lncRNA ANRIL accelerates wound healing in diabetic foot ulcers via modulating HIF1A/VEGFA signaling through interacting with FUS
Abstract
Background
Diabetic foot ulcer (DFU) is a frequently diagnosed complication of diabetes, and remains a heathcare burden worldwide. However, the pathogenesis of DFU is still largely unclear. The objective of this study is to delineate the function and underlying mechanism of lncRNA antisense non coding RNA in the INK4 locus (ANRIL) in endothelial progenitor cells (EPCs) and DFU mice.
Methods
The DFU mouse model was established, and EPCs were subjected to high glucose (HG) treatment to mimic diabetes. qRT-PCR or western blot was employed to detected the expression of ANRIL, HIF1A, FUS and VEGFA. CCK-8 and Annexin V/PI staining were used to monitor cell proliferation and apoptosis. Wound healing, Transwell invasion and tube formation assays were conducted to assess cell migration, invasion and angiogenesis, respectively. The association between ANRIL and FUS was verified by RNA pull-down and RIP assays. Luciferase and ChIP assays were employed to investigate HIF1A-mediated transcriptional regulation of VEGFA and ANRIL. The histological alterations of DFU wound healing were observed by H&E and Masson staining.
Results
ANRIL was downregulated in peripheral blood samples of DFU patients, DFU mice and HG-treated EPCs. Mechanistically, ANRIL regulated HIFA mRNA stability via recruiting FUS. VEGFA and ANRIL were transcriptionally regulated by HIF1A. Functional experiments revealed that HG suppressed EPC proliferation, migration, invasion and tube formation, but promoted apoptosis via ANRIL/HIF1A axis. ANRIL accelerated DFU wound healing via modulating HIF1A expression in vivo.
Conclusion
ANRIL accelerated wound healing in DFU via modulating HIF1A/VEGFA signaling in a FUS-dependent manner.
Abbreviations
-
- ANRIL
-
- antisense non coding RNA in the INK4 locus
-
- ChIP
-
- chromatin immunoprecipitation
-
- DFU
-
- diabetic foot ulcer
-
- EPC
-
- endothelial progenitor cell
-
- FISH
-
- fluorescent in situ hybridization
-
- FITC
-
- fluorescein
-
- FUS
-
- fused in sarcoma
-
- H&E
-
- hematoxylin and eosin
-
- HG
-
- high glucose
-
- LDL
-
- low density lipoprotein
-
- LECs
-
- lymphatic endothelial cells
-
- lncRNA
-
- long non-coding RNA
-
- RBPs
-
- RNA-binding proteins
-
- RIP
-
- RNA immunoprecipitation
-
- UEA I
-
- Ulex europaeus agglutinin I
-
- VEGF
-
- vascular epithelial growth factor
-
- VEGFR2
-
- vascular epithelial growth factor receptor 2
Highlights
- ANRIL was downregulated in DFU.
- ANRIL regulated HIFA mRNA stability via recruiting FUS.
- VEGFA and ANRIL were transcriptionally regulated by HIF1A.
- HG impaired EPC function via the ANRIL/HIF1A axis.
- ANRIL accelerated DFU wound healing via modulating HIF1A expression in vivo.
1 INTRODUCTION
Diabetic foot ulcer (DFU) is a frequently diagnosed complication of diabetes with high incidence, mortality and recurrence rates.1 Approximately 6.3% in the people with diabetes develop DFU annually worldwide, while the lifetime incidence of DFU is estimated to be 15–20% among diabetic patients.1 It has been reported that >50% DFUs become infected and ~20% diabetic foot infections progress to amputation.1, 2 There is an urgent need to investigate the pathogenesis of DFU. DFU development has been attributed to decreased angiogenesis, dysregulated chemokines/cytokine/growth factors and impaired macrophage activation.3-5 However, the mechanisms underlying the development of ulceration are still largely undefined.
Some new convincing evidence suggests that the common etiology of DFU may involve dysfunction of endothelial progenitor cells (EPCs).6, 7 Endothelial progenitor cells circulate in peripheral blood, and play a crucial role in endothelial differentiation and production of angiogenic growth factors, thereby facilitating angiogenesis.8 It has been illustrated that high glucose (HG) suppresses cell proliferation and promotes apoptosis in EPCs,9 thus slowing the diabetic wound healing process. It is well accepted that the proliferation, migration and differentiation of EPCs are critical for neovascularization and wound healing.7 In addition, hypoxia is also implicated in the delayed wound healing in DFU. HIF-1A signaling is suppressed in diabetes, leading to impaired angiogenesis and EPCs recruitment, thereby slowing down the wound healing process in DFU.10, 11
Long non-coding RNA (lncRNA) is a class of ncRNA >200 nt in length. Bioinformatics analysis has identified a number of differentially expressed lncRNAs in DFU12; however, the functional roles of these lncRNAs are yet to be investigated. lncRNAs are implicated in angiogenesis during the diabetic wound healing process.13 lncRNA antisense non-coding RNA in the INK4 locus (ANRIL) is encoded in chromosome 9p21.3, a genetic region which is associated with various human diseases, including different cancers, cardiovascular disease and type 2 diabetes.14-16 ANRIL is associated with coronary artery disease in patients with type 2 diabetes.17 It is also involved in structural and functional alterations in diabetic nephropathy and diabetic cardiomyopathy via modulating extracellular matrix proteins and vascular epithelial growth factor (VEGF).18 A recent study has demonstrated that silencing of ANRIL ameliorates diabetic retinopathy by suppressing the NF-κB pathway.19 More importantly, ANRIL is downregulated in DFU mice, and it accelerates diabetic wound healing by enhancing lymphangiogenesis through miR-181a/Prox1 axis.20 However, the biological functions of ANRIL in EPCs remain elusive.
