Treatment outcomes and costs of a simplified antiretroviral treatment strategy for hepatitis C among Hepatitis C Virus and Human Immuno deficiency Virus co-infected patients in Ukraine
EQUIP is a consortium of local African Non-Governmental Organizations, which was funded by USAID to assist countries through innovations to meet the UNAIDS 90, 90 90 targets.
Declaration of conflict of interest: Kara W Chew has received a research grant to the institution from Merck Sharpe & Dohme. The other authors who have taken part in this study do not have anything to disclose regarding funding or conflict of interest with respect to this manuscript.
Author contribution: Ian Sanne, Sydney Rosen, Clint Cavenaugh, Kara W Chew, Charles van der Horst, Tetiana Barnard, Charles S Chasela and Svitlana Antonyak conceived the study; Svitlana Antonyak, Yulia Stopolianska, Tetiana Barnard, Svitlana Antonyak, Maria Liulchuk, Sofiane Mohamed, and Matthiue Barralon conducted the study; Constance Wose Kinge and Charles S Chasela analyzed the data; Charles S Chasela, Sydney Rosen, Kara Chew, Sergie Antoniak, Fadzai Marange, and Constance Wose Kinge drafted the manuscript. All authors reviewed and approved the final manuscript.
Financial support: This work was supported by the USAID EQUIP Grant No. AID-OAA-A-1500070 and the Luxemburg Business Partnership Facility 2017, Grant No. MAE/014 – 171 620.
Abstract
Background and Aim
To demonstrate the use of a standard dose of ledipasvir (LDV) and sofosbuvir (SOF), with or without ribavirin, to treat hepatitis C and hepatitis C/HIV co-infection in Ukraine.
Methods
Eligible HCV viraemic adults from two clinics in Kyiv were treated with LDV/SOF with or without weight-based ribavirin for 12 weeks. Clinical assessments were performed at screening and at week 24, and as needed; treatment was dispensed every 4 weeks. The primary outcome was sustained virologic response (SVR) 12 weeks after treatment, with analysis by intention to treat. Cost per patient was estimated in USD (2018) over the 24-week period.
Results
Of 868 patients included in the study and initiated on therapy, 482 (55.5%) were co-infected with HIV. The common genotypes were 1 (74.1%) and 3 (22%). Overall, SVR was achieved in 831 of the 868 patients (95.7%). SVR in patients with hepatitis C alone and hepatitis C/HIV co-infection was 98.4% and 93.6%, respectively. Adverse events were infrequent and usually mild. Using generic medication, cost per patient was estimated at US$680.
Conclusion
A standard dose of LDV and SOF, with ribavirin as per protocol, resulted in good outcomes for patients with both hepatitis C alone and co-infected with hepatitis C/HIV. Program costs in Ukraine were modest using generic medication.
Introduction
Globally, an estimated 71 million people are living with chronic hepatitis C virus (HCV) infection and are at risk for significant morbidity, including liver cirrhosis, liver failure, hepatocellular carcinoma, and death.1-3 Even in low-HCV prevalence settings, HCV infection remains common among people who inject drugs (PWID) and HIV-infected men who have sex with men (MSM).1, 4 Despite the development of highly effective direct-acting antiviral (DAA) treatment, which can cure HCV infection with 8–12 weeks of therapy, HCV remains a leading cause of mortality worldwide, causing more than 350 000 deaths each year.5, 6 Of these, 85 000 are in Eastern Europe,7 including Ukraine. The WHO's Global Health Sector Strategy has set goals of a 90% reduction in new HCV infections and a 65% reduction in HCV-related mortality by 2030.8, 9 Progress in HCV treatment scale-up has been encouraging, with more than 3 million treated globally with DAAs since 2015. Testing and diagnosis rates are still less than 10% in low and middle income countries (LMICs), however.10
In Ukraine, roughly 3–5% of the population—as many as 2 million people—are estimated to be HCV-infected.11 Those most at risk are PWID, people living with HIV (PLHIV), sex workers (SW), MSM, prisoners, members of the military, populations in conflict zones, and sexual partners of PLHIV. A lack of adequate facilities and free-to-client services, combined with fear of prosecution for drug use and stigma suffered by key populations, particularly PWID and MSM, means that most individuals who present for treatment for HCV and HIV are already well advanced in their illness.12
Low-cost, scalable, integrated HIV and HCV testing and treatment strategies offer a chance to achieve WHO targets for HIV and HCV elimination in regions with both epidemics. We describe the treatment outcomes and estimated costs for an integrated HIV and HCV treatment program that offered HCV treatment with 12 weeks of ledipasvir/sofosbuvir (LDV/SOF) with or without weight-based ribavirin (WBR) in Ukraine.
