Linoleic-acid-substituted polyethylenimine to silence heat shock protein 90B1 (HSP90B1) to inhibit migration of breast cancer cells
Funding information: Discovery Grant from Natural Sciences and Engineering Council of Canada (NSERC); Innovation Grant from the Canadian Breast Cancer Foundation (CBCF)
Abstract
Introduction
Breast cancer continues to be one of the leading causes of death in women, and the lack of treatment options for distant metastasis warrants the need to identify and develop more effective approaches. The aim of this study was to identify and validate targets that are associated with the survival and migration of the breast cancer cells in vitro through RNA interference (RNAi) approach.
Methods
Linoleic-acid-modified polyethylenimine (PEI) polymer was used to screen a short interfering RNA (siRNA) library against numerous cell adhesion and cytoskeleton genes in MDA-MB-231 triple-negative breast cell line, and the functional outcome of silencing was determined by growth and migration inhibition with further target validation studies.
Results
Heat shock protein 90B1 (HSP90B1) was identified as a crucial gene that is known to be involved in various breast cancer machineries, including uncontrolled proliferation and brain metastasis. The success of this approach was also due to the use of hyaluronic acid (HA) additive in lipopolymer complexes that showed a profound impact in reducing the cell viability (~50%), migration (~40%), and mRNA transcript levels (~80%) with a physiologically relevant siRNA concentration of 60 nM. The use of Dicer-substrate siRNA proved to be beneficial in target silencing, and a combinational treatment of integrin-β1 (ITGB1) and HSP90B1 was effective in reducing the migration of the MDA-MB-231 and MDA-MB-436 breast cancer cells.
Conclusion
This study demonstrates the potential to identify and silence targets using a lipid-modified PEI/siRNA system and highlights the importance of HSP90B1 in the growth and migration of breast cancer cells.
1 INTRODUCTION
Breast cancer is one of the four most commonly diagnosed cancer types along with lung, colorectal, and prostate cancers, according to the most recent Canadian Cancer Statistics 2020. It accounts for 25% of new cancer cases and 13% of cancer deaths among Canadian women.1 The metastatic ability of breast cancer to bone, lung, liver, brain, and lymph nodes makes its treatment challenging, even though its diagnosis and therapy has improved significantly in recent years, extending patients’ survival.2 Specifically, the treatment of the more aggressive and highly metastatic, triple-negative breast cancer (estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 negative) remains a formidable task, necessitating the identification of novel targets for a better outcome. In addition, triple-negative breast cancer (TNBC), accounting for 10–15% of all breast cancer cases, exhibits high heterogeneity, which also acts as stumbling blocks in its successful treatment.3
The process of breast metastasis is a complex, multistep cascade that depends on the ability of cancer cells to abandon the primary site and navigate through the circulation while evading the immune system, to reach a distant site. The cells navigate and survive through various distant organs and the bone environment and subsequent re-emergence after establishment.4 The treatment of metastatic breast cancer is challenging, as evident by the poor 5-year survival rate of 22% (according to Canadian Cancer Society). In this intricate process, numerous genes are activated, contributing to each step of metastasis, especially cell adhesion molecules.5 Some of these cell adhesion molecules include cadherins (CDH), which are vital in the interaction and signaling between cells. In particular, in breast cancer the impaired expression of E-cadherins was associated with enhanced invasiveness and decreased survival of patients with breast cancer.6 In addition, protocadherins (PCDH), which are the largest subfamily of cadherins, are shown to play diverse role in breast cancer metastasis. For example, overexpression of PCDH-7 was observed in breast cancer bone metastasis, whereas PCDH-17 was identified as a tumor suppressor gene through the reduction of β-catenin levels, which also highlights the crucial role of catenins in breast cancer invasiveness through their association with cadherins.7-9 Integrins are another major type of cell adhesion molecule involved in breast cancer metastasis. Numerous integrins have been identified to be upregulated during the metastatic process by the activation of various intracellular signaling pathways. For example, the expression of integrins α5β1, α6β4, and αvβ3 displayed strong correlation with the metastatic progression of breast cancer.10 The expression of various cytoskeleton components such as actin, dynein, tubulin, and laminin has additionally been shown to contribute in the complex metastatic process and even provide resistance to chemotherapy.11 Finally, other intrinsic intracellular targets such as the heat shock proteins (HSP) that govern proper protein folding, apoptosis regulators such as the BCL2 family and survivin, and cell-cycle-regulatory proteins such as cell-division cycle protein 20 (CDC20) facilitate the survival, proliferation, and anti-apoptotic property of breast cancer cells. Hence, the successful downregulation of such metastasis-associated genes, either alone or in combination, will have strong beneficial outcome in addressing breast cancer progression and metastasis.
