The CHST11 gene is linked to lung cancer and pulmonary fibrosis
Funding information: This study was supported by the Genomics Research Center and National Science and Technology Council, Taiwan (MOST- 109-2320-B-001-015-MY3) to Michael Hsiao.
Funding information: Academia Sinica; National Science and Technology Council, Grant/Award Number: MOST- 109-2320-B-001-015-MY3
Abstract
Background
The abnormal modification of chondroitin sulfate is one of the leading causes of disease, including cancer progression. During chondroitin sulfate biosynthesis, the CHST11 enzyme plays a vital role in its modification, but its role in cancer is not fully understood. Therefore, understanding the relationship between CHST11 and pulmonary-related diseases through clinically relevant information may be useful for diagnosis or treatment.
Methods
A variety of pulmonary fibrosis clinical gene expression omnibus (GEO) datasets were used to assess the association between CHST11-related manifestations and fibrosis. Multiple lung cancer-related databases, including The Cancer Genome Atlas, GEO datasets, UCSC Xena, GEPIA2, Cbioportal and ingenuity pathway analysis were used to evaluate the clinical correlation between CHST11 and lung cancer and potential molecular mechanisms. For drug repurposing prediction, the molecules that correlated with CHST11 were subjected to the LINCS L1000 algorithm. A variety of in vitro assays were performed to evaluate the in-silico models, including RNA and protein expression, proliferation, migration and invasion.
Results
Clinical analyses indicate that the levels of CHST11 are significantly elevated in cases of pulmonary-related diseases, including fibrosis and lung cancer. According to multiple lung cancer cohorts, CHST11 is the only member of the carbohydrate sulfotransferase family associated with overall survival for lung adenocarcinomas, and it is highly related to smoking-induced lung cancer patients. Based on the results of in vitro experiments, CHST11 expression contributes to tumor malignancy and promotes multiple fibrotic activators. Correlation-based ingenuity pathway analysis indicated that CHST11-related molecules contributed to pulmonary fibrosis or lung adenocarcinomas via similar upstream stimulators. Based on known molecular regulatory relationships, CHST11 has been associated with the regulation of TGF-β and INFγ as important molecules contributing to fibrosis and cancer progression. Interestingly, WordCloud analysis revealed that CHST11-related molecules are involved in regulation primarily by integrin signaling, and these relationships were consistently reflected in the analysis of cell lines and the clinical correlation. A CHST11 signature-based drug repurposing analysis demonstrated that the CHST11/integrin axis could be targeted by AG-1478 (Tyrphostin AG 1478), brefeldin A, geldanamycin and importazole.
Conclusions
This study provides the first demonstration that CHST11 may be used as a biomarker for pulmonary fibrosis or lung cancer, and the levels of CHST11 were increased by TGF-β and INFγ. The molecular simulation analyses demonstrate that the CHST11/integrin axis is a potential therapeutic target for treating lung cancer.
1 INTRODUCTION
According to the American Cancer Society in 2021, lung cancer ranks number one in cancer deaths, with statistics indicating that 22% of all cancer deaths are caused by lung cancer [1]. Among them, 82% of lung cancer deaths are caused by cigarette smoking [2, 3], which is considered one of the major environmental factors that lead to lung inflammation and pulmonary fibrosis [4]. In comparison with lung cancer, there is a very high mortality rate associated with pulmonary fibrosis of approximately 93% [5, 6]. A number of studies have also shown that pulmonary fibrosis is one of the risk factors for lung cancer and that individuals with pulmonary fibrosis have a worse prognosis for lung cancer [7]. According to recent studies, patients with idiopathic pulmonary fibrosis (IPF) have a higher mortality rate (p < 0.001) than patients with squamous cell carcinoma (p = 0.03) [8]. Therefore, identifying pathological and mechanistic causes will be beneficial for the accurate diagnosis and treatment of related diseases.
It is believed that the epithelial–mesenchymal transition is responsible for many cellular functions, including embryogenesis and carcinogenesis [9]. During tissue damage repair processes, fibroblasts are a typical cell type that is activated via epithelial to mesenchymal transition (EMT) to assist with wound healing. Pulmonary fibrosis is a typical example of fibroblast EMT activation. Specifically, it refers to the chronic process that occurs in the lung when alveolar type II (AT2) epithelial cells are stimulated by an extracellular proteoglycan matrix, which initiates myofibroblast transdifferentiation and performs repeated wound healing [10, 11], a process associated with IPF [12]. In cancer, extravasation to metastasis is closely related to the process of EMT [9]. Based on the pathological classification of lung adenocarcinomas (LUADs), it appears to be caused mainly by dysregulation of AT2 epithelial cells through processes involved in EMT [12-14]. While the mechanism of pathological formation remains a subject of debate, several studies have shown that pulmonary fibrosis is a risk factor for non-small cell lung cancer (NSCLC) formation [8, 15, 16]. Accordingly, it is essential to identify specific biomarkers that can reflect the relationship between pulmonary fibrosis and NSCLC.
