatal branch has enlarged to reach the vomer, but the vomer is not so different compared to stage S5 (PQ97). The left and right dentaries are fused with each other, and the symphyseal suture is no longer visible at this stage. The jugal has separated from the maxilla and the squamosal. The alisphenoid is not yet in contact with the basisphenoid. The caudal region of the alisphenoid is more ossified. The pterygoid is attached to the basisphenoid, but not attached to the palatine yet. The left and right palatines are still separated from each other. The palatine is still not in contact with the maxilla. The orbitosphenoid has not yet formed in this specimen. The ossification of the petrosal is more advanced. The crista parotica has become thicker than in the previous stage (PQ97).
3.1.7 Skull stage 7 (PQ105)
CRL is 15.63 mm. This specimen is similar to “stage 22” of Hipposideros armiger in Wang et al. (2010). The metopic suture separating left and right frontal elements becomes obscure. The parietal ossifies towards the dorsal region, and the open space between the left and right parietals become smaller (Figure 2d–f). However, the ossification of the interparietal is less advanced than in stage S6 (VC40), and the interparietal is still separated into left and right elements. The growth of the supraoccipital proceeds and the dorsolateral edges of the supraoccipital are in contact with the interparietal. Both superior part and inferior part of the supraoccipital become smoothly curved. The exoccipital becomes more enlarged. The basioccipital and basisphenoid are in contact with each other. The premaxillary palatal branch extends to the vomer, contacting the maxilla. The basisphenoid is attached to the alisphenoid. The alar process is formed by the contact between the basisphenoid and alisphenoid. The alisphenoid is more developed and now in contact with the parietal and squamosal. The palatine is in contact with the maxilla. The left and right palatines are fused with each other and the suture in-between is invisible. The orbitosphenoid is present in this specimen, but it has no contact with other bones. The suture between the parietal and squamosal has become obscure. The crista parotica of the squamosal projects ventrally.
3.1.8 Skull stage 8 (VC68)
CRL is 16.24 mm. This specimen is similar to “stage 23” of Hipposideros armiger in Wang et al. (2010). In this specimen, all elements are more advanced than in stage S7 (PQ105) except for the state of the parietal (Figure 2g–i). The left and right parietals are not in contact with each other. The fusion between the left and right interparietals is completed and no suture is visible. The interparietal grows rostrally, approaching the parietal. The interparietal and supraoccipital are in contact with each other, but the suture in-between is still visible. The exoccipital is expanded, making the spaces between the supraoccipital and exoccipital and between the exoccipital and basioccipital much smaller. The left and right lateral edges of the nasal are now in contact with the maxilla. The maxilla also has grown towards the vomer and is now in contact with it. The premaxillary palatal branch grows towards the maxilla and is slightly attached to it. The orbitosphenoid is not yet ossified in this specimen. The goniale has started its expansion. The rostral region of the cochlear canal is still unossified and open.
3.1.9 Skull stage 9 (VC62)
This is the latest fetal specimen in our study (Figure 2j–o) and is similar to the “fetal stage” of Hipposideros armiger in Wang et al. (2010). This was presumed to be near birth, given the characters such as all digits, carpals, and tarsals being ossified, digit I being hooked and pigmented, nose-leaves achieving adult appearance (Wang et al., 2010; Koyabu & Son, 2014). CRL is 20.58 mm. The metopic suture of the frontal became obscure. The left and right parietals are clearly in contact with each other. The rostral region of the parietal is more developed and more in contact with the frontal. The space between the parietal and interparietal is smaller, but the interparietal and supraoccipital are not fully fused to each other. The exoccipital and basioccipital are still not in contact. The premaxillary palatal branch has enlarged. A pair of the canine is observed. The vomer has also enlarged and grew rostrally, now reaching the premaxilla. The pterygoid hamulus is enlarged and becomes sharper. The palatine grows rostrally, and the roof of oral cavity is flattened. The presphenoid is now ossified at the ventral floor of the left and right orbitosphenoids. The squamosal shows more contact with the parietal, and the suture in-between becomes more obscure. The crista parotica of the squamosal is extended ventrally and covers more portions of the petrosal. The rostral region of the petrosal is now ossified, forming a smooth dome.