One of the most promising approaches to interfere with the functioning of oncogenes is the use of short interfering ribonucleic acids (siRNA) to silence their in situ expression. Since the discovery of siRNA two decades ago, it is now possible to safely silence the gene of interest without affecting other targets, which is also dependent on its sequence specificity as well as the type of delivery system used.12, 13 For nucleic acid delivery, nonviral systems have been considered a safe alternative to viral methods,14 although they suffer from poor delivery efficiency. Especially with polyethylenimine (PEI) systems, low molecular weight PEIs (< 5 kDa) have been shown to be less toxic but also exhibit low delivery efficiency of siRNA; hence, lipid modifications have been employed to increase their capacity.15 We have successfully designed such delivery systems in our research group and demonstrated their therapeutic usefulness in a wide range of cancer types16-23
Here, we used a linoleic-acid-substituted PEI polymer to deliver siRNA in the metastatic breast cancer cell line MDA-MB-231. We screened a siRNA library targeting various cell surface and cytoskeleton genes to identify new targets that could inhibit the growth and migration of breast cancer cells. Out of the 496 genes silenced from the siRNA library using our delivery system, we observed that knockdown of HSP90B1 was able to inhibit cell growth effectively, and a decrease in migration was observed with the silencing of ITGA4, ITGA6, and ITGAX genes. We further investigated the potential of the identified targets for which Dicer-substrate siRNAs were used with HA as additives in the complex preparation process. Different weight ratios (siRNA to PEI-LA to HA) were explored to obtain the optimal condition for effective silencing, and a weight ratio of 1:5:0.15 was the most successful in this study with HSP90B1 reduction. Given the critical role of HSP90B1 in metastasis to brain, which has also been reported by different research groups, this approach could be a valuable alternative treatment as we were able to show a significant reduction in the cell growth as well as the migration of breast cancer cells. We achieved strong silencing of HSP90B1 with a low concentration and single dose of siRNA, which was adequate to exhibit significant functional outcomes. Given the important role of integrin-β1 (ITGB1) in the migration of breast cancer cells from our previous studies, the combinational silencing of integrin-β1 (ITGB1) along with HSP90B1 could be more advantageous to effectively reduce migration as well as cell growth.24 Hence, we present a promising alternative to address breast cancer metastasis with a simpler and more feasible approach.
2 MATERIALS AND METHODS
2.1 Materials
Polyethyleneimine (PEI, 1.2 kDa), purchased from Polysciences (Warrington, Pennsylvania), was used for linoleic acid (LA) substitution through N-acylation, exhibiting 6 LA per PEI, as assessed by 1H-NMR spectroscopy. Thiazolyl blue tetrazolium bromide (MTT), anhydrous dimethyl sulfoxide (DMSO), bovine serum albumin (BSA), chloroform, and hyaluronic acid (HA) 300 kDa were purchased from Sigma-Aldrich (St. Louis, MO). Dulbecco’s Modified Eagle Medium (DMEM), Hank’s Balanced Salt Solution (HBSS), trypsin/EDTA, penicillin, streptomycin, UltraPure DNase/RNase-free dH2O, Coomassie brilliant blue, and TRIzol were procured from Fisher Scientific (Ottawa, Canada).
2.2 Cell models
Human metastatic breast cancer cell lines, MDA-MB-231-WT and MDA-MB-231-GFP cells, were obtained from Dr. Judith Hugh (Faculty of Medicine and Dentistry, University of Alberta, Edmonton). Additional TNBC cell lines MDA-MB-436 and SUM-149PT were a gift from Dr. Afsaneh Lavasanifar (Faculty of Pharmacy and Pharmaceutical Sciences, University of Alberta). Cells were cultured in DMEM supplemented with 10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin until they covered 80% of the culture flask. Subsequently, cells were washed with HBSS and incubated with 0.05% trypsin/EDTA for 2 min at 37°C. Cells were collected by centrifugation at 600 rpm for 5 min and allowed to culture for 24 h before its utilization for studies.
2.3 Polymer/siRNA complex preparation
Serum-free DMEM was used to prepare complexes at siRNA-to-1.2PEI-LA6 polymer weight ratio of 1:5 for siRNA library studies and incubated for 30 min at room temperature. For the preparation of additive complexes using HA, siRNA was mixed with HA in serum-free DMEM, which was followed by the addition of polymer and incubated as mentioned above. These complexes were prepared at various siRNA-to-polymer-to-HA weight ratios, using siRNA concentrations of 30 nM and 60 nM. In combination studies, different siRNAs were mixed, prior to HA and polymer addition.
2.4 Human cell adhesion and cytoskeleton siRNA library screening
Screening of the siRNA library targeting 496 genes of cell adhesion and cytoskeleton (MISSION siRNA Human Cell Adhesion and Cytoskeleton Panel; SI05100, Sigma-Aldrich) was performed in MDA-MB-231-GFP cells. Briefly, the cells were dispensed into 96-well plates using a Perkin Elmer Automated Workstation and allowed to grow for 24 h. Then, 18 μL of the siRNA for a final concentration of 50 nM was added to a new 96-well plate followed by 18 μL of 1.2PEI-LA6 polymer solution at a weight ratio of 1:5. Each treatment group had three replicates, with each well containing 10 μL of the prepared complex solution. After 3 days of treatment at 37°C, the suspended/dead cells in the medium were removed, MTT solution was added to the attached cells, and cell viability was calculated relative to no-treatment group. Each plate contained a negative control siRNA from the library, a Dicer-substrate control siRNA (DsiRNA), and a positive control BCL2L12 siRNA. A similar experimental setup was carried out to study the migration of the cells following the siRNA library treatment.25, 26 After 2 days of treatment, the monolayer of cells was scratched using a 200 μL pipette tip, and the cells were washed with complete DMEM to remove the detached cells in the medium. Further, the cells were allowed to migrate for 24 h after which they were stained with Coomassie brilliant blue, fixed, and scanned. The migration (wound closure percentage) of the cells was determined on the basis of the open wound area and was compared with the no-treatment well. For this study, CDC20 siRNA and ITGB1 siRNA were included as additional positive controls, and doxorubicin (5 μg/mL) was included in both the siRNA library screening studies owing to its ability to inhibit cell growth and migration.