Patients and methods
Study sites and population
Enrolment into the HCV/HIV demonstration project was conducted in 2018 at two clinical facilities in Kyiv, Ukraine: the Clinic of the L.V. Gromashevsky Institute of Epidemiology and Infectious Diseases and Treatment, and Kyiv City Clinical Hospital No. 5. Patients were recruited to the study from HCV treatment waiting lists available at the sites, were referred by partner organizations, or had a positive HCV antibody or RNA test at one of the sites during the enrolment period. Target populations for study enrolment were PWID, MSM, SW, and sexual partners of HCV-infected individuals, though these characteristics were not required for enrolment.
Study eligibility
Eligible participants included in the treatment study were HCV viraemic, HCV treatment naïve or experienced (prior pegylated interferon [PegIFN] and ribavirin [RBV] only), and 18 years or older, with HCV genotype 1, 2, 3, 4, 5, or 6, and with or without HIV-1 co-infection. Patients with compensated cirrhosis (Child–Pugh Class A) and hepatitis B virus (HBV) infection were eligible; those with decompensated cirrhosis (Child–Pugh Class B or C) or prior treatment with HCV DAAs were not, and were referred to other treatment centers for care. All participants provided written informed consent.
Intervention
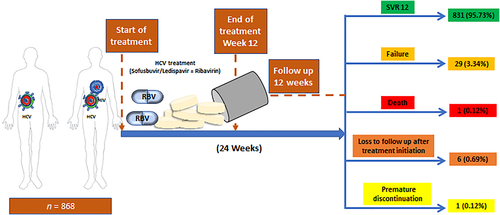
The intervention combined HCV and HIV testing, HCV treatment, simplified HCV treatment monitoring, and HIV treatment initiation for those with HIV co-infection not yet on antiretroviral therapy (ART). HCV treatment was with fixed-dose combination LDV/SOF 400 mg/90 mg ± weight-based ribavirin (1000 mg for patients <75 kg and 1200 mg for those ≥75 kg administered orally in two divided doses) for 12 weeks and provided by the project. Ribavirin was included in the regimen for treatment-experienced cirrhotic genotype 1 and 4 participants and all participants with genotype 3 HCV. HIV treatment was per national guidelines, within the National HIV treatment program.13 ART is recommended to all HIV-infected patients regardless of their CD4 count. The recommended first-line ART regimes in Ukraine has been NNRTU +2 NRTI, PI/rtv +2NRTI, EFV +TDF/FTC or EFV +TDF + 3TC, LPV/rtv +TDF/FTC. Alternative regimes are NNRTI: NVP +2NRTI PI/rtv: ATV/rtv + 2NRTI and NRTI backbone +AZT/3TC or +ABC/RTC.14 Participants with HBV co-infection and hepatitis B surface antigen positivity were concurrently treated with tenofovir. Participants were followed for 24 weeks (through 12 weeks after treatment completion), including assessments of HCV and HIV treatment outcomes and safety. The intervention steps are illustrated in Figure 1. A 4-week supply of study medications was dispensed at weeks 0, 4, and 8, and psychosocial and peer support were provided at entry and at 4, 8, 12, and 24 weeks. Participants not already on medication-assisted treatment (MAT) were referred for harm reduction, including MAT programs.
Clinical and laboratory evaluations
Clinical evaluations and drug dispensing
A complete clinical assessment including medical history, physical exam, and review of concomitant medications was performed at screening. Subsequently, clinical assessments were driven by reported symptoms and required only at entry and week 24. Liver disease stage (presence or absence of cirrhosis) was defined by any of the following and by the study physician's judgment: cirrhosis on liver ultrasound; liver histology (liver biopsy); aspartate aminotransferase (AST)-to-platelet ratio index (APRI) >1.0 and FIB-4 >3.25; or liver stiffness by transient elastography (Fibroscan) ≥12.5 kPa. Child–Pugh scores were calculated for all cirrhotic patients, with compensated cirrhosis defined as Child–Pugh score ≤6 (Class A).
Laboratory evaluations
Pre-treatment laboratory tests included HCV antibody if not already available, HCV RNA for HCV antibody-positive participants, hemoglobin, platelets, AST, alanine aminotransferase (ALT), total bilirubin, albumin, creatinine, blood urea nitrogen, pregnancy testing, prothrombin time/international normalized ratio (INR), HBV serologies (hepatitis B core Ab [HbcAb], hepatitis B surface antigen [HbsAg], hepatitis B surface antibody [HBsAb]), and CD4+ T-cell count for HIV-infected participants. Laboratory results were also obtained from the medical record if available within the specified window.