3.1.10 Skull stage 10 (VN11_0230)
This is an adult specimen which experienced pregnancy. SL is 20.37 mm. Some bony elements are completely fused with one another; thus some sutures are obscure in the scanned macerated skull (Figure 2p–r). Therefore, the set of the frontal, maxilla, vomer, orbitosphenoid, and presphenoid, the set of the parietal and interparietal, the set of the basioccipital, exoccipital, and basisphenoid, and the set of the petrosal and squamosal are not separated in the segmentation procedure. The parietal and interparietal are fully fused with each other. While the median suture is definite, the suture between the parietal and interparietal is obscure. The left and right maxillae and nasals are fused in the dorsal region of the cranium, forming an arch-like structure. The canines extended more ventrally than in stage S9 (VC62). The typical shape of the premolars is recognizable on the ventral side of the maxilla. The orbitosphenoid and presphenoid observed in stage S9 (VC62) are now fused to the vomer, frontal, and maxilla. The expanded nasal fossa is formed in the nasofrontal region. The angular process is more extended towards the outer side. The vomer has extended dorsally, reaching the dorsal side of the frontal. The ectotympanic is inflated and hypertrophied compared to the fetal skull, being tightly fused to the petrosal. From the lateral view, the ectotympanic covers the petrosal. The squamosal is tightly fused to the parietal. The cochlear canal is inflated, forming the basicochlear fissure between the basioccipital and petrosal.
3.2 Ossification sequence in Rhinolophus thomasi
The presence/absence of each bone is summarized in Figure 3. The overall relative ossification sequence is as follows: (a) premaxilla, maxilla, frontal, and dentary, (b) nasal, jugal, lacrimal, parietal, interparietal, squamosal, vomer, palatine, basisphenoid, pterygoid, alisphenoid, supraoccipital, basioccipital, exoccipital, and ectotympanic, (c) petrosal, goniale, and stylohyal, (d) orbitosphenoid, and lastly (e) presphenoid. The presence/absence of the orbitosphenoid shows slight intraspecific variation against CRL. This bone was present in PQ97 (skull stage 5), while it was not present in the larger PQ116 (skull stage 3) and VC40 (skull stage 4).
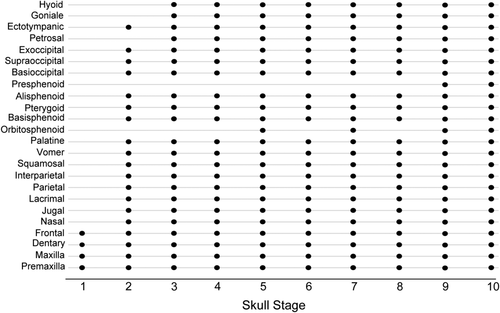
Ossification sequence of skull bones in all specimen. The horizontal axis represents skull stage of each specimen. Skull stages were assigned according to the overall size of the skull (GM), therefore the presence of bones does not necessarily follow skull stages
3.3 Petrosal development in Rhinolophus thomasi
The development of the petrosal is shown in Figure 4. The developmental order of the petrosal components, outlined in the following, did not necessarily agree with the overall sequence of cranial elements. Therefore, for Figure 4 the specimens were reordered according to the degree of the developmental stage of the petrosal instead of GM of the skull. The earliest specimen NH31 showed no petrosal bone and none of the neurocranial elements. The second earliest PQ109 showed ossification of the ectotympanic and occipital bones but no ossification of the cochlear canal. We confirmed that later on the ossification within the petrosal starts from the midbasal location and proceeds in both directions, rostrally and caudally. In VC50 (cochlear stage 1), the onset of ossification of the petrosal starts from the future primary bony lamina. Then, this tiny bony element is curved and the bony labyrinth has started to form in cochlear stage 2 (PQ116). In this stage 2, the future anterior semicircular canal ampulla is formed above the primary bony lamina. This small anterior semicircular canal ampulla is extended in cochlear stage 3 (VC40), and the elliptical recess of the vestibule start their ossification. The two main ossification centers observed in cochlear stage 3 (VC40) are now fused into one bone in cochlear stage 4 (PQ97). In addition, the bony channel for the common crus has started to form. The common crus is formed near the anterior semicircular canal ampulla. In cochlear stage 5 (PQ105), the development of the spiral morphology is advanced, and the secondary bony lamina started its formation. The secondary turn has started to form and the foramen acusticum inferior is formed. The elliptical recess of the vestibule is also extended and more curved than in cochlear stage 4 (PQ97). The fenestra cochleae is formed near the primary bony lamina. From this stage and later, the promontorium initiates its ossification and starts to cover the inner structures such as the primary bony lamina. The common crus is more extended than in cochlear stage 4 (PQ97). In cochlear stage 6 (VC68), the primary and secondary bony laminae are thicker than in cochlear stage 5 (PQ105). The development of the promontorium is more advanced, covering the primary and secondary bony laminae almost completely, in cochlear stage 7 (the near-birth specimen VC62). The suprafacial commissure is formed near the elliptical recess. However, there is still open space at the rostral top of the petrosal. At this stage, the semicircular canals have started their extension. While the posterior semicircular canal is present, the anterior- and lateral semicircular canals were not observed in this specimen. In the adult specimen (cochlear stage 8, VN11_0230), the promontorium completely covers the primary and secondary bony laminae. The open space at the rostral tip of the petrosal in cochlear stage 7 (VC62) is now closed. The posterior semicircular canals became thicker than they were in the fetuses.