2.5 Validation of targets
The identified targets, ITGA4, ITGA6, ITGAX, and HSP90B1, from the siRNA library were further validated for cell growth and migration inhibition, for which Dicer-substrate siRNA sequences were procured from IDT. The following combination of siRNA:polymer:HA weight ratios at 30 nM and 60 nM siRNA was used in this study along with the negative control siRNA (CsiRNA), and CDC20 siRNA, BCL2L12 siRNA, and ITGB1 siRNA as positive controls.
| Polymer | ||||
|---|---|---|---|---|
| siRNA | 5 | 7.5 | 10 | HA |
| 1 | 1:5:0 | 1:7.5:0 | 1:10:0 | 0 |
| 1:5:0.15 | 1:7.5:0.15 | 1:10:0.15 | 0.15 | |
| 1:5:0.3 | 1:7.5:0.3 | 1:10:0.3 | 0.3 | |
| 1:5:1 | 1:7.5:1 | 1:10:1 | 1 | |
| 1:5:3 | 1:7.5:3 | 1:10:3 | 3 | |
2.6 Quantification of mRNA levels
The silencing of the target genes at the mRNA level was evaluated by reverse-transcription quantitative polymerase chain reaction (RT-qPCR). Following 3 days of treatment with HA additive complexes at ratios 1:5:0, 1:5:0.15, and 1:5:0.3 in six-well plates, MDA-MB-231-WT cells were collected, and the total RNA was extracted using TRIzol. SensiFast cDNA synthesis kit (Bioline Meridian Bioscience) was utilized to convert RNA to cDNA according to the manufacturer’s protocol. Then, PCR was performed on StepOnePlus Real-Time PCR system with the below listed genes and their respective primers:
| Gene | Forward | Reverse |
|---|---|---|
| B-Actin | 5′-GCGAGAAGATGACCCAGAT-3′ | 5′-CCAGTGGTACGGCCAGA-3′ |
| HSP90B1 | 5′-CAGTTTGGTGTCGGTTTCTATTC-3′ | 5′-TGTGCTGGGTATCGTTGTT-3′ |
| ITGB1 | 5′-CCGCGCGGAAAAGATGAAT-3′ | 5′-TGAGCAAACACACAGCAAACT-3′ |
| CDC20 | 5′-CGCTATATCCCCCATCGCAG-3′ | 5′-GATGTTCCTTCTTGGTGGGC-3′ |
| BCL2L12 | 5′-CCCGCCCCTATGCCCTTTTT-3′ | 5′-ATAACCGGCCCAGCGTAGAA-3′ |
The experiment was performed in triplicate with a total of 10 μL reaction mixture volume, composed of 5 μL SYBR Green master mix, 2 μL of 10 μM primers, and 3 μL of 5 ng/μL cDNA template. MicroAmp Fast Optical 96-well plates were used for this assay, and the setup was heated to 95°C for 10 min followed by 40 cycles of denaturation at 95°C for 15 s and an annealing/elongation at 65°C for 30 s. Following the completion of the cycles, the ∆CT, ∆∆CT, and relative quantity (RQ) of mRNA were calculated with B-actin as the reference gene and relative to the no treatment (NT) group. The expression of ITGB1, CDC20, and BCL2L12 was also calculated following HSP90B1 silencing.
2.7 Combination therapy
Combinational siRNA therapy using HSP90B1 siRNA and ITGB1 siRNA was performed in MDA-MB-231 and MDA-MB-436 cells at four siRNA concentrations (total concentration), (10 + 10) 20 nM, (20 + 20) 40 nM, (30 + 30) 60 nM, and (40 + 40) 80 nM. Each of these targets was treated in combination with CsiRNA as control. The effect of silencing was evaluated as inhibition of wound closure as described above.
2.8 Statistical analysis
All results were summarized as mean ± standard deviation, and unpaired Student’s t-test was employed to determine the statistical difference between the group mean. Significance (p < 0.05) was determined in comparison with the respective control siRNA treatment groups.
3 RESULTS
3.1 siRNA library screening to identify targets for breast cancer treatment
An siRNA library targeting various cell adhesion and cytoskeleton genes (496 siRNAs) was screened in the TNBC cell line MDA-MB-231 to identify targets that could inhibit cell growth. 1.2PEI-LA6 polymer was used to deliver the siRNAs at a concentration of 50 nM, and cell growth was assessed by MTT assay after 3 days. An siRNA against BCL2L12, which was previously reported to exhibit significant cell growth inhibition, was included as a positive control.27, 28 BCL2L12 in this screen showed 28% of growth inhibition, which was considered as cutoff to shortlist successful hits from the 496 targets. We observed two targets that exhibited ≥28% cell death: cadherin-9 (CDH9) at 34% and heat shock protein 90B1 (HSP90B1) at 29% (Figure 1A).