lncRNAs contribute to various biological processes via association with RNA-binding proteins (RBPs).21 Bioinformatics analysis from ENCORI predicted putative interactions among ANRIL, HIF1A and RBP fused in sarcoma (FUS). Interestingly, FUS is highly expressed in patients with type 1 diabetes,22 and it participates in circFNDC3b-regulated angiogenesis through VEGFA signaling.23 VEGFA is a well-known downstream molecule of HIF1A.24 More importantly, HIF1A/VEGFA is implicated in the diabetic wound healing process,25 raising the possibility that ANRIL might accelerate wound healing in DFU via HIF1A/VEGFA signaling. It is worth noting that the potential binding sites of HIF1A at the promoter region of ANRIL were predicted by JASPAR, suggesting a mutual regulation between HIF1A and ANRIL.
In this study, we demonstrated that ANRIL was downregulated in the peripheral blood samples of DFU patients, DFU mice and HG-treated EPCs. Mechanistically, ANRIL regulated HIFA mRNA stability via interacting with FUS. On the other hand, VEGFA and ANRIL were transcriptionally regulated by HIF1A. Functional experiments revealed that ANRIL/HIF1A axis could promote cell proliferation, migration, invasion and tube formation, but suppress apoptosis in HG-induced EPCs. Further, ANRIL/HIF1A axis accelerated DFU wound healing via modulating angiogenesis in vivo.
2 MATERIALS AND METHODS
2.1 Clinical specimens
Peripheral blood samples and ulcer marginal skin tissues were collected from DFU patients at the Affiliated Hospital of Yunnan University. Diabetic foot ulcer was diagnosed according to the definition of DFU of the International Working Group on the Diabetic Foot.26 Written consents were obtained from all patients. This study was approved by the Ethics Committee of the Affiliated Hospital of Yunnan University.
2.2 Animal study
Male C57BL/6 J mice (8-week-old, 25–35 g) were from Shanghai Laboratory Animal Center, Chinese Academy of Sciences (Shanghai, China). The animal study was approved by the Ethics Committee of the Affiliated Hospital of Yunnan University. The DFU model was established as described in the literature.20 Briefly, mice were administrated streptozotocin (45 mg/kg b.w.) intraperitoneally for 5 days. The serum glucose level was monitored for 2 weeks after the last treatment. Mice with high fasting blood glucose levels (>280 mg/dl) were considered diabetic. For the skin wound healing study, the DFU model mice were randomly divided into 3 groups (n = 6 per group): vector, ANRIL and ANRIL+sh-HIF1A. Full-thickness wounds (10 mm diameter) were made on the back of feet using a sterile punch. A 500 μl aliquot of phosphate-buffer saline (PBS) containing 1 nm plasmid was administered at the wound edges via intradermal injection. Images of the wounds were photographed on 0, 7 and 14 days.
2.3 Isolation and characterization of mouse EPCs
Mouse EPCs were isolated from male C57BL/6 J mice (8 weeks old) as described in the literature.27 In brief, mice were sacrificed, and mononuclear cells were isolated from the tibia and femur by Ficoll density gradient centrifugation. Mouse mononuclear cells were cultured in EGM-2 medium (Lonza, Basel, Switzerland) containing EGM-2 MV SingleQuots, 20 ng/ml mouse VEGF, 4 ng/ml insulin-like growth factor-1, 4 ng/ml fibroblast growth factor and 5% FBS (Gibco, Grand Island, NY, USA). Cells were cultured at 37°C/5% CO2 for 4 days, and non-adherent cells were removed by rinsing with PBS. Adherent cells were further cultured for 3 days, and stained with Dil-Ac-LDL (low density lipoprotein) and fluorescein–Ulex europaeus agglutinin I (FITC-UEA-1; Thermo Fisher Scientific, Waltham, MA, USA). Endothelial progenitor cells were characterized as adherent cells positive for Dil-Ac-LDL and FITC-UEA-1. Images were acquired with a Nikon confocal microscope (Nikon, Tokyo, Japan). The identity of EPCs was also defined by flow cytometry. The EPC surface markers CD34 (ab81289, 1:50, Abcam, Cambridge, MA, USA), CD133 (ab271092, 1:50, Abcam) and vascular epithelial growth factor receptor 2 (VEGFR2; ab233693, 1:500, Abcam) were stained as previously described,28 followed by flow cytometry analysis using the FACSCaibur system (BD, San Jose, CA).