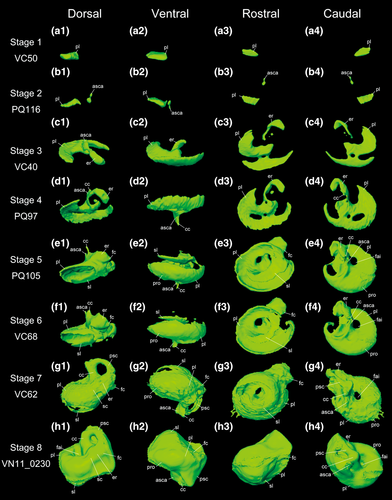
Rhinolophus thomasi, reconstruction of the left petrosal in the fetuses and adult (cochlear stage 1–8). (a1–a4) dorsal, ventral, rostral, and caudal views of cochlear stage 1. (b1–b4) dorsal, ventral, rostral, and caudal views of cochlear stage 2. (c1–c4) dorsal, ventral, rostral, and caudal views of cochlear stage 3. (d1–d4) dorsal, ventral, rostral, and caudal views of cochlear stage 4. (e1–e4) dorsal, ventral, rostral, and caudal views of cochlear stage 5. (f1–f4) dorsal, ventral, rostral, and caudal views of cochlear stage 6. (g1–g4) dorsal, ventral, rostral, and caudal views of cochlear stage 7. (h1–h4) dorsal, ventral, rostral, and caudal views of cochlear stage 8. See text for abbreviations
3.4 Morphometric patterns of the petrosal and comparisons with other mammalian species
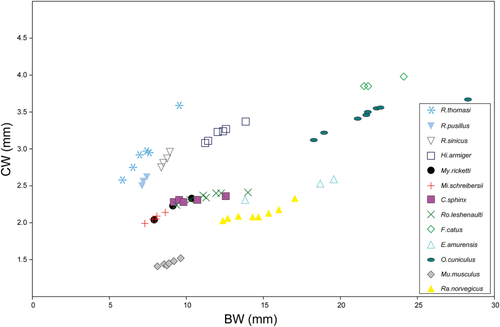
The results of the RMA regression analysis of the PV against the cubed GM are given in Figure 5a. Petrosal volume is highly correlated with the cubed GM (r = 0.92, p = .0014). The regression slope is 3.38 (95% CI from 0.91 to 4.521) and the y-intercept is −8.35 (95% CI from −11.45 to −2.46). The relationship between CW/BW and GM is given in Figure 5b, demonstrating that CW/BW value consistently decreases with the increase of skull size. The ratio of CW is plotted against BW and is compared with values of other bats and mammals reported by Wang et al. (2017) (Figure 6). CW/BW of R. thomasi was higher than in all species reported by Wang et al. (2017). The adult shows the lowest CW/BW among our studied specimens (Table 1), which is higher than that of fetal R. pusillus and fetal R. sinicus (Table 2; Wang et al., 2017). The relationship between CW and BW is shown in Figure 7. The slope of the CW against BW in R. thomasi is similar to those of R. sinicus and R. pusillus and higher than those of non-Rhinolophus bat species.