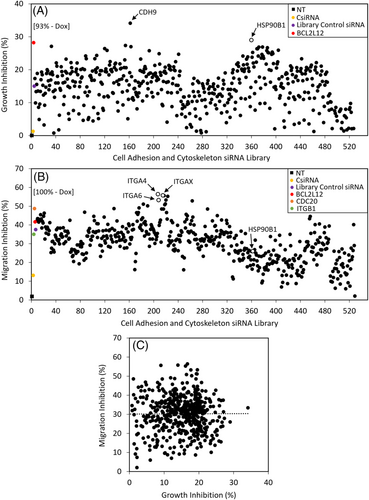
A migration assay was also performed using the same library to identify targets that can inhibit cell migration into a “wound” area. In this study, we included two positive control siRNAs, namely ITGB1- and CDC20-specific siRNAs, that were previously shown to decrease migration in breast cancer cells.24, 29 Three integrin genes appeared to inhibit the migration effectively, integrin-a4 (ITGA4), integrin-a6 (ITGA6), and integrin-aX (ITGAX), with migration inhibition percentages of ~56% (Figure 1B). HSP90B1, which showed 30% inhibition in cell growth, also showed ~30% inhibition in migration studies (Figure S1). We also explored any correlation between cell growth inhibition and migration inhibition in this assay format, but we did not observe a positive correlation among the siRNAs in this library (Figure 1C).
3.2 Validation of targets for inhibition of cell growth
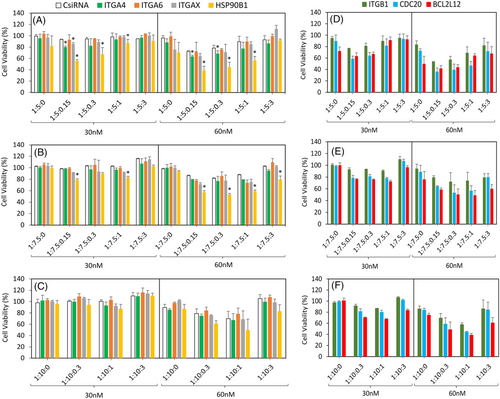
The identified targets ITGA4, ITGA6, ITGAX, and HSP90B1 were further validated by the delivery of specific siRNAs at different ratios and siRNA concentrations of 30 nM and 60 nM. The previously used positive controls ITGB1, CDC20, and BCL2L12 siRNAs were also included, and the inhibition in cell growth was assessed by MTT assay after 3 days of treatment (Figure 2D–F). Silencing of HSP90B1 significantly and consistently reduced cell growth; formulations at siRNA:polymer ratio 1:5 with three additive ratios gave 55%, 67%, and at 87% cell viability at 30 nM siRNA concentration (Figure 2A). The other identified targets for migration inhibition had less effect on cell growth, as expected (ITGA4 20% and ITGAX 17% cell growth inhibition). At 60 nM, the outcome was more pronounced at similar ratios than observed with 30 nM, although the control scrambled siRNA (CsiRNA) also showed some nonspecific activity (toxicity) (Figure 2A). Altogether, we recorded 40%, 45%, and 56% cell growth with the three additive ratios given (Figure 2A). With respect to nanoparticle formulations at 1:7.5 siRNA:polymer ratio, the overall effect was less in comparison with 1:5 ratio; however, HSP90B1 silencing still resulted in a significant drop in cell viability (Figure 2B), whereas with 1:10, no significant growth inhibition was documented (Figure 2C).
3.3 Validation of targets for inhibition of migration
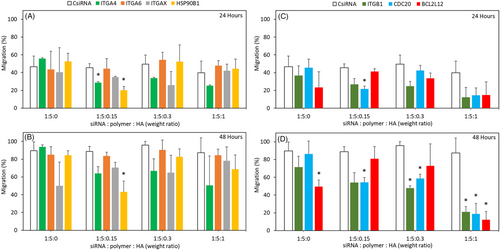
Inhibition of migration was investigated using nanoparticle formulations with 1:5 siRNA:polymer ratio and 60 nM siRNA concentration, as this formulation more effectively inhibited growth. After 24 h, the control siRNA treatment led to 45% of migration, while ITGA4 and HSP90B1 siRNAs showed significant inhibition, with migration values of 28% and 20%, respectively, at 1:5:0.15 formulation. Other targets, ITGA6 and ITGAX, did not manifest any notable reduction irrespective of the ratios employed (Figure 3A). The previously observed reduction at 24 h with HSP90B1 silencing at 1:5:0.15 was prolonged until the 48 h timepoint (Figure S2). Overall, HSP90B1 showed a significant reduction in migration (43%) compared with CsiRNA (88%) after 48 h (Figure 3B). Similar to the cell viability studies, low amount of HA (siRNA:HA ratio of 1:0.15) appeared to be the optimal formulation for reducing the migration (Figure S2). Using higher amounts of HA abolished the inhibitory effects of the specific siRNA on migration. With regard to the positive controls, CDC20 siRNA was significantly effective with 1:5:0.15 weight ratio at 24 h (Figure 3C). At 48 h, the 1:5:1 weight ratio was the most effective for all the positive controls, ITGB1, CDC20, and BCL2L12 siRNAs (Figure 3D).
3.4 Validation at the mRNA transcript level by RT-qPCR
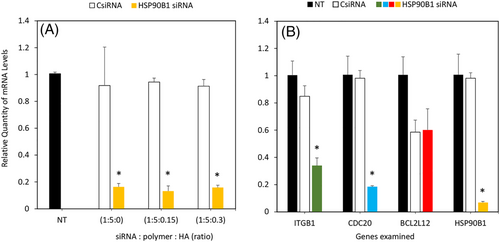
We further validated the extent of silencing by evaluating the specific mRNA reduction using RT-qPCR after 3 days of treatment. A strong decrease in HSP90B1 mRNA levels, ranging between 80% and 85%, was recorded with ratios 1:5:0, 1:5:0.15, and 1:5:0.3 compared with the CsiRNA treatment group, which was consistent with our previous observation with cell viability and wound closure (Figure 4A). We also analyzed the mRNA levels of ITGB1, CDC20, and BCL2L12 after treating with HSP90B1 siRNA. A significant decrease between 60% and 80% was observed in ITGB1 and CDC20 mRNA levels, respectively, which indicates a possible linkage with HSP90B1 (Figure 4B). CsiRNA treatment did not affect the mRNA levels of ITGB1 and CDC20, but BCL2L12 levels were significantly decreased, which was equivalent to HSP90B1 siRNA treatment levels, so the latter changes were attributed to nonspecific effects of the nanoparticles.
