Differences in the genetic backgrounds of patients with alcoholic liver disease and non-alcoholic fatty liver disease
Abstract
Background and Aim
Alcoholic liver disease (ALD) and non-alcoholic fatty liver disease (NAFLD) have common hepatic histological features, but few studies have compared the genomic backgrounds of these two diseases. Here, we compared the genetic differences between ALD and NAFLD.
Methods
This study enrolled 318 Japanese patients with ALD (n = 118; male, 86%; median age, 62 years; liver cirrhosis, 58%; hepatocellular carcinoma [HCC], 31%) and NAFLD (n = 200; male, 55%; age, 61 years; cirrhosis, 19%; HCC, 12%). The genotype frequencies of 10 single nucleotide polymorphisms (SNPs) were analyzed.
Results
The ADH1B genotype GG and ALDH2 genotype GG were observed more frequently, and the percentage of patients with the MTP genotype GG was lower in ALD compared with NAFLD patients (ADH1B, 16 vs 4%; ALDH2 84 vs 44%; MTP 62 vs 72%, respectively; all P < 0.01). Comparing noncirrhosis to cirrhosis, the frequency of the potassium voltage-gated channel subfamily Q member 1 (KCNQ1) genotype TT and adrenoceptor beta 3 (ADRB3) genotype TT was increased significantly in ALD-related cirrhosis. In contrast, the patatin-like phospholipase 3 (PNPLA3) genotype CC was decreased significantly in NAFLD-related cirrhosis. A comparison of patients with and without HCC demonstrated that the KCNQ1 genotype TT was increased significantly in both HCC groups. In addition, associations between the KCNJ15 genotype GG and ALD-HCC and the G allele of PNPLA3 and NAFLD-HCC were identified.
Conclusions
SNPs in genes related to ethanol and lipid metabolism clearly differed between patients with ALD and NAFLD. KCNQ1 might affect the progression and hepatocarcinogenesis in both ALD and NAFLD.
Introduction
Hepatic steatosis and steatohepatitis are becoming an epidemic health problem and are becoming common worldwide. One Japanese cohort study indicated that 29.7% of people had non-alcoholic fatty liver disease (NAFLD).1 The number of overweight individuals is estimated to increase to ~2.0 billion worldwide by 2030.2 Therefore, the prevalence of NAFLD may increase with the increasing levels of obesity. The incidence of hepatocellular carcinoma (HCC) caused by nonviral chronic liver diseases, including NAFLD and alcoholic liver disease (ALD), is also increasing in Japan. Although both ALD and NAFLD patients present with similar pathological findings, the clinical manifestation differs.
The primary enzymes involved in alcohol metabolism are alcohol dehydrogenase (ADH) and aldehyde dehydrogenase (ALDH).3, 4 The activity of these enzymes determines the body's ability to respond to alcohol intake and is associated with liver dysfunction and malignancies, including HCC and gastrointestinal cancer.3, 4 ADH1B activity differs among ethnicities; 5–10% of Whites and 25% of Blacks have elevated ADH1B activity, compared with >85–90% of the Japanese population.4-6 Therefore, it is important to analyze the genetic background of Japanese ALD patients. NAFLD is associated with metabolic syndrome and is considered a hepatic manifestation of metabolic syndrome.7, 8 Thus, genetic factors that play a role in metabolic disease-related processes, such as glucose metabolism, may contribute to disease onset and progression.9, 10
In genome-wide association studies, the rs738409 polymorphism (encoding I148M) in the patatin-like phospholipase 3 (PNPLA3) gene has been consistently recognized as a major genetic factor in the development of NAFLD.11 PNPLA3 is expressed in the liver and adipose tissue and has acyl hydrolase activity.12 The PNPLA3 G allele has been linked to an increased prevalence of NAFLD and is associated with the development and progression of both ALD and NAFLD.13-16 However, no reports have compared single nucleotide polymorphisms (SNPs) linked to disease occurrence and progression between patients with ALD and NAFLD. Here, we conducted a validation study of SNPs proposed to be involved in disease occurrence, progression, and hepatocarcinogenesis and compared their distribution in patients with ALD and NAFLD.