The protocol called for all participants who initiated HCV treatment and remained in care to have the following laboratory tests at week 24: HCV RNA, hemoglobin, platelets, AST, ALT, bilirubin, albumin, creatinine, blood urea nitrogen, prothrombin time/INR, and HIV RNA. Participants receiving ribavirin had hemoglobin monitoring at weeks 2, 4, and 8 and pregnancy testing (for females of childbearing potential) at weeks 4, 8, 12, and 24. Participants with eGFR <60 mL/1.732 at screening and also receiving tenofovir disoproxil fumarate (TDF) had creatinine monitoring at weeks 4 and 12 and subsequently as medically indicated. All other participants had creatine monitoring at screening, post-treatment evaluation, and premature discontinuation. Participants with isolated hepatitis B core Ab (HBcAb+/HBsAg−/HBsAb−) additionally had hepatic function tests (AST, ALT, total bilirubin) measured at weeks 4, 8, and 12 to monitor HBV reactivation. Quantitative HCV viral load test (real-time RT-PCR reagent kit D-0794, Vector Best) and HCV genotyping (hybridization-fluorescence, AmpliSense HCV-genotype-FL reagent kit, Central Epidemiology Research Institute, Russian Federation) were performed at the Synevo central laboratory in Kyiv for all participants.
Outcomes
The primary outcome of the analysis was sustained virology response (SVR) at week 24 stratified by HCV status and HCV/HIV co-infection. We assigned each patient to one of four virologic status outcomes at 24 weeks after treatment initiation: (i) treatment success, defined as SVR at 12 weeks after the end of treatment (week 24); (ii) treatment failure (HCV viremia greater than the lower limit of detection 12 weeks after therapy completion); (iii) loss to follow-up (did not return to clinic for 24-week evaluation); or (iv) death (died within the 24-week study period). Other outcomes included safety (adverse events) during the treatment period.
Data analysis
Outcomes
Baseline sociodemographic, clinical, and laboratory characteristics of the participants were calculated using proportions or means (SDs) or medians (interquartile ranges) for continuous data. The proportion with SVR was calculated by the infection status (HCV mono or HCV/HIV co-infection), by intention to treat (ITT). Differences between those successfully treated and achieved SVR and those who failed treatment were assessed by t-test or chi-squared test as appropriate, as well as by multivariable logistic regression. A P-level of less than 0.2 or priori (known effect) in the bivariate analysis was used to select variables for multivariable analysis. A final model was determined using the stepwise backward elimination method, and only P-level of less than 0.05 was considered for the final model. Multicollinearity test and Hosmer–Lemeshow test (HL test) for logistic regression were undertaken on the final model. Data were analyzed using STATA version 15 SE (StataCorp 2017. Stata Statistical Software: Release 15, StataCorp LLC, College Station, TX, USA).
Costs
Costs were estimated from the provider perspective from initial screening date until assessment of cure 12 weeks after HCV treatment completion (24 weeks total) using standard micro-costing methods described previously.15, 16 Resource costs are reported in four categories: (i) indirect costs (support staff/personnel, building costs, equipment, and office supplies, including education and outreach costs); (ii) event costs (physician visits, counseling visits, and Fibroscans); (iii) laboratory test costs (HCV viral load modeled using the centralized laboratory pricing at the Synevo laboratory, HCV genotyping, HIV and HBV testing, pregnancy testing if applicable, PT/INR, blood counts, chemistries, and imaging); and (iv) medication costs (HCV, HBV, and HIV). Costs incurred solely for research purposes (e.g. obtaining informed consent, data entry, etc.) were not included.
We determined variable patient resource utilization from a de-identified dataset compiled from study participant case reporting forms and estimated resource utilization from average clinic site capacity and total annual visits. We then multiplied the quantity of each resource used by each patient by the associated unit cost to determine the total cost per patient. We evaluated the average resource utilization per patient by cost category and the four HCV treatment outcomes defined for the cost analysis and estimated 95% confidence intervals by the outcome category. Cost estimates utilized data from a subset of all patients enrolled in the study; the subset included sequentially enrolled patients during the first 6 months of the enrollment period.
Study data were managed with HepatiC (ABL SA, Luxembourg). The patient resource utilization matrix for this study was generated using SAS software, Version 9.3 (SAS Institute Inc., Cary, NC, USA). Estimates per patient treated and per successful outcome were made using the HE2RO Healthcare Costs and Outcome Model (http://www.heroza.org/researchtools/the-healthcare-cost-and-outcomes-model-hcom) (HE2RO, Johannesburg, South Africa). Economic models were generated with Excel 2011.
Ethical considerations
The study was reviewed by the Ukrainian Institute on Public Health Policy IRB #1, Institutional Review Board (00007612) and the University of the Witwatersrand Human Research Ethics Committee (M17078). The Boston University Institutional Review Board approved analysis of a de-identified analytic dataset (H-37820). All participants provided written informed consent. The study is registered at ClinicalTrials.gov (NCT04038320).
