Synergistic effect of three-dimensional coculture and photobiomodulation therapy on vascularized liver spheroid formation by stem cells
Abstract
Despite studies reporting functional differentiation of liver cells, a three-dimensional, vascularized liver organ has yet to be developed from mesenchymal stem cells. We investigated whether treatment with photobiomodulation (PBM) before three-dimensional liver spheroid transplantation improved the recovery of liver function via stimulation of angiogenesis and hepatocyte differentiation. Liver spheroids composed of hepatic, endothelial, and mesenchymal cells were subjected to PBM therapy. To evaluate the in vivo therapeutic effect of the liver spheroids treated with PBM, phosphate-buffered saline, liver spheroid, and PBM-treated liver spheroid were transplanted into a damaged host liver using conventional chimeric mouse models. To further characterize the maturation of transplanted PBM-liver spheroid compared with the newly generated non-PBM-liver spheroid or human liver tissues, the expression profiles of mature liver signature genes were analyzed. Liver spheroids expressed hepatocyte growth factors, including vascular endothelial growth factor and angiogenic factors. The cells in liver spheroid compensated for the low viability and improved the function of hepatocytes. Here, we demonstrate the formation of vascularized and functional human liver spheroid from human adipose-derived stem cells by transplantation of liver tissue created in vitro. Albumin secretion by PBM-treated liver spheroid was higher on Day 28 compared with liver spheroid-seeded transplant group. PBM-liver spheroids serve as individual vascularization units, promoting the simultaneous development of new microvascular networks at different locations inside the implanted tissue constructs. The vasculature in the liver spheroid transplants became functional by connecting to the host vessels within 48 h. These PBM-liver spheroids may be useful in designing artificial three-dimensional hepatic tissue constructs and in cell therapy with limited numbers of human hepatocytes.
1 INTRODUCTION
Liver transplantation is a therapeutic option for patients with end-stage liver disease, but is limited by the shortage of donors, the high cost and rates of rejection, and potential risks for living donors (Gary & Becker, 2007, Rauchfuss et al., 2016). Although several types of bio-artificial liver have been described, most are extracorporeal systems indicated for short-term use, with limited clinical success (van de Kerkhove et al., 2004). Recently, stem cells have attracted great attention in the treatment of liver disease. Hepatocyte-based therapies are less invasive than whole-organ or partial liver transplantation, with the possibility of repeated use, and do not require chronic immunosuppression (Baccarani et al., 2003). Hepatocyte transplantation, however, requires a continuous supply of human primary hepatocytes and their in vitro culture with functional maintenance over a period of time. Although primary human hepatocytes derived from partial hepatectomy specimens are more reliable and useful, their poor viability leads to more frequent use (Bhogal et al., 2011; Dandri et al., 2001). Therefore, successful hepatocyte transplantation requires viable hepatocytes in large numbers and improved functional maintenance (Bhogal et al., 2011).
Recent findings suggest that human adipose-derived stem cells (hASCs) can differentiate into cells exhibiting a hepatocyte phenotype (Aurich et al., 2009). Adult stem cell-based therapies represent an alternative or serve as adjunct therapeutic approaches for the treatment of liver diseases (Goldman, 2016; Kopp & Sander, 2016, Takebe et al., 2017; Tsolaki, 2015). Moreover, the estimated fraction of mesenchymal stem cells (MSCs) in adipose tissue is markedly higher than the common MSC sources such as bone marrow (Giai & Oliva, 2012). Unfortunately, the clinical application of stem cells is still limited because of their low therapeutic efficacy (Berardis et al., 2015). Most of the stem cells used die within a week of transplant (Christman & Lee, 2006; Terrovitis, 2010). Paracrine factors released by the transplanted cells enhance host angiogenesis (Wang & Sjöquist, 2006).
