DL-3-n-butylphthalide-induced neuroprotection in rat models of asphyxia-induced cardiac arrest followed by cardiopulmonary resuscitation
#Song Yang and Changxiao Yu contributed equally to this work.
*Ziren Tang and Shen Zhao contributed equally to this work.
Abstract
Most patients that resuscitate successfully from cardiac arrest (CA) suffer from poor neurological prognosis. DL-3-n-butylphthalide (NBP) is known to have neuroprotective effects via multiple mechanisms. This study aimed to investigate whether NBP can decrease neurological impairment after CA. We studied the protective role of NBP in the hippocampus of a rat model of cardiac arrest induced by asphyxia. Thirty-nine rats were divided randomly into sham, control, and NBP groups. Rats in control and NBP groups underwent cardiopulmonary resuscitation (CPR) 6 min after asphyxia. NBP or vehicle (saline) was administered intravenously 10 min after the return of spontaneous circulation (ROSC). Ultrastructure of hippocampal neurons was observed under transmission electron microscope. NBP treatment improved neurological function up to 72 h after CA. The ultrastructural lesion in mitochondria recovered in the NBP-treated CA model. In conclusion, our study demonstrated multiple therapeutic benefits of NBP after CA.
1 INTRODUCTION
DL-3-n-butylphthalide (NBP), originally isolated from the seeds of Apium graveolens, is applicable in therapies against several neurological diseases (Wang et al., 2018). Studies have suggested NBP to have beneficial effects against ischemic stroke (Qin et al., 2019), neurodegenerative diseases such as Parkinson's disease (Xiong et al., 2012) and Alzheimer's disease (Wang et al., 2016), spinal cord injury (Zhao et al., 2017), and traumatic brain injury (Zhao et al., 2017). The target mechanisms for neuroprotection by NBP are multiple, including the alleviation of mitochondrial dysfunction to improve energy metabolism, anti-inflammatory action, reduction of oxidative stress, and inhibition of apoptosis (He et al., 2011; Yang et al., 2018). These studies mainly refer to cerebrovascular diseases (Liao et al., 2019; Niu et al., 2019; Qi et al., 2019). However, for acute diseases, such as cardiac arrest, there is still no comprehensive description of the neuroprotective mechanisms of NBP.
Cardiac arrest (CA) is a devastating event with high morbidity (Moulaert et al., 2017). Only 23.9% of adult in-hospital patients with CA survive hospital discharge (Benjamin et al., 2019), and neurological damage in multiple regions of the brain, including the hippocampus, can cause significant morbidity in patients discharged from the hospital (Horstmann et al., 2010). Due to the poor prognosis and complicated mechanism of neurological function in patients with CA, drug intervention to improve it is still lacking. Although the initial trigger mechanism of post-CA brain injury is different from that of stroke, the pathological signaling pathway and subsequent cell death mechanism of CA are similar to those of ischemic stroke. Therefore, we hypothesized that NBP could have a neuroprotective effect on CA injury as well. An earlier report had shown the number of hippocampal CA1 cells in an NBP group to be significantly higher than that in the untreated group (Zhang et al., 2016). However, whether NBP has other brain-protective mechanisms besides reducing neuronal death remains unclear. Therefore, it is necessary to verify the different protective effects of NBP on CA-related injuries and clarify the cellular and molecular mechanisms underlying the same.
In this study, we hypothesized that NBP had a protective effect on the improvement of neural function in a CA model. On the basis of a model of cardiac arrest after asphyxia, we studied the protective effect of NBP on the hippocampus.
2 METHODS
2.1 Animals
The animal experiments were approved by the Institutional Animal Care and Use Committee of the Tang Wanchun Laboratories of Emergency and Critical Care Medicine at Sun Yat-sen Memorial Hospital, Sun Yat-sen University. Thirty-nine Sprague-Dawley rats, weighing 310–340 g, were randomly divided into three groups: sham group (n = 13), control group (n = 13), and NBP group (n = 13). In each group, eight rats were used for the evaluation of neurological function, and remaining five rats were euthanized for further testing, 24 h after successful CPR treatment.