Methods
Patients and study design
This study was a cross-sectional, single-center study. Overall, 318 patients (118 ALD and 200 NAFLD) were enrolled. The diagnosis of NAFLD was based on the following criteria: (1) macrovesicular steatosis affecting at least 5% of the hepatocytes or causing steatosis on imaging; (2) an intake of <20 g of ethanol/day in women and <30 g/day in men; and (3) the appropriate exclusion of other liver diseases, including viral hepatitis, drug-induced liver injury, Wilson's disease, and α1-antitrypsin deficiency according to the American Association for the Study of Liver Disease guidelines.17 ALD was diagnosed according to the diagnostic criterion of the Japanese Society for Biomedical Research on Alcohol (JASBRA),18 which defines ALD as a habitual alcohol intake of 60 g/day for more than 5 years. Other liver diseases were excluded using biochemical tests and radiological imaging.
Detailed clinical and demographic information, including age, gender, body mass index (BMI), and complications due to lifestyle-related diseases, were collected. Laboratory data were evaluated at the time of liver biopsy or hospitalization for treatment. The homeostatic model assessment for insulin resistance (HOMA-IR) was calculated from glucose and insulin levels to quantify insulin resistance and β-cell function.
Liver biopsy was performed in 42 ALD and 185 NAFLD patients. Pathological findings—that >5% of hepatocytes were steatotic—indicated a diagnosis of NAFLD,17 which was graded S0-3 according to the classification by Brunt et al.19 Intralobular and portal inflammation consists of a mixed inflammatory cell infiltrate (lymphocytes, neutrophils, eosinophils, and Kupffer cells) and was graded A0-3. For fibrosis, staging was based on the location and extent of fibrosis and was graded F1-4 as follows: stage 1, zone 3 perisinusoidal fibrosis; stage 2, portal fibrosis; stage 3, bridging fibrosis in addition to stage 2; and stage 4, cirrhosis.18 The visceral and subcutaneous fat areas were calculated from computed tomography (CT) images.
The diagnosis of liver cirrhosis was defined as stage 4 fibrosis from liver biopsy or typical clinical findings on liver imaging, esophageal and gastric varices formation, and splenomegaly. HCC was diagnosed by histological examination or the detection of imaging findings consistent with the diagnosis using at least two of the following methods: abdominal ultrasound, CT scans, magnetic resonance imaging (MRI), and selective hepatic arteriography.20, 21
This study was conducted according to the principles of the Declaration of Helsinki and the ethical rules of the Tokyo Women's Medical University Hospital (TWMU, Tokyo, Japan). The TWMU Institutional Review Board approved the study protocol.
Gene polymorphism analysis
Ten SNPs associated with key metabolic processes were selected as follows: alcohol metabolism, rs1229984 (ADH1B) and rs671 (ALDH 2 family; ALDH2); lipid metabolism, rs1800591 (microsomal triglyceride transfer protein; MTP) and rs1801282 (peroxisome proliferator activated receptor gamma 2; PPARG2); glucose metabolism, rs2237892 (potassium voltage-gated channel subfamily Q member 1; KCNQ1), rs3746876, and rs743296 (potassium voltage-gated channel subfamily J member 15; KCNJ15); obesity, rs4994 (adrenoceptor beta 3; ADRB3) and rs4986790 (toll-like receptor 4; TLR4); and steatosis, rs738409 (PNPLA3). The MassARRAY® System (Agena Bioscience, San Diego, CA, USA), combined with iPLEX® chemistry, combines mass spectrometry and polymerase chain reaction (PCR). The primers were designed against specific sites in each gene and were extended to determine each allele (Table S1, Supporting Information). The allele frequencies among Japanese healthy controls were determined from the dsSNP database (https://www.ncbi.nlm.nih.gov/projects/SNP/snp_ref.cgi?rs).