Several experimental strategies to improve the tissue survival and engraftment of stem cells have been developed, including transplantation in combination with growth factor delivery, genetic modification of stem cells, and cell liver spheroid culture (Park & Ahn, 2014; Park & Ahn, 2015; Park & Rhie, 2013). We describe here a novel method using differentiated hepatocytes from hASCs to overcome cell shortage and low viability. The key feature of this method is the generation of a three-dimensional (3D) vascularized liver spheroid by coculturing differentiated hepatocytes from hASCs, MSCs, and endothelial cells treated with PBM at the low cell binding surface. Hepatocytes cultured as 3D-liver spheroids show enhanced vitality and hepatic performance, improved viability and function. Based on these findings, we cocultured both cells in 3D liver spheroid expecting a positively outcome. Similar to many other cell types, MSCs aggregate into multicellular liver spheroids when cultured in suspension or on nonadhesive surfaces (Park & Ahn, 2014).
The proliferation and growth factor secretion of hASCs was also enhanced by photobiomodulation (PBM) therapy (Park & Ahn, 2014). Moreover, the use of PBM to stimulate new blood vessel growth has recently received considerable attention. Red and infrared light-emitting diode (LED (660 nm)) enhanced tissue healing by stimulating angiogenesis in various animal models of ischemia (de Sousa et al., 2013). In this study, PBM was used to induce a hypoxic liver spheroid and differentiation of hASCs into hepatocyte by weakening the cell-matrix adhesion. Based on the expression of hypoxia-induced survival factors, inhibition of apoptosis, extracellular matrix (ECM) preservation and vascularization of tissue constructs, and the total volume and hepatocyte potential of various 3D constructs, we developed a vascularized human liver tissue. The vascularized human liver tissues using a 3D aggregation culture facilitate in vitro studies via liver tissue engineering and cell therapy.
2 MATERIALS AND METHODS
2.1 Culture of ASCs
hASCs supplied from CEF were cultured in low-glucose Dulbecco's modified Eagle's medium F-12 (DMEM/F-12; Welgene) supplemented with 10% fetal bovine serum (FBS; Welgene), 100 units/ml penicillin and 100 μg/ml streptomycin at 37.0°C in a 5% CO2 incubator. hASCs between passage 5 and 8 were used for all experiments.
2.2 In vitro hepatic differentiation
hASCs were plated on collagen type I-coated dishes at a concentration of 4 × 104 cells/cm2. When the cells reached confluency, hepatogenic induction was carried out over a period of 2 weeks. First, the cells were treated for 3 days with DMEM (GibcoBRL) (serum free) supplemented with 20 ng/ml Activin A and 20 ng/ml FGF4 (PeproTech EC). Afterwards, the cells were cultured for 10 days in a hepatocyte culture medium (HCM), containing 5 mg/ml transferrin, 10−6 mol/L hydrocortisone-21-hemisuccinate, 0.5 mg/ml bovine serum albumin, 2 mmol/L ascorbic acid, 20 ng/ml epidermal growth factor, 5 mg/ml insulin, 50 mg/ml gentamicin (Cambrex Corp.) and supplemented with 150 ng/ml hepatocyte growth factor (HGF), 100 ng/ml fibroblast growth factor 1 (FGF1), 25 ng/ml FGF4, 30 ng/ml oncostatin M (OsM; PeproTech), (2 × 10−5 mol/L) dexamethasone (Dex; Sigma), 1× insulin-transferrin-selenium (ITS; Gibco), 0.05 mmol/L nicotinamide (Sigma), and 0.1% DMSO (Sigma) (Table S1). For the next few days, the cells were maintained with HCM alone.
2.3 PBM-Liver spheroid
ASCs-ECs and human MSCs (Lonza) were maintained in endothelial growth medium (Lonza) or MSC growth medium (Lonza) at 37°C in a humidified 5% CO2 incubator. To generate human liver spheroid in vitro, 1 × 106 human ASC derived hepatic cells, 1 × 106 human umbilical vein endothelial cells (HUVECs) and 5 × 105 human MSCs were resuspended in the mixture of EGM and HCM (Cambrex) containing with dexamethasone (0.1 mM; Sigma-Aldrich), oncostatin M (10 ng/ml; R&D System), hepatocyte growth factor (HGF; 20 ng/ml; PromoKine), and SingleQuots (Lonza) and plated on pre-solidified ×2 Matrigel diluted with EGM (BD Biosciences) in a 24-well plate. After approximately 4–6 days of culture, self-organized human PBM-Liver spheroid were detached, collected, and transplanted into a mouse.