As the basic substance of the cellular extracellular matrix, chondroitin sulfate can form proteoglycans with core proteins responsible for a range of cellular physiological functions [17]. Recent research has revealed that abnormalities in chondroitin sulfate are associated with the development of diseases, including cancer. Chondroitin sulfate abnormalities may be attributed to enzymes involved in its biosynthesis [18]. The enzyme CHST11, which forms chondroitin 4-sulfate, has been implicated in the progression of several types of cancer. Higher CHST11 expression in colorectal and ovarian cancer patients has been associated with a poor outcome [19, 20]. The abnormal expression of CHST11 promotes abnormal cell proliferation, metastasis and cancer stemness [21-23]. Epigenetic analysis indicates that CHST11 expression is affected by its promoter methylation [24, 25]. The abnormal deposition of proteoglycans in pulmonary fibrosis has been associated with chondroitin 4-sulfate levels [26]. Despite this, there are no studies that indicate the role of the enzyme CHST11 in the sulfate modification of fatty acids in the development of pulmonary fibrosis or lung cancer.
In this study, an integrated clinical data comparison demonstrated a positive correlation between CHST11 expression and the etiology of pulmonary fibrosis or lung cancer caused by smoking. As demonstrated by relevant in vitro and biological assays, CHST11 has independent regulatory mechanisms that modulate the characteristics of pulmonary fibrosis and tumor malignancy, and these two events can also be costimulated by TGF-β and TNFγ. We also present results from molecular simulation models and drug repurposing analyses against the ITGB/CHST11 axis as a therapeutic target.
2 MATERIALS AND METHODS
2.1 Clinical data on lung cancer and pulmonary fibrosis
Gene Expression Omnibus (GEO) datasets, including GSE108134, GSE11906, GSE40839, GSE44723, and GSE31210, were downloaded from NCBI (https://www.ncbi.nlm.nih.gov/gds). GeneSpring GX software was utilized to process all CEL files.
2.2 Correlation of the pathological features of CHST11 in LUAD
Data regarding pathophysiological features and their relationship to CHST11 expression were obtained from UCSC Xena’s website (https://xenabrowser.net/).
2.3 Correlation between gene expression and prognosis in lung cancer
An analysis of the correlation of gene expression in lung cancer was obtained from the GEPIA2 website (http://gepia2.cancer-pku.cn/#index). Kaplan–Meier plots (https://kmplot.com/analysis/) were used to determine the correlation between gene expression and the prognosis of lung cancer patients. The data on gene expression in lung cancers were retrieved from the Cancer Cell Line Encyclopedia (CCLE; https://sites.broadinstitute.org/ccle/).
2.4 Model for the molecular and pharmaceutical simulation of CHST11
Molecules related to CHST11 were downloaded from the Cbioportal website (https://www.cbioportal.org/). The Cancer Genome Atlas (TCGA)-Firehose Legacy, TCGA-Nature 2014, and TCGA-PanCancer Atlas data were used. Correlated molecules were defined as those with Spearman’s correlations of ±0.3. A Venn diagram was used to group molecules that cross between different datasets. Ingenuity pathway analysis (IPA; https://analysis.ingenuity.com/pa/installer/select) provided an analysis of the signaling pathways and gene ontologies that may be impacted by the molecules. Drug repurposing mining used the Venn diagram method to identify genes linked to CHST11, which were then submitted to the L1000 CDS website for analysis.
2.5 Establishment of a two-way model based on CHST11
pLenti-6.3-CHST11 cDNA and pLKO.1-CHST11 shRNA were obtained from DNASU (Arizona State University, USA) and the National RNAi core facility (Academia Sinica, Taiwan). Lentivirus encapsulation was used to produce the virus in HEK293 cells. Cell lines associated with infection-related lung adenocarcinoma, including A549, H441, H358 and H1975, were infected with the virus by a TOOLSFect transfection reagent TTM-TF01 (BIOTOOLS, Taiwan). The infected cells were further selected for puromycin or blasticidin, and the survival of the infected cells was confirmed by immunoblotting.