2.4 Cell treatment and transfection
EPCs were treated with normal glucose (NG, 5.5 mm glucose; Sigma-Aldrich, St Louis, MO, USA), a high concentration of mannose (GM, 5.5mm glucose and 19.5mm mannose) or HG (25mm glucose) for 24 h. Short hairpin RNA control (sh-NC), sh-ANRIL, sh-FUS, sh-HIF1A, pcDNA3.1-HIF1A and pcDNA3.1-ANRIL were purchased from GenePharma (Shanghai, China). Endothelial progenitor cells were transfected using Lipofectamine 3000 reagent (Invitrogen, Carlsbad, CA, USA).
2.5 Cell counting kit-8 (CCK-8) assay
Cell viability was assessed at 24 h post-treatment with a CCK-8 assay (Beyotime, Nanjing, China). In brief, 10 μl of CCK-8 solution was added to each well and incubated for 4 h at 37°C. The absorbance at 450 nm was measured using a microplate reader.
2.6 Cell apoptosis assay
Cell apoptosis was detected using Annexin V-FITC/PI apoptosis detection kit (Beyotime). Briefly, EPCs were resuspended in binding buffer (500 μl) and stained with Annexin V-FITC (5 μl) and PI (5 μl) for 15 min in the dark. The stained cells were then analyzed by flow cytometry using the FACSCaibur system (BD).
2.7 Wound healing assay
Endothelial progenitor cells (2 × 105) were plated into six-well plates 24 h prior to the treatment. After treatment, the cell monolayer was vertically scratched using a 200 μl pipette tip. The detached cells were removed by rinsing with PBS. After 24 h, the scratches were photographed with an inverted microscope (Nikon).
2.8 Transwell invasion assay
Endothelial progenitor cells (2 × 105) were seeded in the Matrigel (Corning Inc., Corning, NY, USA) coated upper Transwell chamber, and grown in serum-free medium. The lower chamber was filled with complete medium. Then the invaded cells were fixed and stained with crystal violet. The stained cells were counted under a microscope (Nikon).
2.9 Tube formation assay
Endothelial progenitor cells (5 × 104) were seeded onto Matrigel (Corning) coated 24-well plates and cultured for 24 h. After treatment, tube-like structures were photographed under a microscope (Nikon). Three random fields were selected for quantification of the area of tube formation. The degree of in vitro angiogenesis was represented as the total area of tube formation.
2.10 qRT-PCR
Total RNA was extracted from EPCs, tissues or blood samples using Trizol or Trizol LS reagent (Invitrogen), respectively. cDNA was synthesized using PrimeScript RT Kit (TaKaRa, Dalian, China). qRT-PCR was performed using SYBR Green PCR MasterMix (ABI, Foster City, CA, USA). The expression of the target gene was calculated using the 2−ΔΔCt method. GAPDH was used as an internal reference. For RNA stability assay, cells were incubated with Actinomycin D (5 μg/ml, Sigma-Aldrich) for 0, 2, 4, 6, 8 and 10 h. The expression of HIF1A was detected by qRT-PCR. Primers are listed in Table 1.
| Gene | Primer sequence |
|---|---|
| ANRIL | Forward: 5′-ATGAGAAGTCGGACAGTGGC-3′ |
| Reverse: 5′-GCTAAAGCCATTGAGTCGGC-3′ | |
| FUS | Forward: 5′-AAGCTACGGGGCCTATCCTA-3′ |
| Reverse: 5′-GCTGTTTTGGGTCTGTCCAT-3′ | |
| HIF1A | Forward: 5′-TCCATGTGACCATGAGGAAA-3′ |
| Reverse: 5′-CTTCCACGTTGCTGACTTGA-3′ | |
| VEGFA | Forward: 5′-GAGAGAGGCCGAAGTCCTTT-3′ |
| Reverse: 5′-TTGGAACCGGCATCTTTATC-3′ | |
| GAPDH | Forward: 5′-AGCCCAAGATGCCCTTCAGT-3′ |
| Reverse: 5′-CCGTGTTCCTACCCCCAATG-3′ |
2.11 Western blot
Proteins were extracted from EPCs using RIPA lysis buffer (Pierce, Rockford, IL, USA). Then, proteins were separated by gel electrophoresis, and transferred onto PVDF membrane. After blocking, the blots were incubated with primary antibodies overnight at 4°C, followed by incubation with secondary antibody (ab205718, 1:10 000, Abcam) for 1 h at room temperature. Signals were detected using ECL substrate (Pierce) and analyzed with ImageJ software (Bio-Rad, Hercules, CA, USA) based on the gray value of target protein and gray ratio of the internal reference (GAPDH). The primary antibodies were anti-FUS (#67840, 1:1000, Cell Signaling Technology, Beverly, MA, USA), anti-HIF1A (#36169, 1:1000, Cell Signaling Technology), anti-VEGFA (ab214424, 1:1000, Abcam) and anti-GAPDH (ab181602, 1:10000, Abcam).