2.4 PBM irradiation
LED (WON Technology Co., Ltd.) was applied for 10 min daily from day 1 to 3. The light source used was LED designed to fit over a standard multi-well plate (12.5 × 8.5 cm) for cell culture. The LED was had an emission wavelength peaked at 660 nm. The irradiance at the surface of the cell monolayer was measured by a power meter (Orion, Ophir Optronics Ltd.). To obtain the energy dose of 6 J/cm2, exposure time for LED array was 10 min under power density of 10 mW/cm2 (1 milliwatt × second = 0.001 J)
2.5 Fluorescence-activated cell sorting (FACS)
Cells were washed with phosphate-buffered saline (PBS) containing 0.5% bovine serum albumin (BSA; Sigma-Aldrich). The cells were stained in PBS containing 1% BSA with either isotype controls or antigen-specific antibodies for 60 min. The antibodies used were human CD34 (BD Biosciences), KDR (Beckman Coulter), CD31 (Beckman Coulter), CD45 (Abcam), CD90 (BD Biosciences), CD105 (Caltac Laboratories), and CD29 (Millipore). The cells were washed three times with PBS containing 0.5% BSA and resuspended in PBS for flow cytometry using an Accuri device (BD Biosciences). Isotype control IgG was used as a negative control.
2.6 Preparation of the experimental animal model
For in vivo transplantation, hepatocyte-like cells were harvested by treatment with a 0.05% collagenase/1000 U/ml dispase solution for 3–5 min, dissolved in PBS (–) and injected intravenously into mice with liver injury caused by CCl4 injection. Experiments involving mice were performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health (NIH Publication No. 85-23, revised 1996). All aspects of the animal care and experimental protocols were approved by the Dankook University Committee on Animal Care. Seven-week-old male BALB/c nude mice (Narabio) were anesthetized with Zoletil (30 mg/kg).
2.7 Dorsal skinfold chamber model and intravital fluorescence microscopy
To study in vivo the vascularization of liver-spheroid transplants, we prepared dorsal skinfold chambers in mice. After the preparation, the animals were allowed to recover for 48 h to exclude deterioration of the microcirculation due to the anesthesia and the surgical trauma. Subsequently, the cover glass of the dorsal skinfold chamber was temporarily removed and one liver-spheroid transplant was placed onto the striated muscle tissue within the center of each chamber, taking care to avoid contamination, mechanical irritation, or damage of the tissue.
For intravital fluorescence microscopy of the chambers, the animals were anesthetized by intraperitoneal injection of ketamine (75 mg/kg body weight; Pharmacia GmbH) and xylazine (25 mg/kg body weight; Rompun). Subsequently, the animals were positioned under a Zeiss Axiotech microscope (Zeiss) with a 100 W mercury lamp attached to an epi-illumination filter block for blue, green, and ultraviolet light.
2.8 Speckle imaging systems
The technique utilizes the speckle pattern that is produced when biological tissues are illuminated by coherent light source, such as laser source. By acquiring raw speckle images of the blurred speckle pattern with a camera at specified exposure times and processing the raw speckle images using custom implemented codes, relative blood flow information can be obtained after the formulation of speckle flow maps. When this speckle pattern is time integrated using a camera with finite exposure times, the pattern becomes blurred and the degree of blurring is quantified by a term called speckle contrast. Computation of speckle flow maps was performed based on raw speckle images obtained from a camera exposure time of 20 ms and spatial correlation analysis. By analyzing speckle flow maps obtained by spatial contrast analysis with a 7 × 7 sliding window, the relative blood flow in the window chamber was averaged into a single mean value.
2.9 Histological staining
Samples were harvested 14 days after treatment. Specimens were fixed in 10% (v/v) buffered formaldehyde, dehydrated in a graded ethanol series and embedded in paraffin. Specimens were sliced into 4 μm-thick sections and stained with hematoxylin and eosin (H&E) to examine muscle degeneration and tissue inflammation.