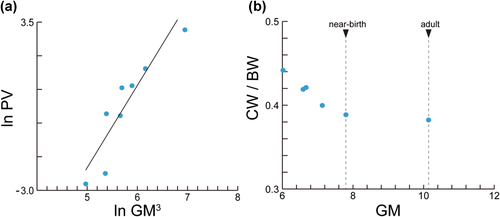
Measurement comparison against geometric mean (GM). (a) The linear regression between petrosal volume (PV) and cubed geometric mean (GM3). Petrosal volume was measureable from skull stage 3, therefore earlier stages were not included. The regression line was obtained by RMA method. (b) Plot of geometric mean (GM) and the ratio of cochlear width to basicranial width (CW/BW). Cochlear width was measureable from skull stage 5, therefore earlier stages were not included
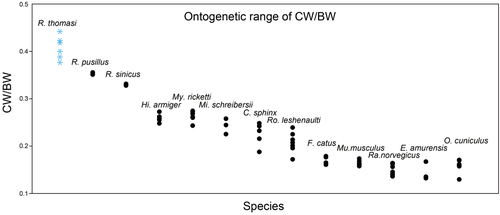
Comparison of the ontogenetic variation of ratios of cochlear width to basicranial width (CW/BW) in Rhinolophus thomasi and other species reported by Wang et al. (2017). CW/BW generally decreases with growth, thus higher values are of younger individuals and lower values are of older individuals. The ranges of CW/BW in Rhinolophus species (R. pusillus and R. sinicus) reported by Wang et al. (2017) are very narrow and skewed compared to R. thomasi (this study). This suggests that their sampling was limited to very few stages and does not capture the overall ontogeny of the cochlea in these species. Note that the upper limit of CW/BW reported by Wang et al. (2017) may increase if earlier fetuses were included for bats and fetuses were included for non-bat mammals
| CW/BW of near-birth | CW/BW of adult | Near-birth CW/BWagainst adult CW/BW | |
|---|---|---|---|
| Rhinolophus thomasi | 0.39 | 0.38 | 0.99 |
| Rhinolophus pusillus | 0.36 | 0.35 | 0.99 |
| Rhinolophus sinicus | 0.33 | 0.33 | 1.01 |
| Myotis ricketti | 0.24 | 0.23 | 0.92 |
| Miniopterus schreibersii | 0.26 | 0.25 | 0.96 |
| Hipposideros armiger | 0.26 | 0.24 | 0.93 |
| Cynopterus sphinx | 0.22 | 0.19 | 0.87 |
| Rousettus leshenaulti | 0.20 | 0.17 | 0.88 |
| Felis catus | 0.18 | 0.16 | 0.90 |
| Erinaceus amurensis | 0.17 | 0.14 | 0.81 |
| Oryctolagus cuniculus | 0.17 | 0.13 | 0.76 |
| Rattus norvegicus | 0.16 | 0.14 | 0.83 |
| Mus musculus | 0.17 | 0.16 | 0.91 |
- CW/BW: ratio of cochlear width to basicranial width.
- Near-birth CW/BW against adult CW/BW: Near-birth CW/BW divided by adult CW/BW.
4 DISCUSSION
4.1 Ossification sequence of cranial bones
The relative ossification sequence of cranial bones in R. thomasi is: (a) premaxilla, maxilla, frontal, and dentary, (b) nasal, jugal, lacrimal, parietal, interparietal, squamosal, vomer, palatine, basisphenoid, pterygoid, alisphenoid, supraoccipital, basioccipital, exoccipital, and ectotympanic, (c) petrosal (ossification starting from the region housing the primary bony lamina), goniale, and stylohyal, (d) orbitosphenoid, and lastly (e) presphenoid. The onset of ossification of the oral region was found to be relatively earlier, a shared developmental pattern among mammals (Koyabu et al., 2014). Compared to non-chiropteran mammals, the onset timing of the petrosal appears to be relatively earlier in R. thomasi. The petrosal ossifies earlier than the presphenoid in R. thomasi whereas in Mesocricetus auratus (Beyerlein, Hillemann, & Van Arsdel, 1951), Mus musculus (Johnson, 1933), Rattus norvegicus (Strong, 1925), Oryctolagus cuniculus (Bruce, 1941), Homo sapiens (de Beer, 1937), Macaca nemestrina (Sirianni & Newell-Morris, 1980), and Ovis aries (Harris, 1937) the petrosal ossifies after or simultaneously to the presphenoid. Generally in mammals, both the petrosal and presphenoid are the last bones to ossify among all cranial bones (Koyabu et al., 2014). Among bats, the overall cranial ossification sequence for Hipposideros armiger, Hipposideros galeritus, and Rhinolophus affinis has been reported by Pedersen (1996), and that for Hesperoptenus blanfordi, Rousettus aegyptiacus, Kerivoula sp., Myotis sp., and Cynopterus sphinx has been reported by Koyabu et al. (2014). However, it is unclear whether the accelerated onset of petrosal development is shared among all bats or specific to certain taxa including R. thomasi as the ossification timing of auditory bones relative to others has not been well studied.