2.6 Immunoblotting and qPCR assays
All lung cancer cell lines and human pulmonary fibroblast cell lines were obtained from ATCC (The Global Bioresource Center, USA) and ScienCell (ScienCell Research Laboratories, USA). The cells were cultured according to official guidelines. Culture medium containing 0.05% penicillin–streptomycin antibiotics (Gibco, USA) and fetal bovine serum (Gibco, USA) was used to maintain the tumor cells, which were cultured in a constant temperature at 37°C and 5% CO2. The cells were treated with different drugs and recombinant proteins, including nicotine (Sigma–Aldrich, USA), particulate matter (PM; 1649b, NIST® SRM®, Gaithersburg, USA), bleomycin (MedChemExpress, USA), recombinant TGF-β protein (Sino Biological, USA), recombinant INFγ protein (Sino Biological, USA) and ITGB inhibitors, including AG-1478, brefeldin A, geldanamycin, importazole and NVP-TAE 684 (MedChemExpress, USA). RNA was collected using an RNA kit (Qiagen, Germany) and translated into cDNA using a TOOLSQuant II Fast RT Kit (Biotools, Taiwan), and mRNA levels were calculated using qPCR. RIPA buffer was used to collect the proteins, and immunoblotting was used to analyze them.
2.7 Proliferation and motility assays for tumor cells
Alamar blue (Sigma–Aldrich, Germany) was used to measure the effect of CHST11 on the proliferation of cells. The appropriate number of cells was seeded on 96-well plates, and the growth trends were assessed over a period of 6 days. Cell migration and invasion were evaluated in a Boyden chamber. In the same manner, appropriate cells were relysed in serum-free medium and seeded in an upper chamber. In the lower chamber, fibronectin (Sigma–Aldrich, Germany) and Matrigel (Corning, USA) were coated with attracting serum medium to evaluate the migration or invasion ability of cells mediated by CHST11. A solution of Giemsa was used for visualization and the polycarbonate membrane filter was exposed under an optical microscope (400×, three randomly selected fields per/well).
2.8 Statistical analysis
Excel was used to perform the statistical analysis using the two-tailed Student’s t-test. The t-test was used to assess the statistical significance of the difference between groups as follows: * p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001.
3 RESULTS
3.1 The expression of CHST11 is associated with smoking-related lung cancer
To identify whether an elevation of CHST11 correlates with lung cancer progression, we analyzed several lung cancer cohorts to determine the correlation between CHST11 expression and overall survival. An analysis of the Kaplan–Meier plot demonstrated that the expression of CHST11 was significantly associated with poor prognosis in lung cancer (p = 0.0000053; Figure 1A). Furthermore, the results revealed that CHST11 was only associated with a poor prognosis for subtypes of LUAD (p = 0.0000026) but not lung squamous cell carcinoma (LUSC; p = 0.31). CHST11 is an enzyme involved in the biosynthesis of CS. Among the other enzymes that contribute to chondroitin sulfate modification are CHST3, CHST7, CHST10, CHST12, CHST13, CHST14 and CHST15 [19]. To determine whether CHST11 is the only enzyme involved in LUAD progression, other vital enzymes involved in the biosynthesis of chondroitin sulfate were also examined. Based on Kaplan–Meier plot analysis, CHST11 was the only molecule associated with poor prognosis in LUAD (p = 0.0000026; Figure 1B). Other clinical cohorts (GSE31210) also yielded consistent results (Figure S1).
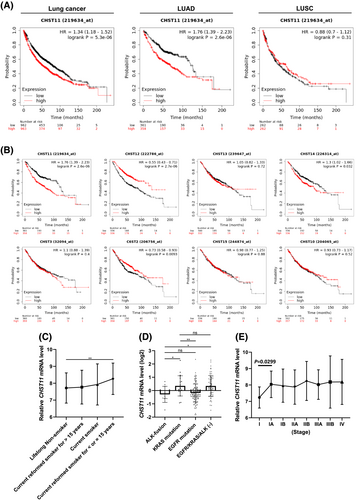
To determine whether the increased level of CHST11 was due to environmental or genetic changes, two LUAD datasets (TCGA and GSE31210) were used. As shown in Figure 1C, smoker lung cancer patients had higher levels of CHST11 expression than non-smokers (p = 0.0076; Supplementary Table 1), especially those who had smoked for 15+ years. In terms of genetic changes, mutations of the EGFR, KRAS and ALK genes were highly associated with the occurrence of spontaneous lung cancers. As indicated by the analysis of LUAD clinical data (GSE31210), CHST11 levels were decreased in patients who had ALK fusions and EGFR mutations and increased in patients who had KRAS mutations and EGFR/KRAS/ALK triple-negative cases (Figure 1D; Supplementary Table 2). However, CHST11 expression did not differ significantly between KRAS mutations and non-genetic variations. Thus, the level of CHST11 may be related to smoking rather than genetics. Furthermore, data from TCGA indicate that the expression of CHST11 increases with stage progression, especially between stage I and stage IA (p = 0.0299; Figure 2E; Supplementary Table 3). These results suggest that CHST11 may be associated with smoking-induced fibrosis but also participates in the progression of smoking-related lung cancer, particularly LUAD.