2.10 Immunofluorescence staining
Indirect immunofluorescence staining was performed using a standard procedure. Briefly, tissues cryosectioned at 4 μm thickness were fixed with 4% paraformaldehyde, blocked with 5% BSA/PBS (1 h, 24°C), washed twice with PBS, treated with 0.1% Triton X-100/PBS for 1 min and washed extensively in PBS. The sections were stained with specific primary antibodies and fluorescent-conjugated secondary antibodies (Table S2) using a M.O.M kit according to the manufacturer's instructions (Vector Laboratories). The primary antibodies were anti-human: CD31, CD34, AFP, CK8/18, FAK, E-cadherin (all from Dako), and ALB (BD Biosciences). The cells were counterstained with DAPI (4,6-diamino-2-phenylindole dihydrochloride; Vector Laboratories). Negative control-mouse IgG (Dako) and –rabbit IgG (Dako) antibody were used as negative controls. Stained sections were viewed with a model DXM1200F fluorescence microscope (Nikon). Processed images were analyzed for fluorescence intensity using ImageJ software (NIH).
2.11 Enzyme-linked immunosorbent assay (ELISA) for angiogenic growth factor production
Angiogenic growth factor production in the spheroid was assayed with a commercially available ELISA kit (R&D Systems) according to the manufacturer's protocols. Concentrations are expressed as the amount of angiogenic growth factor per 104 cells at a given time. After measuring the optical density at 450 nm, the concentrations of FGF and HGF were determined by interpolation from a standard curve, and all data were expressed as nanograms per milliliter.
2.12 Western blot analysis
Samples were solubilized in lysis buffer (20 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 0.1% sodium dodecyl sulfate [SDS], 1 mM phenylmethylsulfonyl fluoride,1 µg/ml leupeptin, 2 µg/ml aprotinin) for 1 h at 4°C. Lysates were clarified by centrifugation at 15,000 g for 30 min at 4°C, diluted in Laemmli sample buffer containing 2% SDS and 5% (v/v) 2-mercaptoethanol, and heated for 5 min at 90°C. Proteins were separated by SDS polyacrylamide gel electrophoresis (PAGE) using 10% or 15% resolving gels followed by transfer to nitrocellulose membranes (Bio-Rad). The membranes were incubated with primary antibody for 1 h at room temperature. For detection, peroxidase-conjugated antimouse IgG or antirabbit IgG and enhanced chemiluminescence (Amersham Pharmacia Biotech) were used as described by the manufacturer. Membranes were scanned to create chemiluminescent images and quantified with an image analyzer (Kodak).
2.13 RNA extraction, complementary DNA (cDNA) synthesis, and quantitative
As a result of microarray, nine genes that are representatively increasing among genes related to liver development were selected for the polymerase chain reaction (PCR) test. Real-time PCR Total RNA was extracted from stem cells or homogenized tissues using illustra™ RNAspin mini kit (GE Healthcare) and the cDNA was generated from 2 mg of total RNA using the Super Script™ First-Strand Synthesis System (Invitrogen). Total RNA content was estimated using a NanoDrop 2000c (Thermo Fisher Scientific). Synthesized cDNAs were subjected to quantitative real-time PCR using the Takara SYBR premix Taq quantitative PCR system (Takara Bio Inc.) and the MyiQ2 real-time PCR machine (Bio-Rad). Primer information and reaction conditions used for our quantitative real-time PCR analysis are listed in Table S3. All quantitative real-time PCR procedures, including the design of primers, the validation of PCR conditions, and the quantification methods were performed according to the MIQE guidelines.
2.14 Microarray analysis
To characterize and compare the gene expression profiles of self-assembled human liver spheroid, we selected genes that were serially upregulated during both human liver development using a Whole Human Genome Agilent 4 × 44 K Oligonucleotide Microarray (Genomictree) according to the manufacturer's instructions. Briefly, total RNAs were extracted from self-assembled human liver spheroid, human liver tissues (Biochain Institute Inc.), or hASCs and subjected to microarray chip hybridization. To compare the gene expression profiles of self-assembled liver spheroid with healthy human liver tissues, signature genes for mature liver tissues were selected to perform a microarray analysis.
2.15 Statistical analyses
All the quantitative results were obtained from triplicate samples. Data were expressed as a mean ± SD. Statistical analysis was carried out using two-sample t test for comparing two groups of samples and one-way analysis of variance for three groups. A value of p < 0.05 was considered to be statistically significant.