Results
Enrolment and patient characteristics
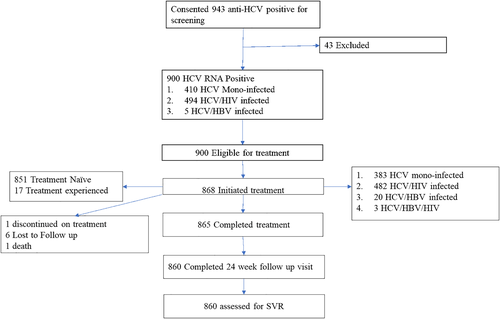
We obtained consent from 943 anti-HCV-positive participants and enrolled those eligible at the Gromashevsky and Kyiv Hospital between 26 March and 2 November 2018. As shown in Figure 1, 43 participants were excluded after obtaining consent, leaving 900 eligible for treatment. Of these, 868 were initiated on treatment, and 860 were assessed for SVR at 12 weeks after treatment. Of those initiated on treatment, 44.1% (383) were HCV mono-infected, 55.5% (482) HCV/HIV infected, and 0.3% (3) HCV/HBV infected. Most HCV mono-infected participants were recruited at the Kyiv City Hospital, while most HCV/HIV co-infected participants were from Gromashevsky. Out of the 868 who started treatment, 87% (755) were PWID, of whom 60.7% (434) were on MAT; most PWID were seen at Gromashevsky. Most participants, 91.8% (797), were not cirrhotic (Table 1). The mean APRI Score was 0.5 (0.3–0.8).
| HCV† | HCV/HIV‡ | Total | ||||
|---|---|---|---|---|---|---|
| Characteristics | n = 386 | % | n = 482 | % | n = 868 | % |
| Age, median (IQR) | 38.5 | 34–45 | 40 | 36–43 | 39 | 35–44 |
| Sex | ||||||
| Female | 138 | 35.8 | 157 | 32.6 | 295 | 34 |
| Male | 248 | 64.2 | 325 | 67.4 | 573 | 66 |
| Education (%) | ||||||
| Primary and below | 124 | 32.1 | 171 | 35.5 | 295 | 34 |
| Beyond primary | 262 | 67.9 | 311 | 64.5 | 573 | 66 |
| Risk groups (%) | ||||||
| PWID | 310 | 88.8 | 405 | 85.3 | 715 | 86.8 |
| No PWID | 39 | 11.2 | 70 | 14.7 | 109 | 13.2 |
| Marital status (%) | ||||||
| Never married | 322 | 83.4 | 377 | 78.2 | 699 | 80.5 |
| Ever married | 64 | 16.6 | 105 | 21.8 | 169 | 19.5 |
| Median BMI (kg/m2) (IQR) | 24.4 | 22.1–27.1 | 23.4 | 21.5–26.1 | 23.9 | 21.6–26.5 |
| Cirrhosis (%) | ||||||
| Compensated cirrhosis | 31 | 8 | 40 | 8.3 | 71 | 8.2 |
| No cirrhosis | 355 | 92 | 442 | 91.7 | 797 | 91.8 |
| Genotype (subtype) (%) | ||||||
| 1 | 285 | 73.8 | 358 | 74.3 | 643 | 74.1 |
| 2 | 6 | 1.6 | 14 | 2.9 | 20 | 2.3 |
| 3 | 88 | 22.8 | 103 | 21.4 | 191 | 22 |
| 4 | 2 | 0.5 | 0 | 0 | 2 | 0.2 |
| Mixed | 1 | 0.3 | 1 | 0.2 | 2 | 0.2 |
| Indeterminant | 4 | 1 | 6 | 1.2 | 10 | 1.2 |
| HCV RNA, log10IU/mL (median, IQR) | 5.9 | 5.5–6.6 | 6.4 | 5.9–7.3 | 6.1 | 5.7–7.1 |
| CD4 T-cell count | 0 | — | 506.5 | 351–704 | 506.5 | 351–704 |
| Hemoglobin, g/dL (median, IQR) | 14.7 | 13.5–15.7 | 14.7 | 13.4–15.7 | 14.7 | 13.5–15.7 |
| Creatinine, mg/dL (median, IQR) | 69 | 59.9–79 | 71 | 61–81 | 70 | 61–80 |
| ALT, U/L (median, IQR) | 53 | 36.1–88.0 | 52.7 | 36–88 | 53 | 36–88 |
| Bilirubin, mg/dL (median, IQR) | 9.8 | 7.1–13.6 | 9.36 | 6.2–14 | 9.7 | 6.6–13.7 |
| Albumin, g/dL (median, IQR) | 48.4 | 46–50.7 | 47.5 | 45–50 | 47.9 | 45.5–50.3 |
| INR (median, IQR) | 1.0 | 0.95–1.03 | 1.0 | 0.94–1.03 | 1.0 | 0.95–1.03 |
| Platelets (×109/L) (median, IQR) | 221.5 | 187–259 | 209.5 | 174–251 | 215 | 179–255 |
| APRI score (median, IQR) | 0.5 | 0.3–0.8 | 0.5 | 0.4–0.9 | 0.5 | 0.3–0.8 |
| Fibroscan (%) | ||||||
| F0–F1 | 162 | 50.3 | 93 | 24.5 | 255 | 36.4 |
| F1–F2 | 109 | 33.9 | 219 | 57.8 | 328 | 46.8 |
| F2 | 12 | 3.7 | 30 | 7.9 | 42 | 6.0 |
| F3 | 37 | 11.5 | 33 | 8.7 | 70 | 10.0 |
| F4 | 2 | 0.6 | 4 | 1.1 | 6 | 0.9 |
- † HCV includes three co-infections with HBV.