4.2 Ossification of the petrosal components
The onset of ossification of the petrosal in R. thomasi started around VC50 (skull stage 4/cochlear stage 1) and PQ116 (skull stage 3/cochlear stage 2). The overall fetal ossification sequence of the petrosal components was as follows: (a) the primary bony lamina region, (b) anterior semicircular canal ampulla region, (c) elliptical recess region, (d) common crus and basicochlear commissure regions, (e) fenestra cochleae, secondary bony lamina, foramen acusticum inferior, and promontorium regions, and lastly (f) posterior semicircular canal. The regions housing the anterior and lateral semicircular canals were not visible in our fetal scans. As an overall pattern, it was confirmed that the ossification proceeded from the caudal region (primary bony lamina region) to the rostral region (secondary bony lamina region) following the formation of the spiral. The onset of ossification of the spiral morphology started around the same timing of the growth of the primary bony lamina (skull stage 6, VC40). The petrosal was first in contact with the basioccipital at the timing of the ossification of the common crus region and then was in contact with the basisphenoid at the timing of the ossification of the part housing the posterior semicircular canal.
As derived mainly from mice experiments, Tbx1 plays an essential role in the determination of the rostrocaudal axis of the cochlea (Vitelli et al., 2003; Raft, Nowotschin, Lial, & Morrow, 2004; Arnold et al., 2006), Wnt signaling from the hindbrain determines the dorsoventral axis (Riccomagno, Takada, & Epstein, 2005), and Fgf3 determines the specification of the mediolateral axis (McKay, Lewis, & Lumsden, 1996; Lin, Cantos, Patente, & Wu, 2005). It is also known that Otx1 is essential for the formation of the lateral canal and ampulla, whereas Otx2 plays a critical role in the patterning of ventral structures of the cochlea and saccule (Cantos, Cole, Acampora, Simeone, & Wu, 2000). Our knowledge on the development of the bony labyrinth is still limited to few species. In Me. auratus the region housing the posterior cochlear center ossifies first, then the median cochlear center region, and lastly, the anterior cochlear center region is ossified (Beyerlein et al., 1951). The ossification of the portion housing the cochlea occurs earlier than the portion housing the semicircular canals in Bos taurus (Costeur, Mennecart, & Schulz, 2016) and Tragulus kanchil (Mennecart & Costeur, 2016a). In Monodelphis domestica and Macropus eugenii, the part housing the semicircular canals starts its ossification on the 8th day after birth and then the cochlear canal ossifies on the 13th day (Clark & Smith, 1993). In R. thomasi, the cochlear canal is ossified first, and then the ossification occurs in the order of ampullae region, vestibule region, and common crus region subsequently. It is known that in several rodents and H. sapiens cytodifferentiation of the cochlea is initiated in midbasal cochlear regions and then proceeds in both apical and basal direction (Kraus & Aulbach-Kraus, 1981; Romand, 1983; Pujol & Uziel, 1988; Roth & Bruns, 1992; Weaver & Schweitzer, 1994; Pujol, Lavigne-Rebillard, & Lenoir, 1998; Vater, 2000; Toyoda et al., 2015). From which region the membranous cochlea develops and from which region the bony cochlear canal ossifies have been unknown for bats, but in agreement with the reported development of membranous cochlea in rodents and H. sapiens, our results show that the ossification of the cochlear canal in bats similarly occurs from the midbasal region and then proceeds in apical and basal directions, suggesting that this can be a shared pattern among mammals.
4.3 The peculiarity of cochlear size growth in Rhinolophus species
The increase of petrosal volume against skull size is nearly linear from fetal period to adult stage (r = 0.92, p = .0014; Figure 5a). In the near-birth fetus (skull stage 9/cochlear stage 7, VC62), the anterior- and lateral semicircular canals were unossified and the inner bony lamina was not fully covered by the bony closure of the promontorium. Our results suggest that the ossification, shape growth, and size growth of the petrosal are not completed during fetal period and continue after birth, whereas the number of cochlear turns is established prenatally. Relative cochlear size against basicranial size (CW/BW) becomes nearly constant when the onset of the ossification of the secondary bony lamina is initiated (Figure 5b). Retaining comparable CW/BW ratios from near-birth (0.39) to adult stage (0.38) suggests that cochlear size continues to increase nearly constantly along skull size growth after birth until maturity (Table 2). This relatively late completion of cochlear size is striking if compared to other placental mammals where generally the cochlea stops growing before or around birth whereas the whole skull continues to grow until maturity. For example in Bos taurus, the bony labyrinth is already fully ossified and reaches adult size volume around the 6th month of gestation (the mid-gestation period of the 9.3-month-long