3 RESULTS
3.1 Characterization of hASCs
Adherent cells obtained from human adipose tissue were expanded in vitro. The cells expressed human MSC markers CD29 (β1 integrin), CD90 (Thy-1), and CD105 (endoglin). However, the cells tested negative for human endothelial cell markers CD34, CD31, KDR (vascular endothelial growth factor [VEGF] receptor) and hematopoietic cell marker CD45 with immunofluorescence staining and flow cytometry analyses (Figure S1). Therefore, the expanded cells included a large population of hASCs without any endothelial cells.
3.2 Hepatic differentiation
After 12 days of culture in the hepatic differentiation medium (Figure S3a), the hASCs stained positive for albumin (ALB) and α-fetoprotein (AFP) (Figure S3b). Undifferentiated hASCs tested negative with ALB and AFP staining. ACSs were differentiated using sequential media formulations containing factors or ECMs that mimic the temporal expression observed during fetal liver development. After 12 days of differentiation, ASCs adopted a hepatic-like morphology (Figure S3b).
3.3 Formation of self-assembled liver spheroid
Cells (human ASC-derived hepatic cells, HUVECs, and human MSCs) were cultured on pre-solidified ×2 matrigel diluted with EGM in a 24-well plate (Figure 1a). The cells formed a floating spheroid via PBM 3 days after seeding (Figure 1b). The diameter of most PBM-spheroids ranged from 1.2 to 1.5 mm (Figure 1c). PBM-liver spheroids showed higher viability and aggregated more rapidly than liver spheroids (Figure 1d). Notably, although cells were plated in 2D conditions, human PBM-liver spheroid self-assembled into macroscopically visible 3D cell spheroids due to an inherent potential, up to 36 h after seeding.
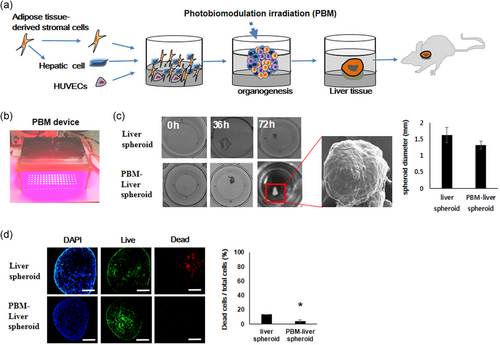
3.4 Intermediate phenotype
Focal adhesion kinase (FAK) and E‑cadherin staining were significantly increased in the liver spheroids treated with PBM compared with untreated control cells (Figure 2a). A stiffness- and FAK-dependent induction of cadherin contributed to the interaction between cellular matrix and intercellular adhesions. Apoptosis induced by a lack of cell-matrix interaction (anoikis), was prevented in hASCs cultured as spheroids. The PBM-liver spheroid showed well-preserved ECMs (Figure 2b).
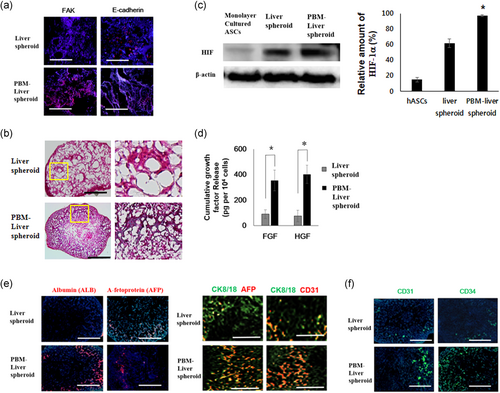
3.5 Production of growth factors by cells in PBM-spheroids
PBM-liver spheroid cultures showed a dramatic increase in the expression of hypoxia-induced survival factors, such as hypoxia-inducible factor (HIF)-1α, relative to cells in the monolayer culture (Figure 2c). Therefore, the PBM-liver spheroid appeared to be more adaptable and resistant to hypoxia compared with hASCs in monolayer cultures. HIF-1α is known to upregulate the expression of growth factors (de Sousa AP, 2013; Park IS, 2015). The PBM-liver spheroid showed a significant expression of HGF and FGF2. The expression of growth factors in PBM-liver spheroid was significantly greater than that of cells cultured in a liver spheroid (Figure 2d). Similar to liver, the human PBM-liver spheroid is composed of proliferating AFP-positive hepatoblasts as well as mesenchymal and endothelial progenitors (Figure 2e). The immunostained image also showed that the human albumin and ALB were expressed strongly in PBM-liver spheroid (Figure 2e). Immunofluorescent staining showed that significantly higher levels of CD31, CD34 were expressed by the human PBM-liver spheroid groups versus the liver spheroid group (Figure 2f). This finding indicated that the human PBM-liver spheroid was composed of cells with ECs phenotypes expressing CD31, CD34 (Figure 2f).