2.2 Experimental procedures
Animals were anesthetized with 3% pentobarbital sodium (45 mg/kg). The trachea was intubated orally with a 14-gauge cannula (Abbocath-T). For measuring the mean arterial pressure (MAP) and for arterial blood gas analysis, a 23-gauge polyethylene 50 (PE-50) catheter (Abbocath-T) was inserted into the left femoral artery. For the determination of right atrial pressure, a PE-50 catheter was inserted into the right atrium through the left external jugular vein. A Capstar-100 CO2 analyzer (IITC Life Science) was interposed between the tracheal cannula and ventilator to continuously measure the end-tidal CO2. Conventional electrocardiogram lead II and hemodynamic data were continuously recorded. A heat lamp was used for maintaining rectal temperature (within 36.5 ± 0.5°C).
Asphyxia model was established by endotracheal tube clamping. An MAP value less than 20 mmHg was defined as cardiac arrest (CA). After 6 min of untreated CA, CPR was implemented, as described previously (Wang, Li, et al., 2018). Epinephrine (0.01 mg/kg) was administered via the right atrium after 2 min of precordial compression (PC). If rats had ventricular fibrillation, defibrillation was attempted with up to three 2 J counter shocks after 4 min of PC. PC was stopped when ROSC failed after 5 min. MAP ≥ 60 mmHg, lasting for a minimum of 5 min, was defined as ROSC. The animals were randomized into three groups after ROCS was achieved successfully.
2.3 Butylphthalide (NBP) administration
The rats in the NBP group were divided into two groups that were submitted to two different injection methods. For the rats needing a neurological function test, NBP, 10 mg/kg, was injected intravenously 10 min after cardiopulmonary resuscitation twice a day for three consecutive days, and then the neurological function was measured; for rats requiring euthanasia within 24 h, the same amount of NBP was injected twice in the first 24 h. The control group was given the same amount of normal saline. In contrast to the animals in the control group, those in the sham group were not treated with ventricular fibrillation and CPR; however, they were injected with the same volume of saline vehicle in the corresponding time. As NBP has been studied for many years as a protective drug, we defined the NBP dose according to the instructions provided by the manufacturer and previous studies (Zhang et al., 2006).
2.4 Evaluation of neurological function
Neurological deficit scores (NDS) were assessed by two investigators unaware of the groups. The method of NDS was used to evaluate neurological function at different time points, including 24, 48, and 72 h after NBP treatment. The scores mainly included exercise, feelings, balance, and reflection. Zero-point was defined as normal neurological function. Higher the neurological deficit, higher was the score (Wang, Li, et al., 2018).
2.5 TUNEL assay
Terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) assay was performed using an in-situ cell death detection kit (Santa Cruz Biotechnology), following the manufacturer's instruction with some modification. Briefly, hippocampal sections were permeabilized with 0.2% Triton X-100. Fragmented DNA was labeled with fluorescein-12-dUTP at 37°C for 1 h. Images were captured using a Nikon ECLIPSE 90i.
2.6 Ultrastructural examination of hippocampal neurons
Samples were collected from hippocampal tissues and fixed in 2.5% glutaraldehyde with 0.1 mol/L cacodylate buffer (pH 7.4). After postfixing in 1% osmium tetroxide, samples were dehydrated and embedded in Epon. Ultrathin sections (60–80 nm) of hippocampus samples were stained with lead citrate and uranyl acetate and subsequently observed under an FEI Tecnai G2 transmission electron microscope equipped with a Gatan 832 CCD camera (Gatan).
2.7 Western blot
Approximately 2 μg lysate from hippocampal tissue was loaded on each lane of 8% polyacrylamide gel, and the protein bands were blotted thereafter on a polyvinylidene difluoride (PVDF) membrane. After blocking with PBST containing 5% nonfat dry milk, the membrane was incubated with antibodies against cleaved Caspase-3 (Abcam, ab49822, 1:500), Caspase-3 (CST, 9662, 1:2000), TNF-α (CST, 6945, 1:1000), PHF-Tau (Thermo, MN1020, 1:10), P-JNK (CST, 9255, 1:500), JNK (CST, 9252, 1:2000), P-p38 (CST, 4511, 1:500), p38 (CST, 8690, 1:2000), and GAPDH (Affinity, t0004, 1:5000). Peroxidase-linked antirabbit IgG (Abcam) was used as a secondary antibody. The proteins were eventually visualized using an ECL Western blot analysis detection kit (Amersham Biosciences).