Statistical analysis
Data are presented as medians and ranges. Differences between ALD and NAFLD, noncirrhosis and cirrhosis, and with or without HCC were assessed using Mann–Whitney U tests or χ2 tests with SPSS software (SPSS Inc., Chicago, IL, USA); P-values <0.05 were considered significant. When comparing ALD and NAFLD patients, genotypes were divided into three groups, and each SNP was compared. In cases of cirrhosis or HCC, genotypes were divided into two groups and compared because of the small sample size.
Calculation of propensity score: We performed a logistic regression analysis with the ALD or NAFLD as the objective variable using gender, age, and presence or absence of cirrhosis as the explanatory variables and calculated PS by fitting the results of this analysis after matching age, gender and the fibrosis status. Propensity score was conducted so that it was balanced across ALD and NAFLD, and we checked that covariates are balanced across ALD and NAFLD within the strata of the propensity score after examining and checking its distribution. We confirmed that there was no significant difference of PS depending on the ALD or NAFLD with respect to gender, age, and presence or absence of cirrhosis. Then, the distribution of ADH 1B, ALDH 2, and MTP were compared between the group with ALD and the group with NAFLD. SPSS Statistics Ver. 23 (IBM Corporation, Armonk, NY, USA) was used for statistical analysis, including the calculation of PS.
Results
Comparison of gene polymorphisms between ALD and NAFLD patients
Table 1 lists the patient demographics. Male gender was dominant in the ALD group. BMI and the incidence of complications due to lifestyle-related diseases, including diabetes, dyslipidemia, and hypertension, were extremely low in the ALD group. A clinical diagnosis of cirrhosis was significantly higher in the ALD group (ALD, 57.6%; NAFLD, 18.5%). HCC was diagnosed in 30.5% of the ALD group, which was significantly higher than the NAFLD group (11.5%). Overall, liver biopsies were performed in 35.6 and 92.5% of patients in the ALD and NAFLD groups, respectively.
| Characteristic | Total (n = 318) | ALD (n = 118) | NAFLD (n = 200) | P-value (ALD vs NAFLD) |
|---|---|---|---|---|
| Age (years) | 61 (17–94) | 62 (29–80) | 61 (17–94) | 0.84 |
| Male gender (%) | 211 (66.4) | 101 (85.6) | 110 (55.0) | <0.01 |
| Body mass index (kg/m2) | 26.3 (12.8–46.0) | 23.8 (15.9–33.8) | 27.9 (12.8–46.0) | <0.01 |
| Visceral fat area (mm2) | 16 457 (183–48 868) | 16 144 (4488–48 596) | 16 628 (183–48 868) | 0.97 |
| Subcutaneous fat area (mm2) | 23 484 (192–257 408) | 17 184 (3336–43 917) | 24 586 (192–257 408) | <0.01 |
| Liver cirrhosis (%) | 105 (33.0) | 68 (57.6) | 37 (18.5) | <0.01 |
| Diabetes (%) | 130 (40.9) | 31 (26.3) | 99 (49.5) | <0.01 |
| Dyslipidemia (%) | 139 (43.7) | 22 (18.6) | 117 (58.5) | <0.01 |
| Hypertension (%) | 172 (54.1) | 37 (31.4) | 135 (67.5) | <0.01 |