3.2 Increased expression of CHST11 is associated with pulmonary fibrosis
There is still controversy regarding the detailed mechanism of how pulmonary fibrosis and lung cancer develop. The presence of lung fibrosis is currently considered one of the risk factors for lung cancer [8]. It may be caused by environmental factors such as smoking and is also one of the factors that contribute to lung cancer [27]. By investigating whether CHST11 is involved in both fibrosis and cancer, we may be able to distinguish between the two symptoms or find a target that can be used in conjunction to treat both conditions. To investigate the relationship between CHST11 and lung fibrosis, a number of clinically relevant GEO datasets with primary lung lesions were utilized, including smoker (GSE108134), chronic obstructive pulmonary disease (COPD; GSE11906) and usual interstitial pneumonia (UIP; GSE40839; Supplementary Table 4). To confirm that these samples were indeed related to pulmonary fibrosis, the levels of fibrosis markers such as fibrosis factor (TGF-β) and activated fibroblast markers (FAP, COL1A1, ACTA2 and FN1) were assessed (Figure S2A). Based on these data, the smoking group had higher CHST11 expression than the non-smoking group (p = 0.0167). The patients with COPD also had higher CHST11 expression than the non-smoking group (p = 0.0575). In pulmonary fibrosis of unknown etiology, such as UPI, CHST11 expression was also found to increase (p = 0.0306; Figure S2A). The heatmap analysis indicated that although there were no significant differences between the COPD groups, the expression of CHST11 was elevated in all pulmonary fibrosis cases, consistent with the smoker and UPI groups (Figure S2B).
To determine whether the upregulation of CHST11 is caused by the stimulation of fibrosis factors, human pulmonary fibroblasts (HPF) were treated with fibrosis inducers, including nicotine, ambient PM and bleomycin. The results demonstrated that diverse stimulators activated the related fibrosis markers (such as a-SMA, FAP, Col I and Col II) as well as CHST11 expression. Overall, these results suggest that CHST11 may also play an essential role in the development of pulmonary fibrosis, in addition to its role as a lung cancer diagnostic marker.
3.3 CHST11 levels affect lung cancer malignancy characteristics
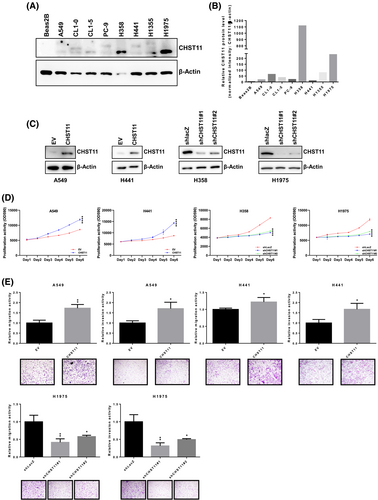
To confirm that CHST11 is involved in lung cancer progression, a CHST11-mediated two-way model was developed. The distribution of CHST11 in cancer cells was evaluated using the cancer model (Figure 2A) (Figure S8). Compared with normal lung fibroblast cells, BEAS-2B lung cancer cells expressed more CHST11 (Figure 2B). To establish a CHST11 two-way model, cells with knockdown and those with overexpression of CHST11 were selected based on their expression of CHST11 (Figure 2C) (Figure S8). For the biological assays, the results showed that when cells overexpressed CHST11, the growth rate increased, while the growth rate of cells with CHST11 knockdown decreased (Figure 2D). The analysis of cell motility also revealed that CHST11 increases the motility of cells, including their ability to migrate and invade. As shown in Figure 2E, knockdown of H1975 with different shCHST11 constructs also reduced the motility of cells. Based on the results presented above, CHST11 can not only function as a diagnostic factor but also participate in lung cancer malignancy.
3.4 CHST11 regulation is involved in the processes of pulmonary fibrosis and cancer development
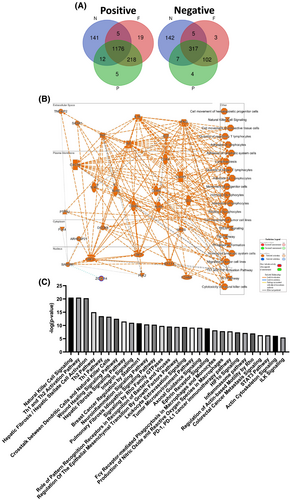
The molecular mechanism of CHST11 in pulmonary fibrosis or lung cancer has not been studied to date. To reveal the signaling hallmark that CHST11 may be responsible for regulating pulmonary fibrosis and lung cancer progression, a molecular simulation analysis was conducted [28]. This analysis used three TCGA-LUAD datasets: TCGA-Firehose Legacy, TCGA-Nature 2014 and TCGA-PanCancer Atlas. In lung cancer, the Venn diagram was integrated with CHST11 based on a Spearman’s correlation greater than ±0.3 as a valid basis parameter. In total, 1,176 positive and 317 negative genes were collected (Figure 3A; Supplementary Table 5). By utilizing the IPA database, we were able to simulate possible gene ontologies and the mutual regulatory relationships between the molecules (Figure 3B). These signaling hallmarks are illustrated in Figure 3C based on the ranking of the p-values (Supplementary Table 6). The signaling hallmark ranking suggests that CHST11-related molecules participate in both tumor-related signaling and pulmonary fibrosis idiopathic signaling as well as hepatic fibrosis/hepatic stellate cell activation and hepatic fibrosis signaling pathways. These results support the relevance and usefulness of this molecular simulation by emphasizing CHST11’s potential mechanisms in pulmonary fibrosis and LUAD progression.