2.8 Statistical analysis
Raw data were analyzed using SPSS19.0 software and presented as mean ± standard deviation. Band gray values were determined by ImageJ software. Statistical difference was determined by unpaired Student's t test. Results with p-value <.05 were considered significantly different.
3 RESULTS
3.1 NBP alleviated neurological dysfunction in a rat model of asphyxia-induced cardiac arrest
There was no significant difference in baseline outcomes across the three groups (Table 1). All the animals of cardiac arrest were successfully resuscitated. There was no significant difference in survival rate among the three groups. Eight rats in each group were used to evaluate the neurological function, and the other five rats were euthanized 24 h after successful CPR, and the number and distribution of animals included in this study are shown in Table S1. We evaluated whether NBP could improve neurological function in the asphyxia model, based on neurological deficit scores. As shown in Table 2, the score significantly decreased from 218 ± 26 to 172 ± 20, 186 ± 28 to 136 ± 19, and 165 ± 35 to 100 ± 27 in asphyxia models, after NBP treatment for 24, 48, and 72 h, compared with that in the control group. Thus, NBP could improve neurological function in asphyxia-induced cardiac arrest models.
| Variables | Sham group | Control group | NBP group |
|---|---|---|---|
| Bodyweight, g | 496 ± 29 | 505 ± 30 | 515 ± 40 |
| Mean aortic pressure, mmHg | 130 ± 18 | 128 ± 20 | 136 ± 26 |
| End-tidal carbon dioxide, mmHg | 45 ± 5 | 48 ± 7 | 45 ± 6 |
| PH | 7.42 ± 0.10 | 7.49 ± 0.12 | 7.48 ± 0.10 |
| PaO2, mmHg | 90 ± 18 | 102 ± 16 | 96 ± 15 |
| Asphyxia time, min | \ | 10.8 ± 1.3 | 10.2 ± 1.5 |
| PC time, min | \ | 3.6 ± 2.1 | 3.8 ± 1.6 |
| DFs, n | \ | 1.9 ± 0.7 | 1.7 ± 0.6 |
| Total epinephrine, μg | \ | 4.8 ± 0.8 | 4.6 ± 0.7 |
- Note: Values are presented as mean ± SD.
- Abbreviations: DFs, times of defibrillation; PC, precordial compression; PH, pondus hydrogenii; ROSC, return of spontaneous circulation.
| NDS at 24 h | NDS at 48 h | NDS at 72 h | |
|---|---|---|---|
| Sham group | 0 | 0 | 0 |
| Control group | 218 ± 16* | 186 ± 22* | 165 ± 30* |
| NBP group | 172 ± 12*, ** | 136 ± 19*, ** | 100 ± 20*, ** |
- * p < .05 versus the sham group.
- ** p < .05 versus the control group
- Abbreviation: NDS, neurological deficit score.
3.2 NBP inhibited apoptosis in the hippocampal region of cardiac arrest model
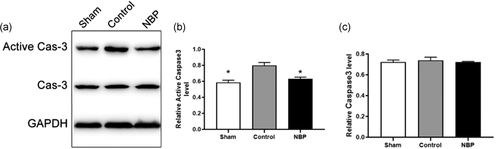
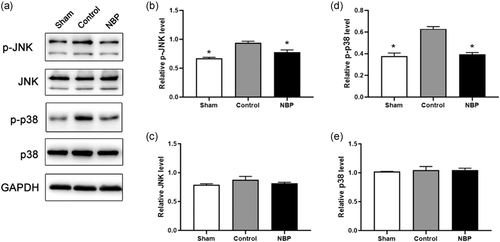
We determined whether NBP could affect neuronal apoptosis in the hippocampus of asphyxia model. The TUNEL assay indicated the ratio of TUNEL-positive cells to be drastically increased in the hippocampal CA1 region of cardiac arrest models, compared with that in sham rats (Figure 1a,b,d, p < .01). In the presence of NBP, the proportion of TUNEL + cells was significantly decreased than in the control rats (Figure 1b–d, p < .01). Besides, the protein level of active Caspase-3 was significantly increased in the hippocampal region of asphyxia-induced cardiac arrest model, demonstrating the neuronal death after resuscitation to be mediated via the Caspase 3-dependent pathway; cell viability was impaired in the hippocampal region of cardiac arrest models (Figure 2a,b, p < .05). In contrast, a significant reduction of active Caspase-3 level was observed in rats treated with NBP (Figure 2a, b, p < .05), whereas the protein level of Caspase-3 was not affected across the different groups (Figure 2a, c, p > .1). Next, we elucidated the signaling mechanism underlying the antiapoptotic role of NBP in hippocampus of cardiac arrest model after return of spontaneous circulation (ROSC). The expression level of p-JNK or p-p38 was aberrantly higher in the control than in sham, implying the activation of JNK/p38 signaling in hippocampal region (Figure 3a–c, p < .01). In contrast, both p-JNK and p-p38 levels were decreased in the hippocampus of NBP-treated cardiac arrest models, suggesting JNK/p38 signaling to mediate the antiapoptotic effect of NBP (Figure 3a–c, p < .05 for P-JNK, p < .01 for P-p38). However, we did not observe any difference in the level of JNK or p38, as an internal control (Figure 3a,d,e). Collectively, these data demonstrated the preventive effect of NBP on neuronal apoptosis in the hippocampal region of asphyxia-induced cardiac arrest model.