3.5 Combinational treatment
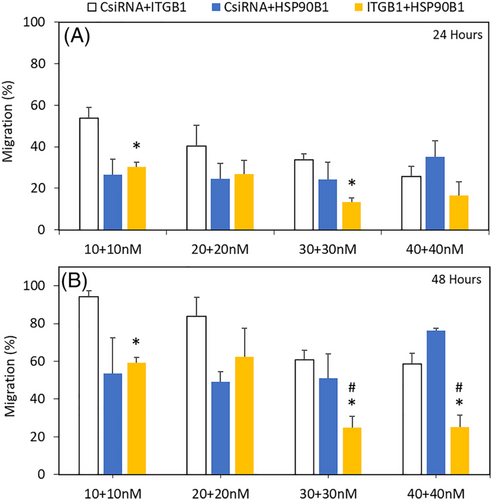
We had shown the importance of ITGB1 in the migration of breast cancer in a previous report.24 Since it is also partially downregulated due to HSP90B1 silencing, we wanted to combine HSP90B1 silencing with ITGB1 silencing to explore the benefit of using both siRNAs to inhibit migration. We employed equal amounts of ITGB1 and HSP90B1 siRNAs in the treatments, along with the CsiRNA as reference. At 24 h, 10 + 10 nM of CsiRNA + HSP90B1 siRNA combination showed equivalent effect to that of ITGB1 + HSP90B1 siRNA combination; however, the latter showed significant difference compared with CsiRNA + ITGB1. Similarly, with 20 + 20 nM siRNA combination, both treatments showed equivalent levels of inhibition. At 30 + 30 nM, only ITGB1 + HSP90B1 showed significant decrease, and at 40 + 40 nM siRNA combinations, a slight difference was observed, but this was not significant (Figures 5 and 6). After 48 h, 10 + 10 nM and 20 + 20 nM CsiRNA + HSP90B1 siRNA combinations as well as ITGB1 + HSP90B1 siRNA combinations showed similar levels of migration; however, only 10 + 10 nM of ITGB1 + HSP90B1 exhibited significant difference. At the higher 30 + 30 nM and 40 + 40 nM siRNA combinations of ITGB1 + HSP90B1, the inhibition of migration was more significant when compared with the CsiRNA combinations with ITGB1 as well as HSP90B1 siRNAs (Figure 5 and 6).
3.6 Validation studies in additional breast cancer cells
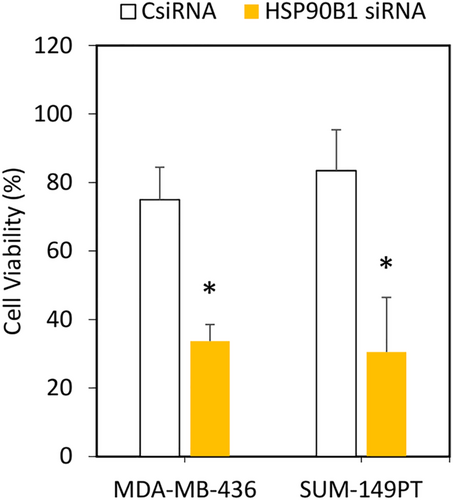
Following successful functional evaluation in the highly metastatic MDA-MB-231 cells, we further validated the findings of cell viability in two additional TNBC cell lines, MDA-MB-436 and SUM-149PT cells. In both the cell lines, compared with CsiRNA treatment, silencing of HSP90B1 with 1:5:0.15 siRNA complexes resulted in significant loss of cell viability (~30% versus ~80%) after 3 days of treatment, further supporting the validity of HSP90B1 as an essential target (Figure 7).
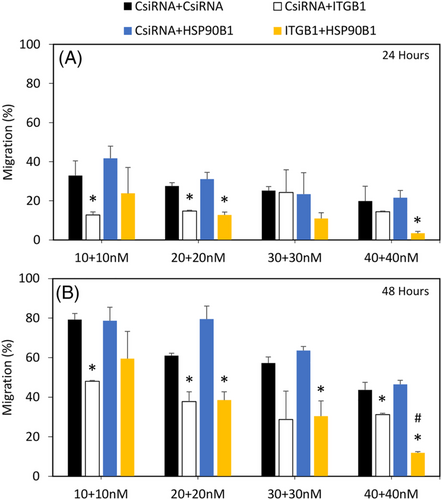
Furthermore, the siRNA combinational studies were also assessed in MDA-MB-436 cells. At 24 h with 10 + 10 nM siRNA concentration, only CsiRNA + ITGB1 showed significant inhibition compared with CsiRNA + CsiRNA, whereas at 20 + 20 nM, CsiRNA + ITGB1 and ITGB1 + HSP90B1 treatment exhibited significant difference. At 40 + 40 nM of siRNA combination, only ITGB1 + HSP90B1 indicated a much stronger inhibition of migration (Figure 8A). After 48 h, a similar trend in inhibition was observed with CsiRNA + ITGB1 at 10 + 10 nM as well as at 20 + 20 nM and ITGB1 + HSP90B1 at 20 + 20 nM alone. At a higher 30 + 30 nM, only ITGB1 + HSP90B1 displayed significant decrease. With a further increase in siRNA concentration at 40 + 40 nM, both CsiRNA+ITGB1 and ITGB1 + HSP90B1 showed a decrease; however, the difference was much higher with ITGB1 + HSP90B1 combinational treatment (Figure 8B).