- ‡ HCV/HIV includes three co-infections with HBV.
- BMI, body mass index; HBV, hepatitis B virus; HCV, hepatitis C virus; INR, international normalized ratio; IQR, interquartile range; MAT, medication-assisted treatment; MSM, men who have sex with men; partner+, sexual partner of HCV-infected person; PLHIV, people living with human immunodeficiency virus (HIV) infection; PWID, people who inject drugs; SW, sex worker.
The most common HCV genotypes were 1 (74.1%) and 3 (22.0%) (Table 1). The median (interquartile range, IQR) HCV RNA was 6.1 (5.7–7.1) log10IU/m. Among those HIV-infected (n = 482), the median CD4 count was 507 cells/mm3 (351–704), and the majority had HIV viral suppression (<200 copies per milliliter of blood) at entry. Twenty had detectable viral load with a median (IQR) of 72 (52.5–267) copies per milliliter of blood; and five were unsuppressed (>200 copies per milliliter of blood). At 24 weeks, 12 weeks after the completion of HCV treatment, only two had unsuppressed viral loads.
Treatment outcomes
Outcomes of treatment for either HCV mono or HCV/HIV co-infection are reported in Table 2. Of the 868 patients who initiated HCV treatment, 214 were treated with ribavirin. There were 80 adverse events reported, with 29, 20, 19, and 12 events at weeks 4, 8, 12, and 24 weeks, respectively. Of the 80 adverse events, 76.5% were mild and the rest moderate; 92.6% were either anemia or hyperbilirubinemia, 3 were due to skin rash and itching, and 1 had lobar pneumonia. The majority, 86.3% (69), were deemed study-drug-related; 89.9% (62/69) ribavirin-related events were managed by reduction of the dose, and all SOF/LDV events were self-resolved.
| Outcome (n, %) | HCV† | HCV/HIV‡ | Total |
|---|---|---|---|
| Treatment success (SVR) | 380 (98.4%) | 451 (93.6%) | 831 (95.73%) |
| Treatment failure (retained in care) | 3 (0.8%) | 26 (6.7%) | 29 (3.34) |
| Loss to follow-up after treatment initiation | 3 (0.8%) | 3 (0%) | 6 (0.69) |
| Premature discontinuation | 0 | 1 (0%) | 1 (0.12) |
| Death | 0 (0%) | 1 (0.2) | 1 (0.12) |
| Total | 386 (100%) | 482 (100%) | 868 (100%) |
- † HCV includes three co-infections with HBV.
- ‡ HCV/HIV includes three co-infections with HBV.
- SVR, sustained virologic response.
Following treatment initiation, six participants were lost to follow-up (LTFU), one discontinued treatment prematurely, and one died due to myocardial infarction between weeks 12 and 24. Eight-hundred and sixty were assessed for SVR at 24 weeks. By ITT analysis, which defined all non-successful outcomes as failures, 37 participants failed therapy, giving an overall treatment success rate of 831/868 (95.7%) (Fig. 2). The SVR rates for participants with genotypes 1 and 3 HCV were 95.5% and 96.3%, respectively (Table 3). SVR rates differed significantly by site and by HIV co-infection status. Most treatment failures happened among the HCV/HIV co-infected patients. All loss to follow-up and the treatment discontinuation were from the Gromashevsky clinic; treatment failures were from Kyiv City Hospital (n = 29). Of the 755 PWID assessed at 24 weeks, 95.2% achieved SVR.