3.6 Angiogenic efficacy
To analyze the vascularization of liver tissue transplant using intravital fluorescence microscopy, tissues were implanted into dorsal skinfold chambers of mice (Figure 3a). Interestingly, the liver spheroid progressively lost its round shape during the course of the experiment and resulted in microvessel growth in the transplant (Figure 3b). Quantitative analyses of PBM-liver spheroid vascularization suggested that the liver spheroid induced a poor angiogenic host tissue response with the formation of only a few microvessels in their border zones and a complete lack of blood-perfused microvessels in the center throughout the 28-day observation period (Figure 3b). In contrast, the PBM-liver spheroid markedly promoted the ingrowth of microvessels into the implants, which were finally detected in the central surface areas of the transplant. Importantly, this effect was more pronounced in the PBM-liver spheroid, with a higher number of centrally perfused ROIs between Days 14 and 28 when compared with transplants seeded with liver spheroid. In addition, the density of the newly formed microvascular networks in the center of PBM-liver spheroid on Day 28 was significantly higher compared with the group carrying liver spheroid-seeded transplants (Figure 3c).
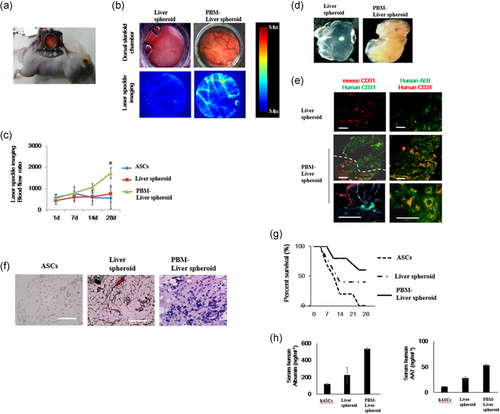
At the end of the in vivo experiments, we further analyzed the vascularization of the liver spheroid-seeded transplants via immunohistochemistry. In line with microscopic results, we found that the group carrying PBM-liver spheroid exhibited a higher microvessel density in the border and center zones (Figure 3e). The liver spheroid did not induce human microvessel response with the formation of only a few mouse microvessels in their border zones. We further assessed the fraction of CD31-positive microvessels in transplants, which were seeded with liver spheroid or PBM-liver spheroid. In both groups, we detected CD31-positive microvessels, which were primarily localized in the center of the implants. However, most of the microvessels in the border zones were also hCD31-positive, suggesting that during the implantation a number of microvessels were associated with the transplants and the surrounding granulation tissue at the implantation site. These hCD31-positive microvessels developed connections with the mouse CD31-positive microvessels in the host tissue. Moreover, we found that the PBM-liver spheroid transplant presented with a significantly higher proportion of microvessels in the center when compared with the non-PBM-liver spheroid transplant.
3.7 Functional characterization of human liver spheroid
Similar to human organs, the PBM-liver spheroid transplants consisted of hepatic cord-like structures characteristic of adult liver after 28 days, and cells expressing ALB (Figure 3f). Notably, the transplanted conventional human PBM-liver spheroids produced higher levels of albumin, suggesting the importance of 3D vascularized tissue formation for successful engraftment and maturation at ectopic sites. Transplantation of human PBM-liver spheroid improved the survival of mice after gancyclovir-induced liver failure compared with sham-operated mice, and mice transplanted with liver spheroids (Figure 3g). We analyzed the liver-specific functions of these liver spheroids based on albumin and ALB secretion. Quantitative analysis showed that the PBM-liver spheroid secreted about 2.5-fold more albumin and ALB than the liver spheroid (Figure 3h). Thus, we successfully generated vascularized and functional human liver via transplantation of human PBM-liver spheroid.