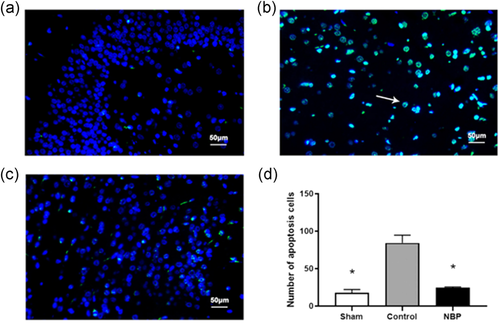
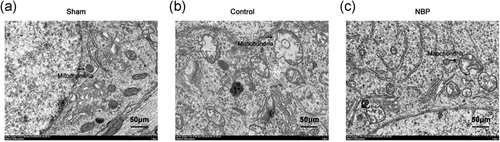
3.3 NBP alleviated ultrastructural mitochondrial damage in hippocampal neurons
We determined whether NBP could recover the ultrastructural damage in hippocampal neurons of asphyxia model. In sham group, normal mitochondrial ultrastructure was observed in hippocampal neurons, with intact mitochondrial morphology and obvious mitochondrial ridge in order (Figure 4a). In contrast, severe swelling of mitochondria was observed in hippocampal neurons of control rats, and the ridge displayed apparent disorders (Figure 4b). Meanwhile, autophagosomes were observed in the cytoplasm of rats in control group, demonstrating the neurons to have undergone severe lesion and subsequent apoptotic process (Figure 4b). In the presence of NBP, mitochondrial swelling was alleviated in cardiac arrest models, with reduced number of autophagosomes in the cytoplasm (Figure 4c). Thus, we concluded that NBP treatment could alleviate the ultrastructural lesions in hippocampal neurons of asphyxia-induced cardiac arrest models.
3.4 NBP treatment reduced NF-κB activation and early inflammatory response after CA
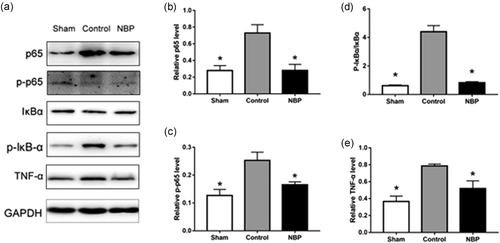
In western blot analysis, the level of NF-κB p65 in nuclear protein extracts from pericontusion region was significantly increased 72 h after CA, compared with that in sham rats. The CA-induced increase of nuclear NF-κB p65 was significantly decreased by NBP treatment (Figure 5a). We also found an apparent increase in p65 and p-p65 levels in the control group, whereas NBP treatment drastically decreased their expression (Figure 5a–c). In addition, the cytoplasmic level of IκBα protein was examined by western blot analysis (Figure 5a). Results are expressed as the ratio of p-IκBα versus total IκBα, which was significantly elevated in CA saline control rats than in sham rats. However, CA rats receiving NBP treatment showed a significantly reduced p-IκBα/total IκBα ratio as compared with CA saline controls (Figure 5d). Next, we assessed the effect of NBP on the expression of inflammatory mediators. Western blot analysis revealed an increase in the expression of tumor necrosis factor-alpha (TNF-α) in CA brain (Figure 5a). The expression of TNF-α in NBP-treated CA rats were significantly decreased than in CA saline controls (Figure 5e).