3.5 The CHST11 gene primarily regulates fibrosis and cancer motility events related to cell motility
Fibrosis is defined as the transformation of epithelial cells into myofibroblasts following the EMT, which is also important in the metastatic progression of tumor cells [11]. To determine whether the increased CHST11 level in pulmonary fibrosis correlates with cellular motility, datasets from GSE44723 were analyzed. The clinical data (GSE44723) showed that CHST11 was expressed at a high level in the fibroblast-rapid group compared with the fibroblast-low group (Figure S3A) (Supplementary Table 7). Cells stimulated by different fibrosis activators also transactivate factors associated with EMT, such as vimentin (Figure S3B), which supports the level of CHST11 associated with EMT processes during fibrosis occurrence. Consistently, TCGA-LUAD data demonstrated that CHST11 was positively correlated (Spearman’s correlations >0.3) with several mesenchymal factors in LUAD, including VIM, TWIST1, TWIST2, SNAI1 and SNAI2, suggesting a role for CHST11 in EMT (Figure S3C). In cancer cells overexpressing CHST11, mesenchymal factors, including snail1, slug, twist, zeb1, zo1, goosecoid, mmp2 and mmp9, are also increased (Figure S3D). Accordingly, the molecular simulations supported that CHST11-related molecules are indeed involved in many EMT-related signaling pathways, including the Wound Healing Signaling Pathway, Integrin Signaling, Signaling by Rho Family GTPases, Regulation of the Epithelial Mesenchymal Transition by Growth Factors Pathway, Axonal Guidance Signaling, Regulation of Actin-based Motility by Rho, Colorectal Cancer Metastasis Signaling, Actin Cytoskeleton Signaling and ILK Signaling (Figure 3C). Thus, CHST11 may contribute to LUAD progression during the EMT process and may be involved in pulmonary fibrosis events.
3.6 CHST11 expression is mainly induced by TGF-β and INFγ
For an unbiased analysis, EMT signaling was first aligned with molecules that are known to play a role in CHST11 regulation. Four molecules were identified, ERBB2, IFNG, MMP9 and TGF-β (Figure 4A). According to clinical correlation, these molecules are correlated with CHST11 in LUAD. The molecules with Spearman’s correlation values greater than 0.3 were IFNG (R = 0.41) and MMP9 (R = 0.45) (Figure 4B). A comparison of IFNG and MMP9 as signatures with EMT-related molecules revealed a significant correlation with LUAD (R = 0.59) (Figure 4C). To confirm that clinical correlations can be seen in LUAD cell lines, we utilized CCLE datasets. The results indicated a strong correlation between IFNG (R = 0.787) or MMP9 (R = 0.47) and CHST11 in LUAD at the cellular level (Figure 4D; Supplementary Table 8). As a result of treatment with recombinant TGF-β and INFγ proteins, cells were able to further activate the expression of CHST11 (Figure 4E).
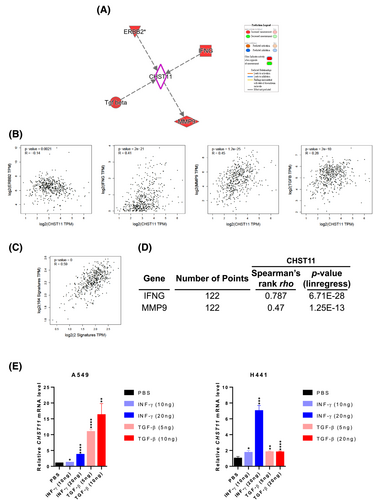
To assess whether such regulatory relationships are similar to those of the fibrosis model, GEO datasets related to fibrosis (GSE11906, GSE108134, GSE40839) were used to identify CHST11-related molecules (Figure S4A). Based on the Venn diagram results, approximately 13 molecules are collectively involved in different fibrosis events caused by CHST11. As analyzed by IPA, TGF-β and INFγ share a molecular relationship with CHST11 in both fibrosis and cancer models. It was found that CHST11 expression was increased when HPF cells were stimulated by both TGF-β and INFγ (Figure S4C), which is consistent with the tumor cell response. Additionally, molecules involved in the CHST11 pathway were consistently activated by fibrosis activators (Figure S4D). It should be noted, however, that downstream regulators of CHST11 are distinct from upstream regulators in fibrosis (Figure S4D) and tumor events (Figure 4A). These findings suggest that CHST11 may contribute to pulmonary fibrosis or LUAD progression by regulating EMT, which can be stimulated by TGF-β or INFγ, triggering different downstream CHST11 effectors.