2.12 Subcellular fractionation
Subcellular fractionation was conducted using the PARIS kit (Invitrogen). Briefly, EPCs were harvested and lysed with cell fractionation buffer. After centrifugation, the cytoplasmic lysates (supernatants) were collected, and the nuclear lysates were prepared with cell disruption buffer (pellets). The level of ANRIL was detected using qRT-PCR. U1 and GAPDH were employed as the nuclear and cytoplasmic markers, respectively.
2.13 RNA fluorescent in situ hybridization
The subcellular localization of ANRIL was assessed by RNA fluorescent in situ hybridization (FISH). The Cy3-labeled ANRIL probe was synthesized by RiboBio (Guangzhou, China). Endothelial progenitor cells were fixed with 4% paraformaldehyde and permeabilized with 0.3% Triton X-100. RNA FISH was conducted using the FISH kit (RiboBio). The localization of ANRIL was observed using a Nikon confocal microscope (Nikon).
2.14 RNA pull-down assay
The RNA pull-down assay was performed using the Pierce RNA Pull-Down kit (Pierce). The biotin-labeled ANRIL probe was conjugated to streptavidin magnetic beads, followed by the incubation with cell lysates. The detailed procedure strictly followed the manufacturer's guide. An ANRIL anti-sense probe served as a negative control. The enriched RNA-protein complex was then eluted and subjected to western blot.
2.15 RNA immunoprecipitation assay
The direct association between FUS and ANRIL was detected using a Magna RNA immunoprecipitation (RIP) kit (Millipore, Billerica, MA, USA). Briefly, anti-FUS (#67840, 1:100, Cell Signaling Technology) antibody conjugated beads were incubated with cell lysates at 4°C overnight. Normal rabbit IgG served as a negative control. The enriched ANRIL was detected by qRT-PCR.
2.16 Chromatin immunoprecipitation assay
The interaction between HIF1A and the promoter regions of VEGFA and ANRIL was verified by chromatin immunoprecipitation (ChIP) assay using a Pierce agarose ChIP kit (Pierce). In brief, EPCs were cross-linked with 1% formaldehyde, and subjected to MNase digestion. Antibodies against HIF1A conjugated beads were incubated with chromatin fractions. DNA was purified and analyzed using qRT-PCR. Normal rabbit IgG served as a negative control.
2.17 Dual luciferase reporter assay
The putative binding sites of HIF1A in the promoter regions of VEGFA and ANRIL were cloned into pGL-3 luciferase reporter vector (Promega, Madison, WI, USA). Endothelial progenitor cells were co-transfected with pcDNA3.1 or pcDNA3.1-HIF1A and corresponding luciferase constructs using Lipofectamine 3000 reagent in accordance with the manufacturer's instructions. After transfection, luciferase activity was measured with a dual luciferase reporter system (Promega).
2.18 Immunohistochemistry and immunofluorescence assays
On day 14 post-injury, the mouse skin tissues were collected. The tissues were then fixed with 4% paraformaldehyde and embedded in paraffin. The slides were stained with hematoxylin and eosin (H&E) as previously described.25 Masson staining was conducted using a Masson–Goldner staining kit (Sigma-Aldrich). Images were photographed with an inverted microscope (Nikon). In order to validate histopathological alterations in angiogenesis, tissue sections were allowed for immunofluorescence staining of CD31. Images was acquired using a Nikon confocal microscope (Nikon).
2.19 Statistical analysis
GraphPad Prism Software 8.0 (GraphPad Inc., San Diego, CA, USA) was employed for statistical analysis. One-way ANOVA or Student's t-test was used to analyze differences among multiple groups or between two groups, respectively. A value of p < 0.05 was considered to be statistically significant.
3 RESULTS
3.1 Isolation and characterization of mouse EPCs
Mouse EPCs were isolated as previously described.27 As shown in Figure S1A-B, EPCs exhibited cobblestone-like morphology, and >90% of the adherent cells were double positive stained with Dil-Ac-LDL and FITC-UEA-1, indicating the uptake of Dil-Ac-LDL and the binding of FITC-UEA-1. Endothelial progenitor cells were characterized by the surface expression of hematopoietic stem cell markers CD34 and CD133, as well as endothelial marker VEGFR2.29, 30 As expected, the isolated cells were positive for CD34, CD133 and VEGFR2 as detected by flow cytometry (Figure S1C). Taken together, these data indicate the successful isolation of EPCs.