3.8 Expression profiles of mature liver signature genes
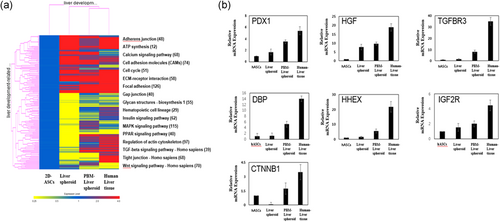
To further characterize the maturation of transplanted PBM-liver spheroid compared with the newly generated non-PBM-liver spheroid or human liver tissues, the expression profiles of mature liver signature genes were analyzed. The results showed that the expression profile of 28-day transplanted PBM-human liver was similar to the human liver than the newly assembled liver spheroid (Figure 4a). We performed a quantitative real-time PCR analysis to validate the expression of nine key markers as PDX1 (pancreatic and duodenal homeobox), HGF (hepatocyte growth factor), TGFBR3 (betaglycan), DBP (D-site of albumin promoter binding protein), HHEX (hematopoietically-expressed homeobox protein), IGF2R (insulin-like growth factor 2 receptor), CTNNB1 (β-catenin) of mature livers, newly generated human liver buds, 28-day transplanted PBM-liver spheroid, human livers, and hepatoma liver cells (Figure 4b). The expression of those genes in transplanted PBM-liver spheroid was similar to that of human livers, and much higher than in non-PBM-liver spheroids (Figure 4b). In conclusion, transplantation with self-assembled PBM-liver spheroid ameliorated liver injury via in vivo maturation.
4 DISCUSSION
We have demonstrated the successful generation of PBM-liver spheroids (Figure 1). Successful generation of uniform 3D hepatocyte liver spheroids has been reported using several approaches, including use of non-adherent surfaces (Bomo et al., 2016, Janani & Mandal, 2018). These liver spheroids were detached easily from the wells without the need for treatment with trypsin or collagenase due to the concave-morphology effect, without affecting cell viability. In contrast to traditional 2D cell cultures, liver spheroids are compatible with physiological conditions due to intensive cell–cell contacts and cell–matrix interactions (Rasheena et al., 2014). The formation of spheroids is affected by the strength of cell–matrix adhesion (Santini & Indovina, 2000). Moreover, PBM facilitates the migration of hASCs (Hou et al., 2008; Mvula et al., 2008; Peplow et al., 2011). In this study, within 3 days of culture on 24-well plates, the hASCs formed spheroids as a result of PBM irradiation (l-spheroids) (Figure 1). The size of PBM-liver spheroid decreased drastically after cell seeding, which might be due to tight cell–cell interactions (Figure 1). The role of cadherins or integrins that connect a cell with its environment has been the main focus of spheroid formation (Smyrek et al., 2019). A spheroid is an in vitro multicellular aggregate that provides a microenvironment similar to that of normal tissue in vivo (Park & Rhie, 2013). The strong homophilic interaction between E-cadherins is a major factor in the morphological transition from loose cellular aggregates to compact spheroids (Byers et al., 1995). The FAK also plays a role in cell adhesion, growth, and migration, especially by transmitting extracellular signals via integrins and modulating cell adhesion and migration via rearrangement of actin filaments and microtubules (Lange et al., 2013; Mitra & Schlaepfer, 2005).
Hypoxia mediates the angiogenic switch in tumor cell agglomerates larger than 200 µm in diameter (Bhang et al., 2011). The expression of HIF-1α was used to confirm hypoxia in spheroid cultures of hASCs (Figure 1). In this context, the hypoxic conditions in the core of liver spheroid facilitate the preconditioning of the cells within the liver spheroid, resulting in an increased resistance to hypoxia-induced cell death after implantation into a defective site (Park & Ahn, 2015). PBM increases the gene expression and release of several angiogenic growth factors, including FGF, VEGF, and HGF from stem cells (Hou et al., 2008; Mvula et al., 2008; Peplow et al., 2011). In this case, the induction does not depend on the oxygen tension, but occurs via activation of a different regulatory mechanism possibly mediated by mitogen-activated protein kinase and phosphatidylinositol 3-kinase/Akt signaling pathway (Hu et al., 2007). Conversely, in spheroid cultures, growth factors secreted from stem cells after 3D cell aggregation may be stored in the cell aggregate and stimulate endothelial differentiation of stem cells in an autocrine or paracrine manner (Park & Kim, 2013). PBM improves cellular metabolism and improves ECM synthesis including collagen by stem cells (Mitra & Schlaepfer, 2005).