3.7 CHST11-related molecules primarily function through integrin signaling
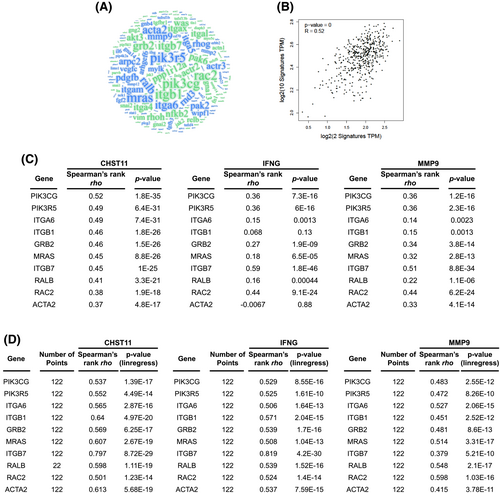
To identify CHST11-mediated regulation involved in specific signaling, a WordCloud analysis was conducted based on EMT-related signaling (Figure 5A; Supplementary Table 9). Based on correlation analysis, the top 10 repeatable molecules with high correlations in LUAD were IFNG and MMP9 (R = 0.52; Figure 5B). In LUAD, we found a strong clinical correlation (greater than 0.30 Spearman’s correlation) between these highly repetitive EMT molecules and CHST11/IFNG/MMP9. Among the IFNG-related genes were PIK3CG (R = 0.36), PIK3R5 (R = 0.36), ITGB7 (R = 0.59), and RAC2 (R = 0.44). Genes associated with MMP9 included PIK3CG (R = 0.36), PIK3R5 (R = 0.36), GRB2 (R = 0.34), MRAS (R = 0.32), ITGB7 (R = 0.51), RAC2 (R = 0.44) and ACTA2 (R = 0.33) (Figure 5C). According to the CCLE database, these genes were significantly correlated with CHST11, IFNG and MMP9 gene expression (Figure 5D). Based on these observations, we conclude that most of these highly related molecules play important roles in the integrin signaling process. IPA also revealed that CHST11-related molecules are highly correlated with integrin signaling (red indicates a positive correlation; Figure S5). By analyzing Kaplan–Meier plots, it was discovered that the majority of these integrin-related molecules were associated with a poorer prognosis for LUAD, including IFNG (p = 0.0013), MMP9 (p = 0.009), PIK3R5 (p = 0.0000000000063), ITGA6 (p = 0.00017), ITGB1 (p = 0.0099), GRB2 (p = 0.018), MRAS (p = 0.049), RALB (p = 0.00038), RAC2 (p = 0.000000051) and ACTA2 (p = 0.015) (Figure S6). These results demonstrated that CHST11-mediated IFNG and MMP9 could affect cellular EMT by regulating integrin signaling, which may contribute to a poor prognosis in LUAD.
3.8 CHST11 signature-based in-silico drug repurposing
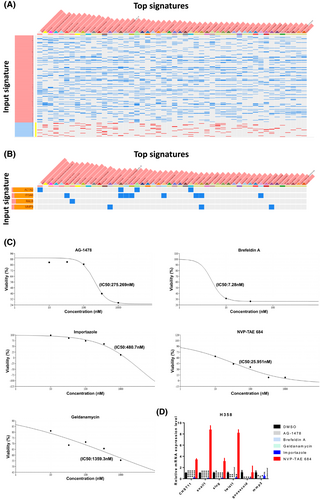
To identify potential pharmaceuticals targeting the CHST11/integrin pathway, 1,176 positive and 317 negative genes that correlate with CHST11 (Figure 3A; Supplementary Table 5) were submitted for analysis as signatures to the ultrafast LINCS L1000 characteristic direction signature search engine. The analysis revealed that approximately 50 drugs were capable of reversing CHST11-positive molecules (Figure 6A; Supplementary Table 10). Most of these drugs have been tested in relative doses in LUAD cell lines, such as A549 and HCC515. The related compounds include sodium dithionite, TG101348, FCCP, WH-4-025, malonoben, IMD 0354, Selamectin, Vorinostat, BRD-A17065207, NVP-TAE684, AG-879, PD-407824, niclosamide, I-BET151, PD-173074, CCCP and CGP 71683 hydrochloride. It is noteworthy that there are a number of drugs that more specifically target integrin signaling-related molecules, including ACTA2, ITGA6, RALB and MMP9 (Figure 6B). Among the drugs in this class that are capable of cross-targeting are 15-deoxy-delta-12, 14-prostaglandin J2 (ITGA6, ACTA2), NVP-TAE684 (ITGA6, MMP9, ACTA2) and brefeldin A (ITGA6, RALB). To investigate the effects of ITGB-related inhibitors on CHST11 and their potential as potential therapeutics, several drugs were tested. Accordingly, the IC50 was determined and considered to be the optimal treatment drug concentration (Figure 6C; Figure S7A). According to the results of qPCR analysis, AG-1478 (Tyrphostin AG 1478), brefeldin A, geldanamycin and importazole effectively inhibited CHST11 or mesenchymal-related factors in H358 cells that expressed the highest levels of CHST11 (Figure 6D), but only geldanamycin and importazole inhibited CHST11 in H1975 cells (Figure S7B), which indicates that different CHST11 levels can respond differently to drugs. Based on these results, these potential drugs could be repurposed by targeting the CHST11 signature and the CHST11/integrin axis to inhibit the progression of LUAD.