gestation), while the petrosal bone and the whole skull continues to grow (Costeur et al., 2016). In Homo sapiens, the bony labyrinth attains adult size between 17 to 19 weeks of gestation whereas the whole skull continues to grow (Bast, Anson, & Gardner, 1947; Toyoda et al., 2015). The fetal bony labyrinth in Homo sapiens shows little or no changes in structure, size, and shape once it is embedded in the ossified otic capsule (Jeffery & Spoor, 2004). Congruently, the slope of the growth curve of CW against BW reaches nearly a plateau in larger individuals in various terrestrial placentals (Figure 7). Thus, terrestrial placentals generally experience a considerable decrease of CW/BW ratio from near-birth period to maturity (Figure 6). Marsupials differ from placentals in giving birth without most cranial bones being ossified but are principally similar to placentals in that the cochlear size arrests its growth at some point while the skull size continues to increase. Ekdale (2010) reported that in Monodelphis domestica the cochlea achieves mature size and shape around day 27, which is also the timing of completing bony labyrinth ossification but the whole skull size continues to grow until day 465. Thus, this arrested growth of the cochlea in comparison to the continued growth of the whole skull may be pointed out as a general pattern among therian mammals (Figure 6). In contrast, the CW/BW ratio of R. thomasi is maintained constantly against skull growth (Figure 5b). The CW/BW ratio of R. thomasi is the highest in the earliest fetus and decreases until near-birth, and then it becomes nearly constant. Cochlear width was defined as the length from the end of the first half turn of the cochlea to the end of the second half turn at the clear outline of the cochlea visible in CT-images (Habersetzer & Storch, 1992), thus reflecting the diameter of the primary bony lamina. Therefore, it is expected that the diameter of the primary bony lamina continues to homogeneously expand until maturity, resulting in constant CW/BW ratios throughout ontogeny after birth.
CW/BW ratio of adult R. thomasi (0.38) is slightly higher than the values of two related species reported by Wang et al. (2017), R. pusillus (0.35) and R. sinicus (0.33; Table 2). Among R. thomasi, the CW/BW ratios are higher in the earlier stages, and those of late-stage fetuses such as skull stage 8/cochlear stage 6 (0.40, VC68) and skull stage 9/cochlear stage 7 (0.39, VC62) are virtually comparable to that of the adult (Table 1). Accordingly, CW/BW ratio decreases while GM increases until near-birth and then become nearly constant in R. thomasi (Figure 5b). In contrast, CW/BW ratios of R. sinicus, which is phylogenetically close to R. thomasi (Xing & Mao, 2016), and R. pusillus were reported to be virtually constant throughout the whole ontogeny from fetal period to adulthood in Wang et al. (2017) (see Figure 6). While CW/BW ratios range between 0.38 and 0.44 during R. thomasi development, it varies surprisingly little as 0.35–0.36 in R. pusillus, and all the individuals were 0.33 in R. sinicus. Given our investigations on R. thomasi (Figure 5b) and the close phylogenetic affinity of R. thomasi with these species (Stoffberg et al., 2010; Xing & Mao, 2016), we assume that the observation on Rhinolophus by Wang et al. (2017) resulted from sampling only final stage fetuses. In fact, basicranial size range between the youngest fetal individual with measureable ossified cochlear canal and adult reported by Wang et al. (2017) was 7.12 to 7.43 mm for R. pusillus and 8.37–8.93 mm for R. sinicus, whereas for R. thomasi it was 5.83 to 9.54 mm, indicating that their sampling was possibly skewed toward late ontogenetic stages. We predict that, if earlier stages were included, the actual CW/BW curves of R. sinicus and R. pusillus might have been similar to R. thomasi as shown in Figure 5b, that is, decreasing until near-birth and then becoming constant from near-birth to adulthood.
While the CW/BW ratio is virtually maintained through postnatal ontogeny in R. thomasi, the CW/BW ratios of adults in all other mammalian taxa except for Rhinolophus reported by Wang et al. (2017) are notably smaller than in near-birth individuals (Table 2). Carter & Adams (2015) reported that the neonate cochlea of a the laryngeal echolocator Artibeus jamaicensis, a member of Yangochiroptera, is not significantly different from the adult cochlea in height, width (basal and apical turns) or morphometrics. Together, these indicate that decrease of CW/BW ratio throughout postnatal ontogeny, caused by postnatal skull size increase with no significant increase of the cochlear size, is a common pattern among mammals except for Rhinolophus (Table 2). Furthermore, current evidence suggests that a nearly constant CW/BW after birth is not necessarily shared among all bats and could be a characteristic feature of Rhinolophus development. We postulate that the growth of the cochlea continues in parallel with skull expansion, resulting in the retention of the relative size of the cochlea against skull size in Rhinolophus (Table 2).