In the present study, we used liver spheroid-seeded transplants or PBM-liver spheroids for implantation into dorsal skinfold chambers of wild-type animals (Figure 3a). The relative blood flow images of the liver tissue (Figure 3b) and quantitative analysis using laser speckle imaging are presented (Figure 3c). Laser speckle imaging is a noninvasive optical technique that allows full-field image acquisition without the need for an external contrast agent (Vaz et al., 2017). Cells such as red blood cells inside the blood vessels scatter the laser and produce a time-varying speckle pattern. In contrast to immunohistochemical staining, which detects all perfused and non-perfused microvessels in a histological section, intravital fluorescence microscopy facilitates selective in vivo visualization of functional RBC-perfused microvessels (Vaz et al., 2016). The perfused microvessels contribute to oxygen supply, and thereby enhance the survival of implants. Importantly, the PBM-liver spheroid may not only contribute to the vascularization of scaffolds by releasing growth factors, but also via differentiation into new microvessels under in vivo conditions (Figure 3). This approach has been used to analyze the origin of newly formed microvessels in the border and central zones of the implants via immunohistochemical detection of human CD31 and mouse CD31 (Figure 3e). In the group of PBM-liver spheroids, a high proportion of all microvessels within the center of the implants originate from human CD31-positive MSCs. These blood vessels also develop interconnections with the ingrowing mouse CD31-positive blood vessels from the surrounding host tissue (Figure 3e). Accordingly, our intravital microscopic results clearly demonstrate an improved functionality of the newly formed microvascular networks in PBM-liver spheroids when compared with liver spheroid-seeded transplant. This finding may be explained by the higher amounts of cytokines secreted by the PBM-liver spheroids when compared with liver spheroid-seeded transplants (Figure 3g). Both types of liver spheroid secreted albumin and alpha-1-antitrypsin (AAT) continuously; however, the PBM-liver spheroid secreted a greater quantity of albumin than the liver spheroid (Figure 3h). We found that PBM-liver spheroid generally functioned better than the liver spheroid. These results indicate that hepatic function and phenotype were enhanced in our 3D model.
Thus, the 3D configuration of the cells with increased cell-to-cell interactions simulates the in vivo environment of a real tissue effectively when compared with conventional monocultures. The PBM-liver spheroid dramatically enhanced the viability of hepatocytes, and liver spheroid formation with a uniform shape and hepatic function. In addition, increased resistance to hypoxia and apoptotic cell death are other advantages.
5 CONCLUSIONS
In summary, we found that hASCs in the PBM-liver spheroid facilitate the formation of compact hepatic liver spheroids, enhance the viability of hepatocytes even during partial hepatectomy, and act like hepatocytes. After implantation, they strongly stimulate angiogenesis. The PBM-liver spheroids may represent useful sources of human liver transplantation and human hepatocyte cell therapy. Furthermore, they can be used to design 3D human liver models and to facilitate studies of human liver physiology and pathophysiology, as well as contribute to the recovery of damaged liver function and the development of bioartificial liver systems, overcoming cell shortage problems. The PBM-liver spheroids may represent a new therapeutic modality and cell transplantation paradigm in the management of patients with end-stage or acute liver failure.
ACKNOWLEDGMENTS
This study was supported by the National Research Foundation (NRF)and the Ministry of SMEs and Startupsfunded by the Korean government (NRF-2020R1I1A1A01058248, SMEs-S2957289).
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
ETHICS STATEMENT
Experiments involving mice were performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health (NIH Publication No. 85-23, revised 1996). All aspects of the animal care and experimental protocols were approved by the Dankook University Committee on Animal Care. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
AUTHOR CONTRIBUTIONS
Conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing: In-Su Park.