| Characteristic | Treatment failure n = 37 | Treatment success (n = 831) | ITT n = 868 | OR (95% CI) for treatment failure | P-value | AOR (95% CI) for treatment failure | P-value |
|---|---|---|---|---|---|---|---|
| Clinics | |||||||
| Gromashevsky | 7 (18.9) | 427 (51.4) | 434 (50.0) | Ref. | |||
| Kyiv City Clinic | 30 (81.1) | 404 (48.6) | 434 (50.0) | 4.5 (2.0–10.4) | <0.001 | 7.0 (3.0–16.5) | 0.000 |
| Age (median, IQR) | 38 (34–41) | 39 (36–44) | 39 (35–44) | 1.0 (0.9–1.0) | 0.087 | ||
| Sex (%) | |||||||
| Female | 8 (21.6) | 287 (34.5) | 295 (34) | 0.5 (0.2–1.2) | 0.092 | ||
| Male | 29 (76.4) | 544 (65.5) | 573 (66) | Ref. | |||
| Education | |||||||
| Primary and below | 13 (34.1) | 282 (34.0) | 295 (34) | Ref | |||
| Beyond primary | 24 (64.9) | 549 (66.1) | 573 (66) | 0.9 (0.5–1.9) | 0.880 | ||
| PWID (%) | |||||||
| PWID | 34 (94.4) | 681 (86.4) | 715 (86.8) | 2.6 (0.6–11.3) | 0.123 | ||
| Non-PWID | 2 (5.6) | 107 (13.6) | 109 (13.2) | Ref. | |||
| PWID (OST) (%) | |||||||
| PWID (OST) (%) | 30 (100) | 404 (95.1) | 434 (95.4) | Ref. | |||
| PWID (no OST) (%) | 0 (0) | 21 (5) | 21 (4.6) | 1 (empty) | — | ||
| Marital status (%) | |||||||
| Never married | 24 (64.9) | 675 (81.2) | 699 (80.5) | Ref | |||
| Ever married | 13 (35.1) | 156 (18.8) | 169 (19.5) | 2.3 (1.2–4.7) | 0.022 | 2.2 (1.1–4.6) | 0.034 |
| Median BMI (kg/m2) (IQR) | 24.3 (21.4–26.0) | 23.9 (21.7–26.5) | 23.9 (21.6–26.5) | 1.0 (0.9–1.1) | 0.624 | ||
| Cirrhosis (%) | |||||||
| Compensated | 3 (8.2) | 68 (8.2) | 71 (8.2) | Ref. | |||
| No cirrhosis | 34 (91.9) | 763 (91.8) | 797 (91.8) | 1.0 (0.3–3.4) | 0.987 | ||
| Infection status (%) | |||||||
| HCV (mono)† | 6 (16.2) | 380 (45.7) | 386 (44.5) | Ref. | |||
| HCV/HIV‡ | 31 (83.8) | 451 (54.3) | 482 (55.5) | 4.4 (1.8–10.5) | 0.0002 | 7.0 (2.7–18.7) | 0.000 |
| Genotype (subtype) | |||||||
| 1 | 29 (78.4) | 614 (73.9) | 643 (74.1) | Ref. | |||
| 2 | 1 (2.7) | 19 (2.3) | 20 (2.3) | 1.1 (0.1–8.6) | 0.917 | ||
| 3 | 7 (18.9) | 184 (22.1) | 191 (22.0) | 0.8 (0.3–1.9) | 0.614 | ||
| 4 | 0 (0.0) | 2 (0.2) | 2 (0.2) | 1 (empty) | |||
| Mixed | 0 (0.0) | 2 (0.2) | 2 (0.2) | 1(empty) | |||
| Indeterminant | 0 (0.0) | 10 (1.2) | 10 (1.2) | 1 (empty) | |||
| Hemoglobin, g/dL (median, IQR) | 147 (131–156) | 147 (135–157) | 147 (134.5–157) | 1.0 (1.0–1.0) | 0.637 | ||
| Log10 viral load (median, IQR) | 5.9 (5.6–6.6) | 6.2 (5.7–7.1) | 6.1 (5.7–7.1) | 0.9 (0.7–1.0) | 0.081 | ||
| Creatinine, mg/dL (median, IQR) | 73 (67–82) | 70 (60–80) | 70 (61–80) | 1.0 (1.0–1.0) | 0.233 | ||
| ALT U/L (median, IQR) | 54 (35–98.2) | 53 (36–87) | 53 (36–88) | 1.0 (1.0–1.0) | 0.800 | ||
| Bilirubin, mg/dL (median, IQR) | 10.2 (6.9–17.5) | 9.6 (6.6–13.7) | 9.7 (6.6–13.7) | 1.0 (1.0–1.0) | 0.021 | ||
| Albumin, g/dL (median, IQR) | 48.2 (46.5–51.6) | 47.9 (45.4–50.3) | 47.9 (45.5–50.3) | 1.0 (1.0–1.1) | 0.914 | ||
| INR (median, IQR) | 1 (1.0–1.1) | 1.0 (1.0–1.03) | 1.0 (1.0–1.03) | 0.9 (0.4–2.3) | 0.883 | ||
| Platelets (×109/L) | 216 (185–254) | 215 (179–255) | 215 (179–255) | 1.0 (1.0–1.0) | 0.619 | ||
| APRI score (median, IQR) | 0.5 (0.4–0.8) | 0.5 (0.3–0.8) | 0.5 (0.3–0.8) | 1.0 (0.7–1.5) | 0.842 | ||
| CD4 cells/μL (median, IQR) | 490 (372–704) | 508 (344–704) | 506.5 (351–704) | 1.0 (1.0–1.0) | 0.862 | ||
- † HCV includes three co-infections with HBV.