| Hepatocellular carcinoma (%) | 59 (18.6) | 36 (30.5) | 23 (11.5) | <0.01 |
| Liver biopsy (%) | 227 (71.4) | 42 (35.6) | 185 (92.5) | <0.01 |
| Fibrosis (F0–2, F3–4) (%) | 124 (54.9)/102 (45.1) | 17 (40.5)/25 (59.5) | 107 (57.8)/77 (41.6) | 0.26 |
| Activity (A1–2, A3) (%) | 132 (58.4)/94 (41.6) | 35 (85.4)/6 (14.6) | 97 (52.4)/88 (47.6) | <0.01 |
| Steatosis (S1–2, S3) (%) | 107 (47.6)/116 (52.4) | 31 (81.5)/7 (18.4) | 76 (41.1)/109 (58.9) | <0.01 |
| Laboratory data | ||||
| Albumin (g/dL) | 4.2 (1.6–5.5) | 3.6 (1.6–5.1) | 4.4 (2.3–5.5) | <0.01 |
| Total bilirubin (mg/dL) | 0.9 (0.3–43.5) | 1.2 (0.3–43.5) | 0.7 (0.3–6.6) | <0.01 |
| Aspartate aminotransferase (U/L) | 48 (13–1024) | 46 (13–1024) | 52 (14–231) | 0.07 |
| Alanine transaminase (U/L) | 50 (7–2609) | 35 (7–2609) | 70 (10–392) | 0.80 |
| γ-glutamyltransferase (U/L) | 76 (15–1982) | 143 (16–1982) | 65 (15–568) | <0.01 |
| Fasting blood glucose (mg/dL) | 103 (59–301) | 110 (63–301) | 100 (59–258) | 0.11 |
| HbA1c (%) | 5.7 (3.7–11.4) | 5.6 (3.7–11.4) | 5.9 (3.9–11.3) | 0.09 |
| IRI (μU/mL) | 11.7 (0.4–204.9) | 9.7 (0.4–123.6) | 12.1 (2.4–204.9) | 0.19 |
| HOMA-IR | 3.09 (0.06–52.80) | 2.61 (0.06–52.80) | 3.36 (0.75–42.40) | 0.47 |
| Triglyceride (mg/dL) | 121 (21–696) | 98 (21–696) | 137 (28–678) | <0.01 |
| Total cholesterol (mg/mL) | 183 (51–331) | 153 (51–331) | 192 (57–328) | <0.01 |
| Iron (μg/dL) | 99 (11–372) | 98 (11–372) | 100 (12–235) | 0.51 |
| Ferritin (ng/mL) | 254 (6–5331) | 263 (6–5331) | 250 (10–1718) | 0.01 |
| Platelet counts (×104/μL) | 18.2 (1.6–43.2) | 12.9 (1.6–34.6) | 20.0 (3.4–43.2) | <0.01 |
| Prothrombin time (%) | 88.3 (9.6–110) | 73.8 (21.9–100) | 91.0 (9.6–110) | <0.01 |
| Alpha–fetoprotein (AFP, ng/mL) | 4 (1–119 130) | 5 (1–3513) | 4 (1–119 130) | 0.15 |
| des-γ-carboxy prothrombin (DCP, mAU/mL) | 22 (8–36 283) | 28 (10–36 283) | 21 (8–26 812) | 0.58 |
- ALD, alcoholic liver disease; HbA1c, hemoglobin A1c; HOMA-IR, homeostatic model assessment for insulin resistance; IRI, immunoreactive insulin; NAFLD, non-alcoholic fatty liver disease.
Ten gene polymorphisms associated with obesity; steatosis; and alcohol, glucose, and lipid metabolism were compared between the ALD and NAFLD groups (Table 2). Higher percentages of the ADH1B genotype GG and ALDH2 genotype GG were observed in the ALD group compared with the NAFLD group (ADH1B genotype GG, 16 vs 4%, G allele frequency, 35 vs 21%; ALDH2 genotype GG, 84 vs 44%, G allele frequency, 93 vs 67%, respectively; all P < 0.01). Conversely, the percentage of patients with the MTP genotype GG was lower in the ALD compared with the NAFLD group (genotype GG 62 vs 72%, G allele frequency, 77 vs 86%, both P < 0.01). There were no significant differences in the prevalence of other SNPs between the two groups. All patients exhibited the TLR4 AA genotype, so this gene was excluded from the study.