4 DISCUSSION
The precise mechanism by which pulmonary fibrosis caused by environmental factors contributes to cancer development remains unclear. [29]. There are reports that gene alterations constitute a significant cause of spontaneous lung tumors [30, 31]. Therefore, there is a need to investigate pulmonary fibrosis and carcinogenesis in relation to related biomarkers. Representative molecules include ALK fusions, KRAS mutations and EGFR mutations [32, 33]. Based on these gene alterations, CHST11 did not show a statistically significant difference by cohort (GSE31210), which may reflect the clinical significance of LUAD compared with the EGFR/KRAS/ALK triple-negative cases (Figure 1D). Accordingly, CHST11 expression is independent of gene alteration and in response to chronic inflammatory stimuli, including tobacco smoke. Using multiple published clinical studies as a basis for this study, the potential role of CHST11 in pulmonary fibrosis was revealed. According to the current etiology and pathological definition of pulmonary fibrosis, IPF and COPD are the two most common classifications. IPF is the most common type of UIP. It has been determined that smoking is a major environmental factor. In the smoker group, CHST11 levels were significantly increased (p = 0.0167; Figure S2A). COPD and UIP both showed consistent increases. This study suggested that CHST11 could be used as a pulmonary fibrosis biomarker. According to the National Institutes of Health, smoking is one of the environmental factors contributing to lung cancer and pulmonary fibrosis [4]. Among patients with LUAD compared with non-smokers, patients who smoked for over 15 years had more significant expression of CHST11 (p = 0.0076). Pulmonary fibrosis is one of the primary causes of the development of NSCLC [16]. Therefore, our results suggest that CHST11 may share the exact same molecular mechanism in response to smoking-induced pulmonary fibrosis or lung cancer.
A variety of fibrosis stimulators have been used to simulate the activation of fibroblasts, confirming that CHST11 is activated when myofibroblasts are formed (Figure S2C), indicating that CHST11 can indeed serve as a meaningful biomarker in pulmonary fibrosis. In cancer, similar results can also be observed. Based on the analysis of TCGA-LUAD data, it can be seen that the expression of CHST11 tends to increase with the increase in LUAD stages, particularly in the early stage I to stage IA (Figure 1E). A Kaplan–Meier analysis also demonstrated that high CHST11 expression only significantly predicted the worst prognosis for LUAD patients (p = 0.00000026; Figure 1A). It is known that CHST11 modifies chondroitin sulfate at the 4S position during chondroitin sulfate biosynthesis. A number of modifications have been made to chondroitin sulfate, including 2S, 3S, 4S and 6S sulfate modifications. The results of our study indicated that based on the analysis of these related enzymes by different cohorts, only CHST11 acted as a unique biomarker independent of other related enzymes in LUAD (Figure S1). Furthermore, biological function assays confirmed that artificially manipulating the expression of CHST11 can influence tumor cell proliferation and motility (Figure 2D and E). All of these results suggest that CHST11 might prove to be helpful as an early diagnostic biomarker and involved in cancer malignancy features.
Currently, no research has been conducted to determine the mechanism by which CHST11 is involved in lung-related diseases, such as pulmonary fibrosis and cancer. We developed a molecular simulation model by aggregating CHST11-related molecules to predict that CHST11 may be involved in the main signaling hallmark of progression in LUAD (Figure 3). This model effectively predicts that CHST11-related molecules are indeed involved in fibrosis-related signaling, including the pulmonary fibrosis idiopathic signaling pathway, hepatic fibrosis/stellate cell activation and the hepatic fibrosis signaling pathway. Fibrosis is usually defined in terms of myofibroblast formation [34], which is induced by abnormal stimulation of alveolar epithelial cells that results in the activation of the EMT process [10, 11]. Compared with slow-motility fibroblasts, CHST11 expression was found to be significantly higher in fibroblasts with rapid motility (p = 0.0110; Figure S3A), showing a very high correlation with EMT. It was also confirmed by in vitro assays that these fibroblast stimulators that stimulate CHST11 upregulation also activate vimentin expression (Figure S3B). Notably, in the cancer model, the results of molecular simulations indicated that CHST11-related molecules are involved in many signaling pathways related to EMT (Figure 3C and Figure S3C). It has also been shown that cells that overexpress CHST11 are able to increase the expression levels of various factors associated with the mesenchymal state (Figure S3D).