- ‡ HCV/HIV includes three co-infections with HBV.
- ALT, alanine aminotransferase; APRI, AST-to-platelet ratio index; AST, aspartate aminotransferase; CI, confidence interval; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; INR, international normalized ratio; ITT, intention to treat; IQR, interquartile range; OST, opoid substitution treatment; PWID, people who inject drugs.
Predictors of SVR
Characteristics of participants are compared between the SVR group and the non-SVR group by ITT population in Table 3. There were significant differences in the odds of achieving a successful treatment response by treatment site, marriage status, and HIV co-infection status in both univariate and multivariate analysis.
Costs
Resource utilization for the first 522 participants enrolled at the Kyiv and Gromashevsky study sites and locally collected unit costs are presented in Table 4; a detailed breakdown by cost component and outcome is provided in Table 5. We note that the estimates in Table 5 assume generic pricing for LDV/SOF of $1.06/tablet; brand pricing for this medication is estimated to cost $10.61/tablet. The results shown in Table 5 are a reasonable proxy for costs under “real world” conditions, with a cost per patient of $680.
| Resource | Mean utilized per patient | Unit cost (2018 USD) |
|---|---|---|
| Resource quantities utilized and unit costs | ||
| Physical exam | 2.0 | $4.04 |
| Counseling visit | 6.0 | $3.91 |
| Pregnancy test | 0.6 | $1.00 |
| Fibroscan | 1.0 | $45.23 |
| HCV RNA | 1.8 | $33.71 |
| HCV genotype | 1.0 | $25.31 |
| Liver tests (ALT, AST, albumin, bilirubin) | 4.1 | $5.80 |
| CBC (Hb, Plt) | 3.7 | $5.30 |
| Creatinine | 4.4 | $1.40 |
| Urea | 1.5 | $1.40 |
| INR/PT | 2.0 | $2.70 |
| HIV screening antibody (OraQuick) | 1.0 | $1.01 |
| CD4 count | 0.86 | $10.00 |
| HIV RNA test | 1.0 | $17.00 |
| HBV testing (Surface Ab, Core Ab, Surface Ag) | 1.0 | $1.86 |
| Medication costs | ||
| HIV medication (mean per HIV+ participant per 24-week study period) | $298.96 | |
| HCV medication using generic pricing (mean per 24-week study period) | $101.69 | |
| HCV medication as procured in the study (mean per 24-week study period) | $912.29 | |
- Ab, antibody; Ag, antigen; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CBC, complete blood count; Hb, hemoglobin; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; INR, international normalized ratio; Plt, platelet count; PT, prothrombin time; RNA, ribonucleic acid.
| Outcome | N (%) | Indirect costs | Events | Labs | Drugs | Mean (95% confidence interval) cost per outcome |
|---|---|---|---|---|---|---|
| Success | 511 (98) | 174 | 77 | 171 | 257 | 679 (653–705) |
| Failure | 7 (1) | 174 | 65 | 182 | 206 | 627 (516–737) |
| Loss | 3 (1) | 141 | 77 | 145 | 243 | 606 (437–775) |
| Death | 1 (0) | 154 | 77 | 135 | 1188† | 1554 (N/A) |
| All outcomes | 522 | 173 | 77 | 171 | 258 | 680 (653–706) |
- † The patient who died was an HIV-infected individual with nevirapine (6 USD per pill) as a component of the antiretroviral regimen, incurring very high drug cost.
The study protocol included some resources that may not be regarded as essential for the intervention, such as a Fibroscan, and many patients received more laboratory tests than were called for in the protocol. The cost of these excess resources is included in the estimate in Table 5. If Fibroscan is removed and the number of liver tests, creatinine tests, and INR/PT are each cut to half, the average cost per patient would be $616.