3.5 NBP inhibited neurotoxicity in the hippocampal region of cardiac arrest model
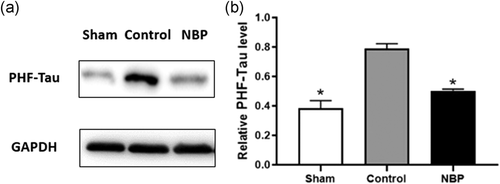
We investigated whether NBP could affect neurotoxicity in the hippocampus of the asphyxia-induced cardiac arrest model. Microtubule-associated protein tau (MAPT), also known as paired helical filaments Tau (PHF-Tau), constitutes the intraneuronal neurofibrillary tangles, commonly observed in neurodegenerative diseases (Pirscoveanu et al., 2017). Thus, this protein could be a marker for neurotoxicity and disorders in neurological function. We found the expression level of PHF-Tau to be drastically increased in the control group as compared with that in the sham group (Figure 6a,b). With NBP treatment, PHF-Tau level was significantly decreased than in untreated models (Figure 6a,b). Thus, neuronal abnormality and neurotoxicity were enhanced in cardiac arrest models, whereas treatment with NBP alleviated the lesion in hippocampal region.
4 DISCUSSION
Our findings provide new evidence of protective effect of NBP against CA-induced brain damage, besides its neuroprotection against stroke, which have been reported previously. We demonstrated neuroprotection by NBP to be mediated by the inhibition of JNK/p38-mediated apoptotic cell death and NF-κB-mediated inflammation in the CA brain. The ultrastructural damage in mitochondria of hippocampal neurons was restored by NBP treatment. Furthermore, we found new evidence of NBP treatment reducing the expression of neurotoxic factor PHF-Tau, after CA insult. Collectively, we concluded that application of NBP could protect the hippocampal neurons from lesion and death in asphyxia-induced cardiac arrest models, suggesting NBP to possibly function as a therapeutic agent against cardiac arrest.
The antiapoptotic role of NBP in central nervous system had been reported previously (Chen et al., 2019; Gao et al., 2019). Nevertheless, there have only been a few reports about the effect of NBP on CA-induced damages. The underlying mechanism of NBP effects has not been clarified yet. In the present study, we found the hippocampal neurons from cardiac arrest models to undergo severe apoptosis as compared with those in the NBP-treated group. This process was mediated by Caspase-3 signaling, suggesting NBP to relieve the Caspase 3-dependent apoptosis. This type of apoptosis has been reported to be associated with mitochondrial abnormality, such as translocation of cyt-c (Jiang et al., 2017), and dysregulation of mitochondrial function (Wang et al., 2019). We found ultrastructural lesions, including disorders in mitochondrial ridge, severe swelling, as well as appearance of autophagosomes, in the hippocampal neurons of asphyxia-induced cardiac arrest model. This further demonstrated caspase-dependent apoptosis to be activated in cardiac arrest-induced neurological deficit. This might be a therapeutic target for NBP, if future studies could confirm the correlation between NBP administration and the critical factors in extrinsic caspase-dependent apoptotic pathway, including Caspase 6 and 7, DFF45/40, and PARP, and those in the intrinsic caspase-dependent apoptotic pathway, such as Bid, Cytochrome C, Apaf1, and IAPs (Yokobori et al., 2014). The molecular mechanism of neuroprotective effect of NBP in acute diseases is not clear. Here, we found JNK/p38 to mediate the effect of NBP on apoptosis of hippocampal neurons. Previous studies have shown that the JNK/p38 pathway is related to cell apoptosis: acupuncture treatment of vascular dementia may work through the inhibition of the ASK1-JNK/p38 pathway and improving the condition through an antiapoptotic mechanism (Zhu et al., 2018); and the PI3K/Akt/JNK/p38 signaling pathway mediates neuronal apoptosis in the prefrontal cortex of mice (Li et al., 2018). JNK/p38 is known to be closely associated with cardiac arrest (Liu et al., 2017). Downregulation of p-JNK expression inhibited neuronal apoptosis in cardiac arrest model. This was consistent with our finding of reduced p-JNK level in the presence of NBP, leading to less neuronal apoptosis in cardiac model.