| Control | ALD (n = 118) | NAFLD (n = 200) | P-value† (ALD vs NAFLD) | |
|---|---|---|---|---|
| ADH1B (rs1229984) | ||||
| Allele frequency | A = 73%, G = 27%* | A = 65%, G = 35% | A = 79%, G = 21% | |
| Genotype AA, n (%) | 54 (46) | 123 (62) | <0.01 | |
| AG, n (%) | 42 (36) | 66 (33) | ||
| GG, n (%) | 19 (16) | 8 (4) | ||
| ALDH2 (rs671) | ||||
| Allele frequency | G = 76%, A = 24%* | G = 93%, A = 7% | G = 67%, A = 33% | |
| Genotype GG, n (%) | 99 (84) | 88 (44) | <0.01 | |
| AG, n (%) | 16 (14) | 88 (44) | ||
| AA, n (%) | 0 (0) | 21 (11) | ||
| MTP (rs1800591) | ||||
| Allele frequency | G = 85%, T = 15%* | G = 77%, T = 23% | G = 86%, T = 14% | |
| Genotype GG, n (%) | 73 (62) | 143 (72) | <0.01 | |
| GT, n (%) | 32 (27) | 52 (26) | ||
| TT, n (%) | 10 (9) | 2 (1) | ||
| PPARG2 (rs1801282) | ||||
| Allele frequency | C = 3%, G = 97%* | C = 4%, G = 96% | C = 3%, G = 97% | |
| Genotype CC, n (%) | 0 (0) | 0 (0) | 0.22 | |
| GC, n (%) | 10 (9) | 10 (5) | ||
| GG, n (%) | 105 (89) | 186 (93) | ||
| KCNQ1 (rs2237892) | ||||
| Allele frequency | C = 62%, T = 38%* | C = 56%, T = 44% | C = 59%, T = 41% | |
| Genotype CC, n (%) | 37 (31) | 63 (32) | 0.46 | |
| CT, n (%) | 55 (47) | 104 (52) | ||
| TT, n (%) | 23 (20) | 29 (15) | ||
| KCNJ15 (rs3746876) | ||||
| Allele frequency | C = 96%, T = 4%* | C = 97%, T = 3% | C = 95%, T = 5% | |
| Genotype CC, n (%) | 107 (91) | 179 (90) | 0.34 | |
| CT, n (%) | 8 (7) | 17 (9) | ||
| TT, n (%) | 0 (0) | 2 (1) | ||
| KCNJ15 (rs743296) | ||||
| Allele frequency | G = 71%, C = 29%* | G = 68%, C = 32% | G = 70%, C = 30% | |
| Genotype GG, n (%) | 53 (45) | 97 (49) | 0.86 | |
| GC, n (%) | 50 (42) | 83 (42) | ||
| CC, n (%) | 12 (10) | 18 (9) | ||
| TLR4 (rs4986790) | ||||
| Allele frequency | A = 100%, G = 0%* | A = 100%, G = 0% | A = 100%, G = 0% | |
| Genotype AA, n (%) | 100 (100) | 100 (100) | - | |
| AG, n (%) | 0 (0) | 0 (0) | ||
| GG, n (%) | 0 (0) | 0 (0) | ||
| ADRB3 (rs4994) | ||||
| Allele frequency | T = 80%, C = 20%* | T = 82%, C = 18% | T = 82%, C = 18% | |
| Genotype TT, n (%) | 78 (66) | 134 (67) | 0.61 | |
| CT, n (%) | 33 (28) | 50 (25) | ||
| CC, n (%) | 4 (3) | 11 (6) | ||
| PNPLA3 (rs738409) | ||||
| Allele frequency | C = 42%, G = 58%* | C = 39%, G = 61% | C = 35%, G = 65% | |
| Genotype CC, n (%) | 19 (16) | 26 (13) | 0.62 | |
| GC, n (%) | 52 (44) | 84 (42) | ||
| GG, n (%) | 43 (36) | 82 (41) | ||
- ALD, alcoholic liver disease; NAFLD, non-alcoholic fatty liver disease.
- * Allele frequencies were cited from dbSNP database.
- † Genotypes were divided into three groups and compared using χ2 tests.