4 DISCUSSION
The use of PEI for gene therapy has gained considerable momentum in the past few years, with several agents currently tested in various stages of clinical trials and in different cancer types.30, 31 Since high molecular weight PEI has been shown to be toxic to cells, its low molecular weight counterparts have been modified to deliver nucleic acid more efficiently at low toxicity. Low molecular weight PEI such as 1.2 kDa PEI exhibits poor cellular internalization of its nucleic acid cargo and thereby is not functional for siRNA delivery; however, functionalization with small lipids improves its overall efficacy to deliver nucleic acid cargo.15 Linoleic acid (LA) modification, for example, improves the binding, cellular uptake, and intracellular release of intact nucleic acid.16-18 Previously, we showed that high cytoplasmic localization of 1.2PEI-LA/siRNA complexes helped to accomplish a strong knockdown of ITGB1 with specific siRNAs.24 A similar effect was observed with HSP90B1 silencing using the same delivery agent in this study. The use of HA as additives was previously shown to further improve cellular uptake of the complexes, which was partly due to high levels of CD44 (i.e., HA binding ligand) on the MDA-MB-231 cells, as well as the persistent intracellular availability of siRNA, thereby increasing the overall silencing.19, 32 Since we used the same polymeric delivery system, we could extrapolate that the complexes carrying HSP90B1 siRNA similarly underwent enhanced delivery, improving the silencing efficiency of the siRNA target. The use of HA can also reduce the ζ potential of the complexes, facilitating lower disruption of the cell membrane while improving the uptake and intracellular release of the siRNA. Optimizing the right formulation of the siRNA, in terms of the ratio of specific reagents used to make up the nanoparticle, is also crucial as higher ratios can reduce the ζ potential, drastically impairing the delivery.32 In our hands, siRNA:polymer ratio of 5, along with a trace amount of HA (siRNA:HA ratio of 1:0.15), performed the best in all the assays. This was similar to previous studies where the use of HA was successful in silencing cell cycle protein CDC20, survivin, and various phosphatases such as PPP1R7, PTPN1, PTPN22, LHPP, and PPP1R12A, showing the wide applicability of this HA additive formulation.19, 32
From the unbiased library screen in this study, we shortlisted two genes, CDH9 and HSP90B1, that showed cell death higher than the previous reference target, the CDC20 when targeted with specific siRNAs. A recent report on the role of CDH9 in breast cancer suggested that its silencing resulted in enhanced lung metastasis and was identified as a crucial gene for tumor metastasis; hence, its knockdown could be detrimental.33 On the other hand, HSP90B1 was reported by several studies to be an important gene that is upregulated in various cancers, including breast cancers, and its overexpression had a strong correlation with poor survival of patients with breast cancer.34 It was interesting that such contrasting targets turned out to be positive hits in our library screens. Needless to say, we chose to pursue further studies with HSP90B1 given its better-established role in breast cancer. HSPs function as molecular chaperons and promote various fundamental cellular programs in normal as well as transformed cells. HSPs facilitate proper folding of the proteins, counteract stress-induced protein misfolding, and have been classified on the basis of their molecular weight into six main families.35 The HSP90 family of proteins consists of five isoforms: two cytoplasmic HSP90α and HSP90β, an endoplasmic-reticulum-localized glucose-regulated protein 94 (HSP90B1), a mitochondrial tumor necrosis factor receptor-associated protein 1 (TRAP1), and a membrane-associated HSP90N.36 HSP90B1 has shown to be overexpressed in various cancers, modulating several cellular functions such as cell proliferation and metastasis, regulation of various apoptotic molecules, and aiding or inhibiting cell death machinery, making it a highly critical protein.37 HSP90B1 overexpression has also been documented in chronic lymphocytic leukemia (CLL) by next-generation sequencing in patient cells and identified as a potential therapeutic target, especially to address CLL drug resistance.38 Studies have also shown a correlation between high HSP90B1 expression and poor overall survival (OS) as well as disease-free survival (DFS) in patients with non-small cell lung cancer (NSCLC).39 In osteosarcoma, the downregulation of HSP90B1 resulted in tumor growth inhibition in vitro and in vivo through the PI3K/Akt/mTOR pathways.40 Similarly, inhibition of HSP90 in myeloma, B-cell lymphoma, and hepatocellular carcinoma by PU-H71, a specific HSP90 inhibitor, was shown to be beneficial to reduce cell growth and induce apoptosis.41-43 In breast cancer, proteomic analysis of patient samples for relative and absolute quantitation (iTRAQ) method revealed a strong correlation between high expression of HSP90B1 to distant metastasis and low overall survival when compared with samples with low expression who had higher survival.44 Similar observations were made by other groups where patients with poor survival had high mRNA and protein expression of HSP90B1.45 In brain metastasis of breast cancer, HSP90B1 overexpression helped the cancer cells survive under hypoglycemic condition when compared with bone and lung metastatic cells. The HSP90B1 overexpression observed in the brain metastatic breast cancer cell line was accompanied by upregulation of anti-apoptotic genes such as B-cell lymphoma 2 (BCL-2), cellular inhibitor of apoptosis protein (HIAP1), and X-linked inhibitor of apoptosis protein (XIAP), facilitating the survival of metastasized cells. In addition, activation of pro-survival autophagy due to HSP90B1 upregulation was also observed in the brain metastatic cells, and the ablation of HSP90B1 reduced the viability of brain metastatic cells in vitro as well as brain metastasis in vivo, increasing the survival of the animals compared with the control groups.46, 47 The overexpression of HSP90B1 among breast cancer stem cells in CD44hi/CD24lo phenotype and in oxidative-stress-resistant breast cancer cells contributing toward enhanced proliferation and migration further strengthens the importance of HSP90B1 in highly aggressive breast cancer cells as a potential therapeutic target.48, 49 Although various drugs have been tested in vivo and in clinical trials to neutralize HSP90B1 in several other cancer types, none has been introduced for breast cancer treatment owing to toxicity- and specificity-related issues.50-53