3.2 lncRNA ANRIL is downregulated in DFU
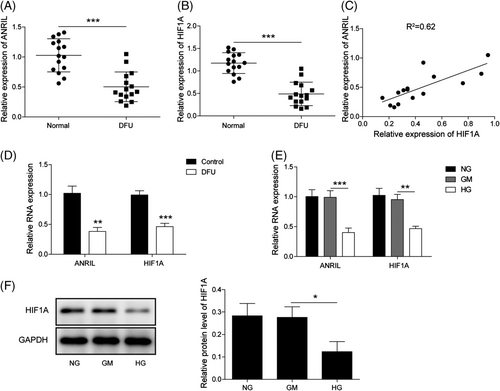
To delineate the biological roles of ANRIL and its putative downstream molecule HIF1A in DFU, we first detected ANRIL and HIF1A in the peripheral blood samples of DFU patients. qRT-PCR showed that ANRIL and HIF1A were remarkably downregulated in peripheral blood samples of DFU patients, compared with those from health people (Figure 1A, B). Pearson correlation analysis revealed that there was a positive correlated between ANRIL and HIF1A in DFU (Figure 1C). In addition, the levels of ANRIL and HIF1A were markedly reduced in the ulcer tissues from DFU patients with a positive correlation (Figure S2A–C). Similarly, low expression of ANRIL and HIF1A was observed in skin tissues of DFU mice (Figure 1D). These findings were further validated in vitro. Endothelial progenitor cells were treated with normal glucose (NG, 5.5 mm), a high concentration of mannose (GM, 5.5 mm glucose and 19.5 mm mannose) or high glucose (HG, 25 mm). As presented in Figure 1E and F, ANRIL and HIF1A levels were dramatically decreased in HG group, while there was no significant difference in ANRIL and HIF1A levels between NG and GM groups. Collectively, these findings suggest that ANRIL is downregulated in DFU, and it might be implicated in the skin wound healing process via modulating HIF1A expression.
3.3 HG suppresses EPC proliferation, migration, invasion and tube formation, but promotes apoptosis
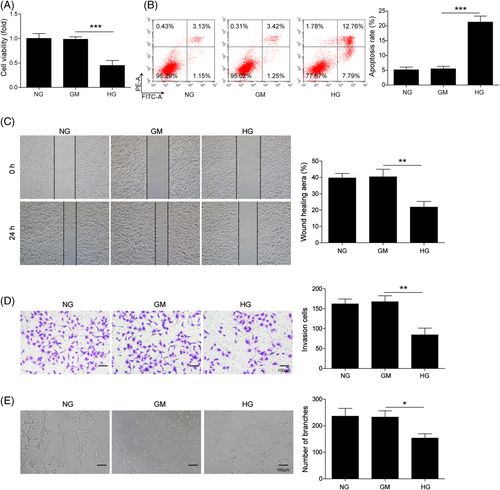
Functional experiments were then performed to assess the effects of HG in EPCs. The CCK-8 assay revealed that HG markedly decreased cell viability of EPCs (Figure 2A). The apoptotic rate of HG-treated EPCs was much higher than that of NG and GM groups as detected by Annexin V-FITC/PI staining (Figure 2B). Wound healing and Transwell invasion assays showed that HG remarkably suppressed the migratory and invasive capacities of EPCs, compared with NG and GM groups (Figure 2C and D). In addition, decreased capability to form capillary-like structures was observed in HG-treated EPCs (Figure 2E). Together, these findings indicate that HG suppressed cell proliferation, migration, invasion and tube formation, but promoted apoptosis in EPCs.
3.4 ANRIL overexpression rescues HG-impaired function of EPCs
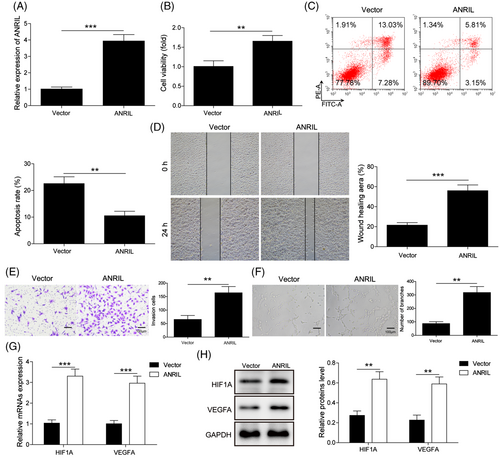
To investigate the function of ANRIL in HG-treated EPCs, gain-of function experiments were next carried out. As expected, transfection of ANRIL overexpression construct caused a dramatic induction of ANRIL in EPCs (Figure 3A). Overexpression of ANRIL reversed HG-impaired cell viability in EPCs (Figure 3B), and HG-enhanced cell apoptosis (Figure 3C) was rescued by ANRIL overexpression. As presented in Figure 3D–F, HG-inhibited cell migration, invasion and tube formation were counteracted by ANRIL overexpression. Interestingly, overexpression of ANRIL resulted in marked induction of HIF1A and VEGFA at both mRNA and protein level (Figure 3G and H). These data indicate that ANRIL is implicated in HG-modulated cell proliferation, apoptosis, migration, invasion and tube formation in EPCs, possible via regulating HIF1A and VEGFA.