Comparison of SNPs between matched populations of ALD and NAFLD patients
Gender, age, and the rate of complications due to lifestyle-related diseases differed between the two groups, as shown in Table 1. To exclude the influence of these factors, we compared ALD and NAFLD patients using a propensity-matched analysis (Table 3). Propensity-matched analysis after matching gender, age, and cirrhosis showed significant differences in these SNPs (ADH1B P < 0.01, ALDH2 P < 0.01, and MTP P = 0.02).
| Name of gene | ALD (n = 118) | NAFLD (n = 200) | P-value |
|---|---|---|---|
| ADH1B | AA 41%, AG 43%, GG 16% | AA 65%, AG 33%, GG 9% | <0.01 |
| ALDH2 | GG 89%, AG 11%, AA 0% | GG 43%, AG 43%, AA 15% | <0.01 |
| MTP | GG 61%, GT 28%, TT 11% | GG 79%, GT 20%, TT 1% | 0.02 |
- Analysis was performed after propensity matching for gender, age, and cirrhosis. ALD, alcoholic liver disease; NAFLD, non-alcoholic fatty liver disease.
Gene polymorphisms associated with cirrhosis and HCC
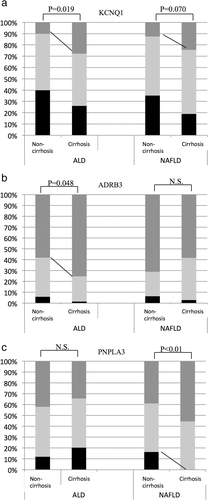
Potential genetic contributions to fibrosis progression were evaluated by comparing noncirrhosis and cirrhosis patients (Fig. 1). We compared all 10 SNPs. Only SNPs showing significant differences were selected. There were 68 cirrhosis patients (57.6%, 55 males) with ALD and 37 (18.5%, 19 males) with NAFLD. Although the median age of cirrhosis and noncirrhosis patients did not differ in ALD, the cirrhosis patients with NAFLD were significantly older (cirrhosis vs noncirrhosis, 69 vs 59 years old, respectively). The frequencies of the KCNQ1 genotype TT and ADRB3 genotype TT were increased significantly in ALD-related cirrhosis compared with noncirrhosis patients (cirrhosis vs noncirrhosis; KCNQ1 genotype TT, 28 vs 10%, P = 0.019, T-allele frequency, 51 vs 35%, P = 0.015; ADRB3 genotype TT, 75 vs 58%, P = 0.048, T-allele frequency, 87 vs 76%, P = 0.023) (Fig. 1a,b). In contrast, the frequency of the KCNQ1 genotype TT was increased (cirrhosis vs noncirrhosis; genotype TT, 24 vs 11%, P = 0.070, T-allele frequency, 53 vs 39%, P = 0.027), and the PNPLA3 genotype CC was significantly decreased in NAFLD-related cirrhosis compared with noncirrhosis patients (cirrhosis vs non-cirrhosis; genotype CC, 0 vs 16%, C-allele frequency, 22 vs 39%, both P < 0.01) (Fig. 1a,c).
 TT,
TT,  TC,
TC,  CC,
CC,  GG,
GG,  GC.
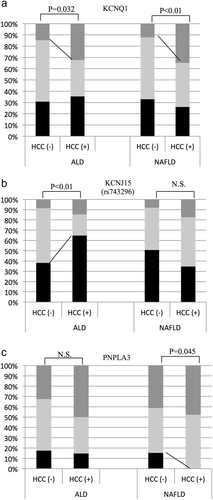
GC.There were 36 HCC patients (30.5%, 33 males) with ALD and 23 (11.5%, 15 males) with NAFLD. The HCC patients were significantly older in both the ALD and NAFLD groups (HCC− vs HCC+: ALD: 57 vs 65 years old and NAFLD: 59 vs 73 years old). When patients with and without HCC were compared, the frequency of KCNQ1 genotype TT was increased significantly in both ALD- and NAFLD-related HCC (ALD HCC− vs HCC+, genotype TT, 15 vs 32%, P = 0.032, T allele frequency, 42 vs 49%, P = 0.36; NAFLD, genotype TT, 12 vs 32%, P < 0.01, T-allele frequency, 40 vs 54%, P = 0.06; Fig. 2a). The KCNJ15 (rs743296) genotype GG was associated with ALD-related HCC (HCC− vs HCC+; genotype GG, 38 vs 65%, P < 0.01, G-allele frequency, 65 vs 75%, P = 0.13, Fig. 2b). The frequency of the PNPLA3 genotype CC was significantly decreased in NAFLD-related HCC (HCC− vs HCC+; genotype CC, 15 vs 0%, P = 0.045, T-allele frequency, 37 vs 26%, P = 0.15, Fig. 2c).