On the basis of our strong outcome obtained by silencing a single integrin target (ITGB1) in our previous report,24 we hypothesized that identifying new integrins could be beneficial to achieving superior inhibition of migration in breast cancers. Hence, from the scratch assay with the library screen, we selected targets that were highly effective as well as a member of the integrin gene family (ITGA4, ITGA6, and ITGAX). ITGA4 was shown to be hypermethylated in breast cancer tissue samples and acts as an important marker for early diagnosis.54 It is also the α-subunit in Very Late Antigen-4 (VLA-4/integrin-α4β1) receptor formed with ITGB1 and binds to fibronectin in the extracellular matrix or to vascular cell adhesion protein-1 (VCAM-1)55 important for migration. Similarly, ITGA6 was found to be overexpressed in breast cancer and contributes toward the resistance to radiation therapy, partially through the activation of PI3K/Akt or MEK/Erk signaling pathways.56 This α-subunit may associate with ITGB1 or ITGB4 forming VLA-6 (α6β1 or α6β4) and act as a laminin receptor.57, 58 Overexpression of ITGAX was associated with aggressive prostate cancer, but we identified only one report where a moderate expression in invasive breast cancer tissue sample was reported.59, 60 The siRNA library also contained siRNA against various other integrin-α (ITGA) genes, including ITGA1, ITGA2, ITGA2B, ITGA3, ITGA5, ITGA7, ITGA8, ITGA9, ITGA10, ITGA11, ITGAD, ITGAE, ITGAL, ITGAM, ITGAV, and ITFG2 (integrin-a FG-GAP repeat containing 2), whose silencing did not manifest any effect on migration or cell growth inhibition in this study. Similarly, silencing of other heat shock proteins (HSP) and glycoproteins (GP) such as HSP90AA2, HSP90AB1, HSPA1A, HSPA2, HSPD1, GP9, and GPA33 did not alter the growth of breast cancer cells. Since each gene was targeted by a pool of three siRNAs in the library format, we can negate the possibility that these genes were not silenced; thereby, it can be concluded that these genes are of low importance for the migration of MDA-MB-231 cells. We did not observe any correlation between cell growth and migration inhibition, which shows the heterogenic functionality of these genes that were included in the study.
On the other hand, since the role of HSP90B1 in regulation of cell death and tumor invasion/metastasis has been well studied,36, 37, 61, 62 various chemical inhibitors of HSP90 have been developed and are under exploration for breast cancer treatment, including ganetespib, 17-allylamino-17-demethoxygeldanamycin (17-AAG), PU-H71, AT-533, BJ-B11, DCZ3112, and TAS-116.41, 50-53, 61-63 Though considerable progress has been achieved with these drugs in different stages of clinical trials, some displayed significant side effects and some failed owing to nonspecific effects in patients, so there is still an urgent need to develop alternate approaches for targeting HSP90B1 in breast cancer.
Following the identification and literature validation of these four targets (ITGA4, ITGA6, ITGAX, and HSP90B1), we used Dicer-substrate siRNAs, which are longer than conventional siRNA (27 base pairs versus 19–21 base pairs, respectively), as the former can undergo cleavage by the Dicer enzyme in the RNA-induced silencing complex (RISC) and has been shown previously to improve the silencing efficiency in some cases.24, 64 Silencing HSP90B1 at the mRNA level was possible even without the HA additive (1:5:0), and its effect on cell growth (30% inhibition) was also observed using 60 nM of siRNA, although the difference from CsiRNA treatment was not significant. The 30% growth inhibition after HSP90B1 silencing was comparable to the effect observed in the initial library screen, which did not utilize the HA additive to employ simpler nanoparticle formulations. The addition of HA at the 1:5:0.15 ratio also caused strong mRNA reduction, which was reflected in cell viability as well as in migration. With the 1:5:0.3 ratio, the decrease in mRNA was accompanied by changes in cell growth, but cell migration was unaffected. Although all the three ratios (1:5:0, 1:5:0.15, 1;5:0.3) displayed excellent mRNA reduction, only 1:5:0.15 (0.15 HA) exhibited the desired comprehensive outcomes. It is possible that a more effective siRNA release was achieved from the complex with this formulation. It is also possible that excess HA in the nanoparticle formulation might have attenuated the effect of HSP90B1 silencing by a yet unknown mechanism. There are many factors that can influence the efficacy of the delivery system, such as its size, ζ potential, cellular uptake pathway, intracellular localization of the nucleic acid, and the type of lipid substitution.16, 24, 32, 65 In addition, the specificity of siRNA, expression level, and turnover rate of the target, cell type, and proliferation rate of the cell line used can also influence the overall results.66-68 In our study, the use of HA could have reduced the ζ potential of the complexes, influencing its interaction with the cell membrane and its cytoplasmic localization, which in turn could have facilitated better silencing of HSP90B1. Another important factor might be the high proliferation rate of MDA-MB-231 cells and the high expression levels of CD44, a primary receptor for HA that could have facilitated better cellular uptake.