3.5 ANRIL regulates HIFA mRNA stability via interacting with FUS
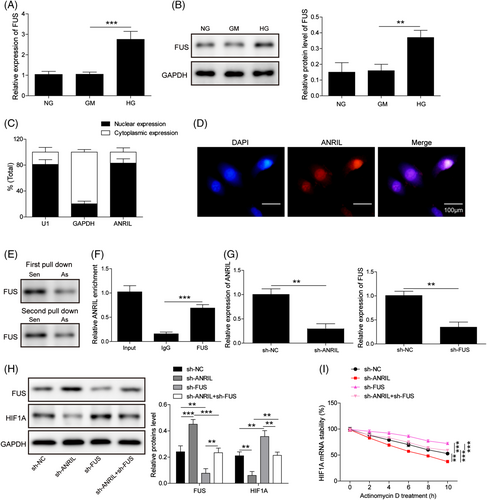
Bioinformatics analysis (ENCORI: https://starbase.sysu.edu.cn/) predicted the putative interactions among ANRIL, HIF1A and FUS (Figure S3). To further unravel the underlying mechanism, we next examined the expression of FUS in EPCs. Fused in sarcoma was notably elevated in HG-treated EPCs, compared with NG and GM groups (Figure 4A and B). Subcellular fractionation and RNA FISH assays unequivocally showed that ANRIL was predominantly expressed in the nucleus in EPCs (Figure 4C and D). Moreover, the RNA pull-down assay revealed that biotin-labeled ANRIL probe successfully pulled down FUS in EPCs (Figure 4E). The RIP assay further confirmed that antibodies against FUS also enriched ANRIL (Figure 4F). Furthermore, loss-of function experiments were performed to further delineate the regulatory network. As anticipated, transfection of sh-ANRIL or sh-HIF1A successfully decreased the ANRIL or HIF1A level, respectively (Figure 4G). Knockdown of ANRIL caused remarkable induction of FUS, while decreasing HIF1A expression in EPCs. In contrast, FUS knockdown increased HIF1A expression. Double knockdown of ANRIL and FUS caused no significant change in FUS and HIF1A levels, compared with the sh-NC group (Figure 4H). These data indicate that sh-ANRIL-mediated FUS upregulation and HIF1A downregulation were abrogated by FUS knockdown. Consistently, mRNA stability assay revealed that ANRIL knockdown decreased HIF1A mRNA stability. Silencing of FUS exerted an opposite effect on HIF1A mRNA stability, and partially reversed the suppressive effect of sh-ANRIL on HIF1A mRNA stability (Figure 4I). Collectively, these findings suggest that ANRIL regulates HIFA mRNA stability via recruiting FUS.
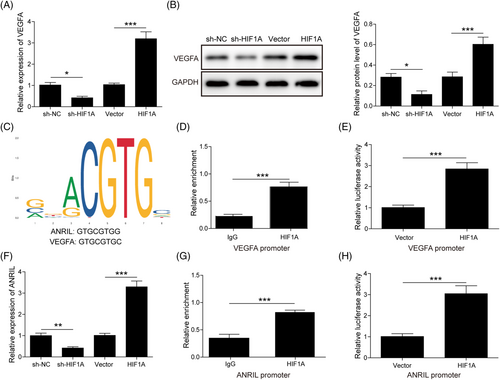
3.6 VEGFA and ANRIL are transcriptionally regulated by HIF1A
A previous study has demonstrated that HIF1A/VEGFA signaling plays a critical role in DFU.25 We next thought to test whether HIF1A/VEGFA acted as a downstream signaler of ANRIL in DFU. As shown in Figure 5A and B, transfection of sh-HIF1A or HIF1A overexpression construct led to a marked reduction or induction of VEGFA mRNA and protein levels in EPCs. The binding motif of HIF1A and binding sites were obtained from JASPAR (http://jaspar.genereg.net/; Figure 5C). The ChIP assay showed a remarkable enrichment of the VEGFA promoter by the anti-HIF1A antibody (Figure 5D). The dual luciferase reporter assay further revealed that co-transfection of the VEGFA promoter–luciferase construct and HIF1A overexpression construct remarkably increased the luciferase activity, compared with the vector alone (Figure 5E). Interestingly, we found that silencing of HIF1A decreased ANRIL level, while overexpression of HIF1A increased the ANRIL expression in EPCs (Figure 5F). Bioinformatics analysis also illustrated the putative interaction between HIF1A and the ANRIL promoter (Figure 5C). As expected, the ChIP assay revealed that antibodies against HIF1A successfully enriched the ANRIL promoter, compared with the IgG control (Figure 5G). In accordance with this result, overexpression of HIF1A dramatically increased luciferase activity in the presence of the ANRIL promoter–luciferase construct (Figure 5H). Taken together, these findings suggest that VEGFA and ANRIL were transcriptionally regulated by HIF1A in EPCs.