Members of genus Rhinolophus are known to emit high duty cycle pulse through the nostril for echolocation (Pedersen, 1998; Fenton, 1999; Lazure & Fenton, 2011). This high duty cycle pulse allows these bats to track and capture more efficiently than bats which depend on the low duty cycle pulse (Lazure & Fenton, 2011). Pteronotus parnellii and members of Hipposideros are also known to employ high duty cycle pulse, but Rhinolophus species emit the longest pulses and have the highest duty cycles among bats (Jones, 1999). Rhinolophus is capable of using Doppler shift compensation that allows the call to return at a frequency to which their ears and auditory neurons are finely tuned, whereas its close relative Hipposideros only shows incomplete partial Doppler shift compensation (Jones, 1999). The characteristic ontogeny that leads to form an enlarged cochlea in Rhinolophus may possibly be related to its hearing behavior. The onset timing of hearing in Rhinolophus is known for R. rouxi from hearing experiments. No acoustic response is elicited in the second to third day-age newborns (Rübsamen & Schäfer, 1990b). Although deaf, newborns are capable of emitting long sequences of loud isolation calls. The first auditory responses emerge during the second week after birth. Then the first sharp tuned responses emerge around the third week, being tuned to frequencies between 57 and 60 kHz, which match the individual's CF-emission frequencies at that age. In parallel with echolocation call development, the frequency of sharply tuned responses shifts upwards with subsequent growth. A light-microscopic study by Vater (1988) reported that the seven half turns are established already at birth in R. rouxi. It was also reported that the height and diameter of the cochlea of newborns are similar to the value of the adult. Our study is in agreement with the prenatal completion of the cochlear turns reported by Vater (1988), but does not confirm her observation that cochlear dimensions are achieved prenatally. This contrast could be due to species difference or could be due to differences in methods. Her observation was based on serial sections in which dimensions such as the maximum width or height of the whole cochlea are generally difficult to measure. However, in comparison to adult Vater (2000) also pointed out that in the newborn, (a) filaments of the basilar membrane are poorly contrasted, and the spiral vessel in the pars tecta of the basilar membrane is patent, and a thick cellular tympanic cover layer is attached to the vestibular surface of the basilar membrane; (b) that there are abundant microvilli on supporting cell phalanges; (c) marginal pillars formed by microvillous protrusions of the phalanges of the outermost row of Deiter's cells attach to the free edge of the tectorial membrane and restrict its freedom of motion; (d) the fluid spaces of the organ of Corti are not fully formed; (e) the cytoskeleton of supporting cells is immature; (f) cytoskeleton outer hair cells are immature; and (g) Hensen and Claudius cells are still small. Together with our results, physiological maturation of hearing function (Vater, 2000) is suggested to be postnatally achieved by changes in these traits and cochlear size increase.
4.4 Prenatal cochlear development among bats
The cochlear canal development in seven species of bats (non-laryngeal-echolocating Cynopterus sphinx and Rosettus leschenaulti, and laryngeal-echolocating Myotis ricketti, Miniopterus schreibersii, Hipposideros armiger, Rhinolophus pusillus, and Rhinolophus sinicus) and five non-chiropteran mammals (Felis catus, Erinaceus amurensis, Oryctolagus cuniculus, Rattus norvegicus, and Mus musculus) were morphometrically compared by Wang et al. (2017) to test whether laryngeal echolocation was gained in the common ancestor of all bats and then lost in Pteropodidae or laryngeal echolocation was gained independently in Rhinolophoidea and Yangochiroptera. They found that all bat lineages have a similarly large cochlea during the fetal period, but that cochlear growth rate in non-echolocating Pteropodidae decreases after birth. It was therefore suggested that this leads non-echolocating Pteropodidae adults to share a similar relative cochlear size as non-chiropteran mammal. They further suggested that the shared large cochlear size of fetuses both in non-echolocating Pteropodidae and laryngeal echolocating bats reflects the growth pattern of the common ancestor of bats and thus vestigially indicates the past laryngeal echolocation capability of the common ancestor.