Previously, RNAi was used to silence HSP90B1, reducing the migration (at 6 and 24 h) as well as the proliferation of MDA-MB-231 and oxidative-stress-resistant MCF-7 cells, which showed similar levels of high HSP90B1 expression. This outcome was achieved by the use of 100 nM siRNA delivered with Dharmafect-1 reagent, a lipid-based delivery agent. In the same study, HSP90B1 expression was shown to be high in tumors compared with normal tissues, and furthermore, it was higher in recurrent tumors from patients who underwent chemotherapy compared with the initial breast tumors. Another study probed the role of HSP90B1 in tumor invasiveness69 under low-glucose conditions, which is a key characteristic of tumor environment. Using the Dharmafect-1 transfection reagent, reduction of HSP90B1 levels lowered the invasive potential of MDA-MB-231 cells by 67%, and expression of HSP90B1 was elevated in low-glucose conditions in MCF-7 cells. Moreover, the sensitivity toward doxorubicin was increased following HSP90B1 silencing with an increase in caspase-3 activity.69 We also used the metastatic breast cancer MDA-MB-231 cells and were able to achieve strong reduction at mRNA levels followed by inhibition in proliferation (cell viability) and migration at 24 and 48 h, but by employing a lower concentration of siRNA (60 nM). This is advantageous to avoid any undesirable effects of the siRNA treatment.
HSP90B1 is known to regulate the functioning of a wide range of ‘client’ proteins, including Toll-like receptors (TLRs) and integrins. Hence, it was not surprising that silencing HSP90B1 reduced the mRNA levels of ITGB1 in this study. In HSP90B1-knockout mice, to study its role in B-cell functioning, the expression of various integrins, including ITGA4, ITGAL, ITGB7, and ITGB2, was downregulated, but not that of ITGB1.70 The inhibition of the αI domain (AID), which is a ligand-binding domain common among all α-integrin subunits, using TAT-α7 helix peptide reduced the maturation and expression levels of various integrins, including ITGAL, ITGAM, and ITGA4, but not ITGB1. That study showed the crucial role of HSP90B1 in the proper maturation and function of integrins.71 Our study noted a relationship between HSP90B1 expression and ITGB1; however, further studies are warranted to confirm these findings. Similarly, the mRNA levels of CDC20, a cell division cycle protein 20 homolog, was downregulated following the knockdown of HSP90B1, which could be probed further and help us to better understand the intracellular consequences of HSP90B1 in breast cancer treatment. Furthermore, the combinational silencing of ITGB1 and HSP90B1 in two TNBC cell lines MDA-MB-231 and MDA-MB436 proved to be beneficial in reducing the migration at higher siRNA concentrations. Interestingly, combinational treatment of control siRNA with ITGB1 siRNA showed significant difference at certain siRNA concentrations only in MDA-MB-436 but not with MDA-MB-231, which highlights the need for target evaluation in different cell lines for cancer treatment. Additionally, these findings can be further evaluated in vivo.
In conclusion, this study has shown that linoleic-acid-substituted PEI and an HA additive siRNA complex was a viable method to effectively deliver Dicer-substrate siRNA and silence HSP90B1 expression. This reduced the proliferation and migration of metastatic breast cancer as observed from different triple-negative breast cancer cell lines (in vitro). Given the wide spectrum of HSP90B1 function in breast cancer invasiveness, aggressiveness, brain metastasis, and resistance to drug treatment, our method is a promising alternative to chemical inhibitors for effective silencing of this important target. Further studies on the relation between HSP90B1 and various other integrins as well as cell cycle proteins could help us to better understand the role of HSP90B1 downregulation in cellular outcomes. In addition, the effect of HSP90B1 silencing on the sensitivity of various anticancer drugs in breast cancer treatment could be crucial information with high clinical translation potential. Finally, the high transfection efficiency of the linoleic-acid-substituted PEI used in this study, along with previous reports on the successful targeting of various oncogenes using the same polymer, provides a unique opportunity to commercialize such lipopolymers as transfection reagents that can be used for various research and development purposes requiring nucleic acid delivery.
ACKNOWLEDGEMENTS
This study was supported by an Innovation Grant from the Canadian Breast Cancer Foundation (CBCF) and a Discovery Grant from Natural Sciences and Engineering Council of Canada (NSERC). We acknowledge the technical help provided by Dr. Bindu Thapa and Mr. Cezary Kucharski from the Uludag Lab and Dr. Joaquin Lopez Orozco from the HCA Core Facility, University of Alberta while performing the siRNA library screening studies. Equipment support was provided by Edmonton Civic Employees Charitable Assistance fund.
DECLARATION OF COMPETING INTEREST
H.U. and R.B.K.C. are founders of a start-up company (RJH Biosciences) commercializing the polymers for nucleic acid delivery. D.N.M.S. declares no conflict of interest.