3.7 HG suppresses EPC proliferation, migration, invasion and tube formation, but promotes apoptosis via the ANRIL/HIF1A axis
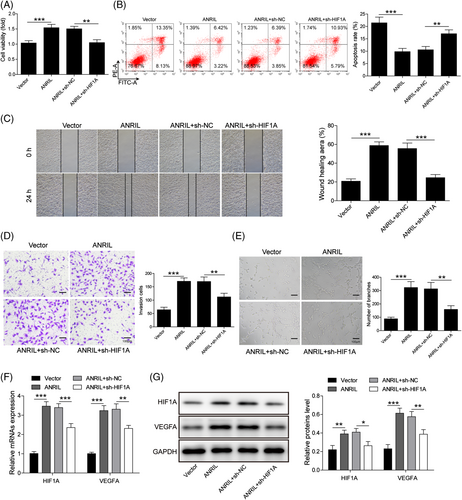
In order to unravel the functional role of the ANRIL/HIF1A axis during DFU, EPCs were transfected with ANRIL overexpression construct or ANRIL+sh-HIF1A, as well as the corresponding controls. As presented in Figure 6A, ANRIL-induced cell viability was attenuated in the ANRIL + sh-HIF1A group upon HG treatment. On the contrary, ANRIL reversed HG-induced apoptosis, while this rescue effect was abrogated by HIF1A knockdown (Figure 6B). Similarly, overexpression of ANRIL promoted the migratory, invasive and tube-forming capacities of EPCs upon HG treatment, whereas these effects were counteracted by HIF1A silencing (Figure 6C–E). In addition, ANRIL overexpression induced HIF1A and VEGFA expression in the presence of HG, while ANRIL-mediated induction of HIF1A and VEGFA was partially abrogated by HIF1A silencing (Figure 6F and G). These data suggest that HG suppressed EPC proliferation, migration, invasion and tube formation, but promoted apoptosis via ANRIL/HIF1A axis.
3.8 ANRIL accelerates DFU wound healing via modulating HIF1A expression
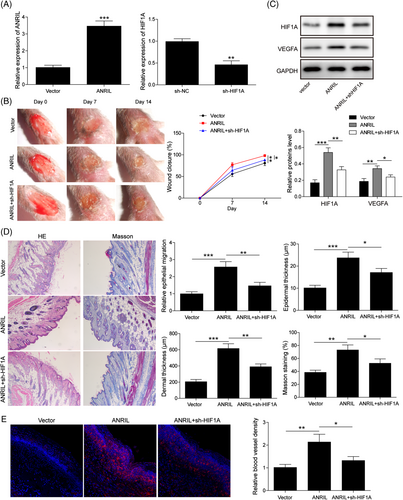
The in vitro findings were next validated in DFU mice. ANRIL overexpression construct or/and sh-HIF1A were administered at the wound edges of DFU mice intradermally. qRT-PCR showed that transfection of ANRIL overexpression plasmid or sh-HIF1A resulted in a notable induction of ANRIL or reduction of HIF1A in skin tissues, respectively (Figure 7A). As shown in Figure 7B, overexpression of ANRIL accelerated wound healing in DFU mice, whereas this effect was abolished by HIF1A knockdown. In consistent with the in vitro findings, ANRIL overexpression upregulated HIF1A and VEGFA expression, while silencing of HIF1A partially attenuated these effects (Figure 7C). H&E coupled with Masson staining revealed that overexpression of ANRIL promoted epithelial migration and collagen fiber formation, and increased epidermal and dermal thickness. ANRIL-induced histological alterations in skin tissues were abrogated by HIF1A silencing (Figure 7D). In addition, we next examined angiogenesis by endothelial marker CD31 staining. Overexpression of ANRIL enhanced angiogenesis in which the CD31-positive vessels were increased, while this effect was partially reversed by HIF1A knockdown (Figure 7E). Collectively, these findings indicate that ANRIL accelerated DFU wound healing via modulating HIF1A expression in vivo.
4 DISCUSSION
Diabetic foot ulcer remains a clinical challenge worldwide, and patients with DFU exhibit much higher risk of death at 5 years than patients who do not have DFU.1 Diabetic foot ulcer-related infection and amputation lead to low life quality and high costs of healthcare.1, 31 In the current study, we found that ANRIL was downregulated in DFU, and ANRIL overexpression rescued HG-impaired EPC functions. Mechanistic studies further showed that ANRIL regulated HIF1A mRNA stability via recruiting RBP FUS. HIF1A also transcriptionally regulated VEGFA and ANRIL. HG impaired EPC functions through ANRIL/HIF1A axis, and ANRIL accelerated wound healing via regulating HIF1A in DFU mice.