Discussion
In a demonstration project of an integrated, simplified protocol for treating HCV and HIV among key populations in Ukraine, of whom 87% (755) were PWID and 55.5% (482) were HCV/HIV co-infected, a high SVR rate of 95.7% was achieved among all who initiated treatment. Treatment success varied slightly by infection status, with 98.4% of those HCV mono-infected and 93.6% of those with HCV/HIV co-infection achieving SVR; both these rates are high. This is unusual success in not only a high-risk population but also for the efficacy of SOF/LDV ± ribavirin, particularly for genotype 3 infection. A previous U.S. trial without ribavirin reported 89% SVR among genotype 3 patients without cirrhosis,17 while a European study using ribavirin combination treatment reported an overall SVR of 94%,18 similar to our findings.
We observed a small but statistically significant difference in SVR rates between HCV mono-infected and HIV co-infected participants, with lower SVR rates in the latter (Table 3). Several other studies did not find a significant difference in SVR rates between HCV mono-infected and HCV/HIV co-infected participants,19-22 while yet others have similarly found a lower SVR rate among HIV co-infected persons or SVR rates similar to ours in the absence of an HCV mono-infected comparator (with different DAA regimens evaluated).23, 24 There are a number of differences between our study and those that did not find a difference in SVR rates between the two populations. One major difference is the small sample size of the HCV/HIV co-infected groups in several of the earlier studies, limiting the precision of the reported SVR rate. For example, in the study by Piekarrska (Epi-Ter2 study), the number of mono-infected was 5533 versus 157 HCV/HIV co-infected.21 In the study by Naggie et al., only 65 co-infected and 62 mono-infected were compared.20 Additionally, the populations enrolled or included in many studies are expected to be very different from our study population: we focused enrolment of PWID, who were likely under-represented in the other studies, particularly clinical trials or real-world treatment in HIV clinics, where it has been repeatedly demonstrated that substance-use history is associated with lower odds of HCV treatment, including in the contemporary DAA era.24, 25 While the proportions of PWID in the HCV mono-infected and HCV/HIV co-infected groups in our study were similar, there may be unmeasured factors among PWID who are also HIV co-infected that negatively influence treatment outcomes. Similarly, genotype 3 infection is well recognized as more difficult to treat, and it may be that it remains harder to eliminate among HIV-infected persons with a regimen of SOF/LDV + ribavirin.
Although PWID status was not associated with treatment failure, 91.9% of those who failed were PWID. While adherence to HCV treatment was not measured, there was minimal loss to follow-up; this may have been due to the community-based treatment model of care, which incorporates a social worker into the clinical management team26, 27 and also provides harm-reduction services at a community level. Even among the PWID, who typically have a high rate of loss to follow-up,28 there were few losses, due perhaps to the community-based treatment and harm-reduction program in Ukraine.27 Other studies have reported high rates of success with HCV treatment co-located with substance use treatment services and among PWID receiving opoid substitution treatment (OST).29, 30
Treatment with LDV/SOF and ribavirin was generally well tolerated, with expected and manageable31 effects and not requiring treatment discontinuation.
In our study, the estimated average cost of treatment per patient initiated on HCV therapy, including the costs for both HCV and HIV treatment, was $680. The handful of patients who did not achieve SVR or did not return for the 24-week evaluation (LTFU) cost slightly less per patient, while the single patient who died cost substantially more, due largely to the expensive HIV drugs. The cost per patient treated reported here ($680/patient) is dependent on utilizing the lowest cost testing platform to confirm HCV viremia and treatment response (the status quo Synevo labs) and securing generic pricing for HCV medications. Other laboratory platforms may cause the cost per patient to go up or down depending on patient volume. Reducing the frequency of laboratory tests will also reduce the cost. If generic drugs cannot be obtained, the cost of treatment will be substantially higher. As HCV treatment moves toward the use of pan-genotypic drugs, further reductions in the laboratory cost component are possible with the elimination of genotype testing, though the cost of medication will likely be higher than generic LDV/SOF. For program budgeting purposes, under the assumption of generic drug pricing but higher laboratory costs, an average cost of $750/patient is likely a reasonable estimate for this intervention. This does not include costs for scaling up or maintaining the treatment program, such as procurement, training, management, and oversight.
Conclusion
In conclusion, an integrated HCV/HIV treatment program in Ukraine achieved excellent outcomes with LDV/SOF ± ribavirin HCV treatment in a patient population in which most individuals were co-infected with HIV and were PWID, and a substantial proportion had genotype 3 HCV infection. Loss to follow-up was very low and cure rates very high. Adverse events due to RBV were minimal. The cost per patient, while relatively high for Ukraine, was modest by international standards. Going forward, a partnership among community-based organizations, private laboratories, and government that can secure generic medications and provide point-of-care testing may be an affordable strategy for making HCV treatment universally available in Ukraine.
Acknowledgments
The authors would like to acknowledge the staff and patients of Gromashevsky Institute of Epidemiology and Infectious Diseases and Treatment and Kyiv City Clinical Hospital # 5 in Ukraine for participating in this study.