Although Wang et al. (2017) said that that they “studied the morphological prenatal development of bat cochleae and compared them with those of outgroup mammals”, actually no fetuses were measured and only postnatal individuals were quantified for the non-chiropteran representatives (see their Supplementary Data, https://media.nature.com/original/nature-assets/natecolevol/2017/s41559-016-0021/extref/s41559-016-0021-s1.pdf). They noted that the cochlea was only X-ray detectable and measurable at postnatal days in non-chiropteran mammals, but this indicates that what they observed with X-ray microradiographs was not the cochlea itself but the bony cochlear canal of the petrosal, which houses the soft-tissue cochlea. The ossification of the cochlear canal within the petrosal generally occurs after birth in small-sized non-chiropteran mammals (Koyabu et al., 2014), but the soft-tissue cochlea forms before birth, preceding the ossification of the cochlear canal (de Beer, 1937; Landford, 1999). This suggests that the soft-tissue cochlea would be formed before birth in Felis catus, Erinaceus amurensis, Oryctolagus cuniculus, Rattus norvegicus, and Mus musculus which were sampled in their study, although it may not be X-ray detectable. As discussed earlier, cochlear size at birth is already comparable to that of adults in various placentals, and relative cochlear size simply decreases after birth in non-chiropteran mammals (Table 2). Wang et al. (2017) only quantified individuals whose cochlear canal was ossified and X-ray detectable, but this would arguably lead to an underestimation of ontogenetic changes of relative cochlear size. In order to conduct meaningful quantitative comparisons, the soft-tissue cochlea which is generally formed before birth, should have been quantified. Therefore, their study which sampled only postnatal individuals captured a limited aspect of the cochlear development of non-chiropteran mammals. They suggested that prenatal cochlear size of non-echolocating Pteropodidae is more similar to laryngeal echolocating bats than to non-chiropteran mammals, but such proposition cannot be fully supported since no fetuses of non-chiropteran mammals were compared in their study.
Furthermore, the fetal cochlear size of non-laryngeal Pteropodidae (Cynopterus sphinx and Rosettus leschenaulti) reported by Wang et al. (2017) shows no overlap with any of the closely related Yinpterochiroptera members (Hipposideros armiger, R. thomasi, R. pusillus, R. sinicus), contradicting to their argument that Pteropodidae shares similar cochlear size to laryngeal echolocating bats during prenatal period (Figure 7). However, it is questionable whether earlier-stage fetuses were sampled broadly enough and to what extent their ontogenetic estimates are resolved for earlier stages. Therefore, their proposition that non-echolocating Pteropodidae shares similar prenatal cochlear size to laryngeal echolocating bats is not conclusive, and we consider the controversy on the origins of laryngeal echolocation is still open for discussion. Following Haeckel's assumption that “ontogeny recapitulates phylogeny”, they asserted that fetal morphology of extant bats can reveal the condition of the last common ancestor of extant bats. Meanwhile, in their concluding remarks it was also stated that “for bats, it appears that Haeckel's theory of recapitulation upholds and that ancestral character states, which reveal past affinities and adaptations, are maintained within the developing fetus”. This circular reasoning should also be taken with caution. Further investigations on multiple prenatal stages from various non-chiropteran mammals, non-laryngeal echolocators, and laryngeal echolocators are awaited to clarify the variation or shared patterns of cochlear development among bats.
5 CONCLUSIONS
Our study provides a detailed description of the cranial and petrosal ossification of a bat in Yinpterochiroptera, providing novel insights into the evolution of the peculiar cochlea of bats. We found that among all cranial bones the onset of the ossification timing of the petrosal is relatively earlier in R. thomasi compared to other reported mammals. While the cochlear canal attains adult size and shape before or around birth in non-chiropteran mammals, its shape growth and size growth are not completed during fetal period and continue until maturity in Rhinolophus. In contrast, the relative size of the cochlear canal against skull size is maintained constant after birth to maturity. The region housing the primary bony lamina continues to expand until maturity, resulting in maintaining constant relative cochlear canal size against skull size throughout postnatal ontogeny to adulthood. Such maintenance of relative cochlear canal size throughout postnatal ontogeny in Rhinolophus appears to be distinctive not only among bats but also among mammals. We suggest that the distinctive developmental patterns in Rhinolophus allow them to form a distinctively large cochlea and facilitate their characteristic echolocation behavior.
ACKNOWLEDGMENTS
We thank D. Fukui, S. Hiryu, K. Kobayashi, T. Saitoh, and L.A.B. Wilson for insightful discussions, and H. Endo and G. Suwa for various supports to this project. We are grateful to the directorates of national parks and nature reserves where we conducted our surveys and Vietnam Administration of Forestry of the Vietnamese Ministry of Agriculture and Rural Development and People's Committees of Provinces for generously supporting the study. The anonymous reviewers are highly appreciated for their constructive comments. Lastly, we thank Z. Wang for kindly providing information on measurement definitions.
CONFLICT OF INTEREST
The authors declare no conflict of interest in relation to the research, results, their interpretation or publication.
AUTHOR CONTRIBUTIONS
This study was designed by D.K. Sampling was conducted by N.T.S., V.T.T., and D.K. CT scanning was conducted and optimized by T.S. and Y.M. Observations were performed by T.N. and D.K. Discussions were conceptualized by T.N., I.W., V.T.T., and D.K. The manuscript was written and approved by all authors.