Accumulating data from basic and clinical studies suggested inflammatory response to be a key mechanism in the development of CA damage, leading to neural death and increased brain edema (Adrie et al., 2004). Post-CA inflammation is characterized by the production of pro- and anti-inflammatory cytokines. Activation of NF-κB signaling is considered a primary regulator of inflammatory responses, and transcriptional activation of NF-κB is tightly regulated by IκBα expression (Gutierrez et al., 2008). NF-κB remains inactive in cytoplasm due to the formation of NF-κB/IκBα complex, and IκBα degradation, induced by stress, allows NF-κB to release and initiate the expression of proinflammatory cytokines (Gutiérrez et al., 2008; Wang et al., 2013). In a rat model of cardiac arrest, inhibition of the NF-κB-mediated inflammatory pathway led to an improvement in neurological outcome (Wei et al., 2015). In the present study, CA damage increased activation of NF-κB and enhanced the expression of proinflammatory cytokines. These observations agreed with the idea that NF-κB signaling pathway participates in the CA-induced inflammatory response. More important, NBP treatment suppressed not only NF-κB activation but also proinflammatory cytokine induction. Our study indicated NBP to significantly inhibit the expression of p65 and p-p65, which were remarkably increased by ischemia–reperfusion, and phosphorylation of p65 to play a key role in the activation of the NF-κB pathway (Kaltschmidt et al., 2019). NBP treatment can also dramatically attenuate the degradation of IκBα. Thus, we considered the potential molecular mechanism underlying the neuroprotective role of NBP to also be associated with the inhibition of the NF-κB pathway, activated by ischemia–reperfusion in a rat model of cardiac arrest.
PHF-Tau protein is a potential pathogenic protein causing abnormal brain structure and function in patients with Alzheimer's disease (AD; Thomas et al., 2012). Thus, this protein is critically associated with abnormalities in specific brain regions. In this study, we reported the PHF-Tau expression level to be reduced with NBP administration in asphyxia-induced cardiac arrest model, indicating the abnormality in hippocampus to be alleviated by NBP treatment. Meanwhile, the PHF-Tau protein might lead to the development of mild cognitive impairment (Cho et al., 2016), hence serving as a potential therapeutic target for the same (Chien et al.). This may explain why NBP could reduce the PHF-Tau protein level and improve neurological function in our study. A previous study had demonstrated alteration in PHF-Tau levels to be parallel to increased levels of reactive oxygen species, causing apparent neurotoxicity (Arora et al., 2015). This may have link with neuronal death in cardiac arrest model; NBP's neuroprotective role was potentially due to the relief of neurotoxic effect, which was the first indication of the correlation between PHF-Tau and NBP's potential clinical application.
There were several limitations in this study. First, we only focused on the antiapoptotic effect, anti-inflammatory response, and antitoxic signaling pathway of NBP; however, other mechanisms deserve attention too: in addition to our findings, NBP may have other protective mechanisms in cardiac arrest that need further study, as NBP has multiple neuroprotective targets. Second, the protective effect of NBP may be time-dependent; however, we only investigated the protein expression after a 24-h treatment. Third, we only studied the effect of NBP treatment on the hippocampus region, considering it the most vulnerable to cerebral ischemic injury; other brain regions also deserve comprehensive investigation.
In summary, our study demonstrated NBP to show multiple therapeutic benefits after CA. It could reduce neuronal damage along with ultrastructural damage, apoptosis through the JNK/p38 pathway, inflammatory response through the NF-κB pathway, and neurotoxicity by reducing the PHF-Tau levels. Therefore, we found NBP to exert a preventive effect on hippocampal injury in a model of asphyxiated cardiac arrest and expect it to be an effective treatment for brain injury caused by cardiac arrest.
ACKNOWLEDGMENTS
We thank all the colleagues who assisted with the experiments.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Y. S., and Y. C. accomplished the most experiments and paper writing; L. Y., and S. X. participated in western blot analysis. Y. Z., C. H., W. Y., and L. Z. participated in the rat model of CA. S. F. assisted in designing the research and statistical analysis. Z. S., and T. Z. designed the research and revised the manuscript. All authors have read and agreed with the final manuscript.
Open Research
DATA AVAILABILITY STATEMENT
The data used to support the findings of this study are available from the corresponding author upon request.