 TT,
TT,  TC,
TC,  CC,
CC,  GG,
GG,  GC.
GC.Discussion
This study found differences in the prevalence of gene polymorphisms among Japanese ALD and NAFLD patients. SNPs in genes related to alcohol metabolism were associated with ALD, and those linked to lipid metabolism were important in NAFLD. A KCNQ1 SNP might contribute to disease progression and hepatocarcinogenesis in both diseases.
The prevalence of SNPs in ADH1B, ALDH2, and MTP differed significantly between ALD and NAFLD patients (P < 0.01). ALD patients exhibit various degrees of liver injury caused by direct and indirect effects of alcohol, especially toxic metabolites and acetaldehyde.22 Several SNPs involved in alcohol metabolism23 and gene variants associated with the accumulation of acetaldehyde may lead to ALD and alcoholism. In the current study, reduced ethanol-metabolizing activity of ADH1B genotype GG and increased activity of the ALDH2 genotype GG were observed in ALD patients. Lower ADH enzyme activity results in a slower conversion of alcohol to acetaldehyde. A wild-type ADLH2 gene encodes an essential active ALDH enzyme that rapidly converts alcohol to acetaldehyde and can consequently drink much alcohol, which leads to liver damage.
MTP regulates hepatic triglycerides and very low-density lipoproteins (VLDL) by exporting lipids from the liver.24 It is expressed in enterocytes and hepatocytes, affects lipid absorption, and is related to NAFLD and diabetes.25 A meta-analysis showed that an MTP polymorphism (genotype GG) was associated with NAFLD development by reducing the export of lipids to serum and triggering the accumulation of lipids inside hepatocytes.26 Moreover, this polymorphism was associated with an atherogenic lipid profile in non-alcoholic steatohepatitis (NASH).27 In NAFLD, SNPs related to lipid metabolism might play a more important role, whereas SNPs related to ethanol metabolism might be more relevant in ALD. The propensity-matched analysis confirmed significant differences in the ADH1B, ALDH2, and MTP SNPs after matching gender, age, and cirrhosis.
When disease progression was compared, the prevalence of the KCNQ1 SNP differed significantly in ALD-related cirrhosis patients. In Japan, it was reported that the frequency of the KCNQ1 C allele is 62% and the T allele is 38%. The KCNQ1 SNP contributes to diabetes and the therapeutic effects of dipeptidyl peptidase-4 (DPP-4) inhibitors.28 KCNQ1 is also associated with the early onset of alcohol dependence29-31 and elevated plasma lipid levels.32 In addition, the KCNQ1 genotype TT was significantly linked to decreased serum triglyceride levels.32 Therefore, we speculated that KCNQ1 accumulates inside hepatocytes and contributes to disease progression. In this study, because there were few patients, propensity score matching could not be performed. A future validation study may be necessary after accumulating more cases.
In NAFLD, the accumulation of lipids, and hence disease progression, is increased in patients with the PNPLA3 genotype GG variant due to reduced ubiquitylation and impaired proteasomal degradation of PNPLA3 protein.33 Several studies have demonstrated that the PNPLA3 SNP influenced both the occurrence and progression of NAFLD.34 The current study suggested that the PNPLA3 SNP was also associated with cirrhosis in NAFLD. In ALD, an association between the PNPLA3 SNP and disease progression has been reported35; however, the PNPLA3 SNP was not related to disease progression in ALD in the current study. Therefore, this phenomenon should be studied in a larger cohort of patients. Nevertheless, we speculate that the PNPLA3 SNP plays a more important role in the progression of NAFLD than ALD in Japanese populations.