IFNG and MMP9 are two of those molecules regulated by CHST11 [35, 36] (Figure 4). As unbiased molecules, IFNG and MMP9 can be used to produce a meaningful signature for EMT-related signaling using the TCGA-LUAD dataset (R = 0.52; Figure 4B), demonstrating the importance of molecular simulation. Notably, we found that CHST11 may mediate LUAD EMT processes via its regulation of integrin signaling-related molecules (Figures 5 and S5). These molecules include PIK3CG, PIK3R5, ITCA6, ITGB1, GRB2, MRAS, ITGB7, RALB, RAC2 and ACTA2. Not only do these molecules have a very high correlation with CHST11 in LUAD-related cells, but they also have clinically significant poor prognoses (Figure S6). Internal signaling plays a role in the progression of LUAD [37-40]. Several studies have suggested that integrin signaling may contribute to pulmonary fibrosis by altering TGF-β expression [41]. In our research, CHST11 was also found to be related to TGF-β (R = 0.28; Figure 4B) and to be involved in EMT-related signaling (Figure 4A). Our results also indicate that CHST11 is stimulated by both TGF-β and INFγ in models of fibrosis and cancer (Figures 4E and S4C). A number of different CHST11-downstream effectors and responses to similar fibrosis stimulators were identified in this study (Figure S4), despite the presence of similar upstream regulators in fibrosis and cancer. In a fibrosis model, ERBB2 expression is stimulated by nicotin, PM, bleomycin and TGF-β, but it is negatively correlated with CHST11 expression in lung cancer. In both fibrosis and cancer, mmp9 has a consistent response to EMT (Figure S3D).
The integrin signaling pathway is also involved in regulating UFNG and MMP9 [42-44], which indicates that both the well-established CHST11-related molecules and the molecules discovered by this model are intimately connected with integrin signaling. Notably, ACTA2 is a mesenchymal marker for fibroblasts [45]. CHST11 is therefore involved in activating and transforming epithelial cells into myofibroblasts through integrin-mediated EMT during pulmonary fibrosis and is also involved in the progression of pulmonary fibrosis and LUAD. Chondroitin sulfate is a component of glycosaminoglycan, which can attach to extracellular matrix proteins [17]. Therefore, our molecular simulation model indicates that abnormal expression of CHST11 may result in abnormal sulfate modification of chondroitin sulfate, which promotes the interaction with extracellular matrix molecules, such as integrin, thereby triggering EMT-mediated pulmonary fibrosis or LUAD carcinogenesis. It was also discovered that ITGB-related drugs identified by drug repurposing significantly inhibited CHST11 expression in cancer cells (Figure 6). These results indicate that targeting the ITGB/CHST11 axis may offer an alternative treatment strategy for lung cancer. However, the precise mechanism still needs to be confirmed by further experiments. Based on current findings, this study highlights CHST11 for the first time as a potential prognostic factor and a potential molecular mechanism contributing to the progression of pulmonary fibrosis and LUAD. Treatment strategies for the treatment of pulmonary diseases may be improved by targeting CHST11.
ACKNOWLEDGEMENT
For the Ingenuity Pathway Analysis and Gene Expression Omnibus datasets analyses, we would like to express our sincere appreciation to Academia Sinica and Genomics Research Center Instrument Core Facilities.
CONFLICT OF INTEREST
The authors have declared no competing interests.
AUTHORS’ CONTRIBUTIONS
Conception and design: Chien-Hsiu Li, and Michael Hsiao
Development of methodology: Chien-Hsiu Li, Ming-Hsien Chan.
Acquisition of data (provided animals, acquired patient information, provided facilities, etc.): Chien-Hsiu Li, Ming-Hsien Chan, Yu-Chan Chang.
Analysis and interpretation of data (e.g. statistical analysis, biostatistics, computational analysis): Chien-Hsiu Li, Yu-Chan Chang, and Michael Hsiao.
Drafting of the manuscript: Chien-Hsiu Li, Ming-Hsien Chan, Yu-Chan Chang, and Michael Hsiao.
Critical revision of the manuscript for important intellectual content: Chien-Hsiu Li, Ming-Hsien Chan, Yu-Chan Chang, and Michael Hsiao.
Fund acquisition and study supervision: Michael Hsiao.
All authors read and approved the final manuscript.
AVAILABILITY OF DATA AND MATERIALS
The manuscript and its supplementary information files provide detailed information.