It is well established that EPCs play important roles in the neovascularization and maintenance of vascular endothelial function and integrity.32 Endothelial progenitor cells are decreased in patients with DFU,33 indicating that decreased EPCs might contribute to non-healing wounds in DFU. In addition, ANRIL is downregulated in DFU mice and HG-treated lymphatic endothelial cells (LECs).20 In accordance with this finding, our data showed that ANRIL was decreased in the peripheral blood samples of DFU patients, DFU mice and HG-treated EPCs. A previous study has illustrated that HG suppresses cell proliferation and promotes apoptosis in EPCs via VEGF-mediated phosphorylation of Akt.9 Besides impaired cell proliferation and apoptosis, our findings also revealed that HG inhibited cell migration, invasion and tube formation in EPCs. In LECs, ANRIL overexpression reversed HG-induced apoptosis, impairment of migratory and the tube-forming capacities of LECs.20 Similarly, functional experiments showed that overexpression of ANRIL rescued HG-impaired EPC functions, suggesting the salient role of ANRIL in EPCs upon HG treatment. The in vitro studies lack in vivo validation owing to the complicated environment in vivo. To further validate the role of EPCs, well-designed in vivo functional studies are needed in future studies.
A previous study has illustrated that ANRIL accelerates wound healing via sponging miR-181a, thereby regulating Prox1 expression in LECs.20 Besides miRNA-sponging activity, lncRNAs participate in various physiological and pathological processes through binding to RBPs.21 For instance, ANRIL regulates VEGF through binding to p300, miR-200B and PRC2 complex in diabetic retinopathy.34 In this study, ANRIL regulated HIF1A mRNA stability via recruiting FUS in EPCs. Consistent with the clinical report which demonstrated the elevation of FUS in patients with type 1 diabetes,22 our data showed that HG resulted in marked induction of FUS in EPCs. A recent report has illustrated the nuclear expression of FUS in EPCs.35 We also found that ANRIL was predominantly expressed in the nucleus in EPCs, indicating the potential interaction between ANRIL and FUS in EPCs. This association was further validated by RNA pull-down and RIP assays. ANRIL positively regulated HIF1A mRNA stability in a FUS-dependent manner. On the other hand, HIF1A was identified as an upstream regulator of VEGFA in EPCs, suggesting that VEGFA might act as a downstream effector in ANRIL-regulated angiogenesis in DFU. HIF1A/VEGFA signaling is activated by lncRNA GAS5 in HUVECs, and contributes to wound healing in DFU,25 supporting the important role of HIFA/VEGFA signaling in DFU wound healing process. Furthermore, we reported that ANRIL was transcriptionally regulated by HIF1A, illustrating a feedback regulation loop in EPCs.
Functional experiments further demonstrated that HG impaired cell proliferation, migration, invasion and tube formation, but enhanced apoptosis via ANRIL/HIF1A axis. These in vitro findings were validated in DFU mice. The alteration of ANRIL or HIF1A further regulated VEGFA expression in vitro and in vivo, thereby modulating angiogenesis and wound healing in DFU. The functional role and detailed mechanism underlying mutual regulation between ANRIL and HIF1A merits in-depth investigation.
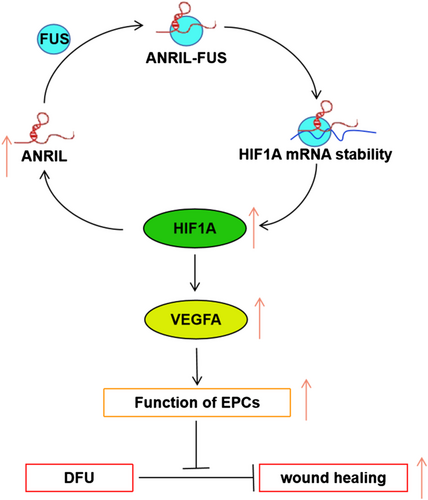
In conclusion, our findings demonstrated that ANRIL accelerated wound healing in DFU via modulating HIF1A/VEGFA signaling in a FUS-dependent manner (Figure 8). Additionally, there was mutual regulation between HIF1A and ANRIL. These findings broaden the understanding of DFU pathogenesis, and ANRIL might be a promising biomarker for DFU.
CONFLICT OF INTEREST
There is no conflict of interest.
AUTHOR CONTRIBUTIONS
Guarantor of integrity of the entire study: Wan Jia, Yang Yong.
Study concepts: Wan Jia, Yang Yong.
Study design: Wan Jia.
Definition of intellectual content: Wan Jia.
Literature research: Bao Yan, Hou Lijuan.
Clinical studies: Ma Zhenhuan, Yang Guokai.
Experimental studies: Hou Yi, Li Zhaoxiang.
Data acquisition: Li Guojian, Du Lingjuan.
Data analysis: Li Guojian, Du Lingjuan.
Statistical analysis: Bao Yan, Hou Lijuan.
Manuscript preparation: Wan Jia, Bao Yan, Hou Lijuan.
Manuscript editing: Wan Jia.
Manuscript review: Wan Jia, Yang Yong.
Open Research
DATA AVAILABILITY STATEMENT
The data used to support the findings of the present study are available from the corresponding author upon reasonable request.