ADRB3 is expressed primarily in adipose tissue and is involved in the regulation of energy metabolism.36 An ADRB genotype variant has been linked to the early development of type 2 diabetes mellitus, a lower resting metabolic rate, abdominal obesity, and insulin resistance.36 In Japan, the frequency of the ADRB3 C allele is ~20%. The percentage of individuals with the T allele was lower in the ALD-related cirrhosis population, but the role that ADRB3 plays in the progression of ALD is unclear.
This study is the first to report a significant association between the KCNQ1 SNP and HCC in both ALD and NAFLD patients. Zhou et al.37 reported that fasting insulin levels and insulin resistance were higher in diabetes patients with the KCNQ1 T allele. KCNQ1 regulates the insulin receptor substrate-2 (IRS-2)/phosphoinositide 3-kinase/Akt pathway. Although the precise mechanism is unknown, the KCNQ1 SNP might affect hepatocarcinogenesis through insulin and these pathways. In addition, the KCNJ15 genotype GG was associated with ALD-related HCC. KCNJ15 is expressed in pancreatic β-cells and affects β-cell function.38 This is the first report demonstrating an association between the KCNJ15 SNP and HCC. Glucose metabolism plays an important role in these effects, although the exact mechanism is unclear.
The PNPLA3 G allele was associated with NAFLD-related HCC in the current study, which is consistent with previous reports. However, it is unclear whether PNPLA3 is directly involved in hepatocarcinogenesis. The genotype-based analysis and allele frequency showed discrepancies, including KCNQ 1 and KCNJ15 in ALD-HCC and PNPLA3 in NAFLD-HCC. Therefore, we need to determine which is correct by obtaining many more samples in the future. Nevertheless, the PNPLA3 SNP was related to liver fibrosis, and fibrosis progression may lead to HCC.
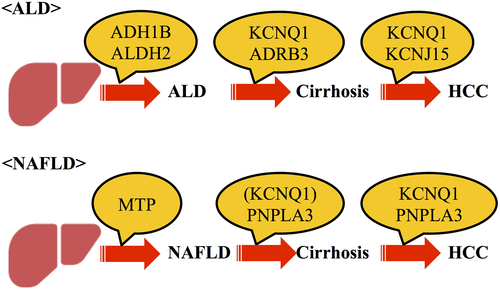
In this study, new hepatocarcinogenesis risk genes were identified in ALD and NAFLD. We used these observations to generate a new hypothesis regarding the effects of genetic background on disease progression and hepatocarcinogenesis in ALD and NAFLD, as shown in Figure 3. Nevertheless, further studies are necessary to confirm this hypothesis using large prospective samples. This study has the following limitations. It was a single-center study with limitations in terms of gender balance and rates of cirrhosis or HCC. In addition, liver biopsy was only performed in 36% of ALD patients. Consequently, the ALD and NAFLD risk SNPs should be validated in larger cohorts in a prospective study.
Conclusions
The activity of enzymes related to alcohol metabolism (ADH and ALDH) might contribute to ALD, and those related to lipid metabolism (MTP) might be associated with NAFLD. Further validation is required in different ethnic populations as well as in comparison with nondiseased groups.
Acknowledgments
This study was supported by a Grant-in-Aid from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (#26461024-0001) to T.K. Professor K.T. received research funding from Sumitomo Dainippon Pharma, Astellas Pharma, Eisai, Taiho Pharmaceutical, Chugai Pharmaceutical, Daiichi Sankyo Pharmaceutical, AbbVie GK, Takeda Pharmaceutical, Asahi Kasei Corporation, Ajinomoto, and Otsuka Pharmaceutical.




