Ten-year update of the international registry on cytokine-induced killer cells in cancer immunotherapy
Abstract
Cytokine-induced killer (CIK) cells represent an exceptional T-cell population uniting a T cell and natural killer cell-like phenotype in their terminally differentiated CD3+CD56+ subset, which features non-MHC-restricted tumor-killing activity. CIK cells have provided encouraging results in initial clinical studies and revealed synergistic antitumor effects when combined with standard therapeutic procedures. We established the international registry on CIK cells (IRCC) to collect and evaluate clinical trials for the treatment of cancer patients in 2010. Moreover, our registry set new standards on the reporting of results from clinical trials using CIK cells. In the present update, a total of 106 clinical trials including 10,225 patients were enrolled in IRCC, of which 4,889 patients in over 30 distinct tumor entities were treated with CIK cells alone or in combination with conventional or novel therapies. Significantly improved median progression-free survival and overall survival were shown in 27 trials, and 9 trials reported a significantly increased 5-year survival rate. Mild adverse effects and graft-versus-host diseases were also observed in the studies. Recently, more efforts have been put into the improvement of antitumoral efficacy by CIK cells including the administration of immune checkpoint inhibitors and modification with chimeric antigen receptorc. The minimal toxicity and multiple improvements on their tumor-killing activity both make CIK cells a favorable therapeutic tool in the clinical practice of cancer immunotherapy.
1 INTRODUCTION
In addition to the conventional therapeutic strategies including surgery, chemotherapy, or radiation, anticancer immunotherapies have been approved as an innovative and effective treatment option for patients with cancer especially in the advanced or recurrence stage. The principal aim of immunotherapy is to promote the activation of body's intrinsic immune response to inhibit tumor growth or achieve tumor eradication (M. Wang, Yin, Wang, & Wang, 2014). Adoptive cell-based immunotherapy, as one of the most encouraging immunotherapies, has demonstrated clinical efficacy and limited side effects in various cancer types (Hinrichs & Rosenberg, 2014). Adoptive cell-based immunotherapy, also called adoptive cell transfer or cellular adoptive immunotherapy, is a treatment strategy in that immune cells from patients or donors are expanded, stimulated or modified ex vivo to achieve large numbers and high antitumor cytotoxicity and then transferred back to patients (Cappuzzello, Sommaggio, Zanovello, & Rosato, 2017). It mainly consists of a T-cell-based therapy like tumor-infiltrating lymphocytes (TILs), engineered T-cell receptor (TCR) or chimeric antigen receptor (CAR)-modified T cells therapy, natural killer (NK) cell- and natural killer T (NKT) cell-based therapy and so forth (Rohaan, Wilgenhof, & Haanen, 2019; Shahrabi, Zayeri, Ansari, Hadad, & Rajaei, 2019). Cytokine-induced killer (CIK) cells, also known as natural killer like T cells, is a promising adoptive cell-based immunotherapy and have indicated encouraging responses preclinically and clinically.
CIK cells are a cluster of heterogeneous cells comprising CD3+CD56− T cells, CD3−CD56+ NK cells, and CD3+CD56+ NKT cells. Schmidt-Wolf, et al. first published the protocol for CIK cells generation in 1991 and their remarkable antitumor activity and minimal toxicity were also reported simultaneously (Schmidt-Wolf, Negrin, Kiem, Blume, & Weissman, 1991). CIK cells can be easily amplified ex vivo from peripheral blood mononuclear cells (PBMCs) with the sequential addition of interferon-γ (IFN-γ) 1000 IU/ml on Day 0, and 50 ng/ml monoclonal antibody against CD3 (anti-CD3 mAb) and 100 IU/ml interleukin-1β (IL-1β) and 600 IU/ml interleukin-2 (IL-2) on the next day (Gao et al., 2017; Schmidt-Wolf et al., 1991). IL-2 and fresh culture medium need to be supplemented regularly until after 2-3 weeks. IFN-γ priming 24 hr before the mitogenic stimulation of anti-CD3 mAb and IL-2 is crucial for the cytotoxic activity of CIK cells, as IFN-γ increases the activation of IL-2-responding cells and activates monocytes to regulate the immunomodulatory factor IL-12. Studies also demonstrated that CIK cells stimulated by or transfected with IL-6, IL-7, IL-12, IL-15, IL-21 or thymoglobulin manifested phenotype alteration, proliferation improvement and cytotoxicity enhancement (Bonanno et al., 2010; G. Lin et al., 2012; Rettinger et al., 2012; Zoll et al., 2000).
It is reported that CD3+ cells accounted for > 90% of CIK cells and the amount of the main effector cells CD3+CD56+ cells ranged between 7.6% and 65% with a median of 35.3% (Y. Linn, Lau, & Hui, 2002). It is interesting to find that the proportion of CD3+CD8+ subset increased faster than its CD3+CD56+ counterpart after IL-2 and anti-CD3 mAb stimulation as the latter kept a low proliferation until after Day 7. The CD3+CD56+ subset exerts its anticancer activity in a major histocompatibility complex (MHC)-unrestricted manner with a higher portion of CD8+ cells and granzyme content. This double-positive population shows a more terminally differentiated effector phenotype CD27+CD28− or CD27−CD28− than its CD3+CD56− precursors (Y. C. Linn et al., 2009).
Besides the polyclonal TCR repertoire, CIK cells also express NK-like structures, including natural killer group 2 member D (NKG2D), DNAX accessory molecule-1 (DNAM-1) and low densities of NKp30, but lack expression of NK-specific activating receptors (NKp44, NKp46) and inhibitory receptors (KIR2DL1, KIR2DL2, KIR3DL1, NKG2A, CD94; Franceschetti et al., 2009). A transcriptomic study found that CD8 and Lck kinase, a member of the Src kinase family, contributed largely to the cytotoxicity of CIK cells (Meng et al., 2018). When mature CIK cells and the ones in the early stage are compared, the expression of tumor cytotoxic molecules, including perforin, granzyme, Fas ligand (FasL) and CD40 ligand (CD40L) expectedly increased, while immune checkpoints PD-1, CD28, CD137, and VSIR were downregulated with the upregulation of LAG3, CTLA4, and TIM-3 (Meng et al., 2018).
Researchers are still exploring the decisive molecules and pathways involved in CIK cell cytotoxicity. One key mechanism is the engagement of NKG2D. NKG2D is a member of the c-type lectin-activating receptor family that is originally identified in NK cells but also expressed by activated CD8+ T cells, γδT cells, and NK1.1+ T cells (Jamieson et al., 2002). The interaction between NKG2D and its ligand MHC class I-related molecules A and B (MIC A/B) and members of the UL16-binding protein family (ULBP1–4), whose expression are relatively restricted to malignancies, accounts for the majority of the MHC-independent cytotoxicity of CIK cells (Verneris, Karami, Baker, Jayaswal, & Negrin, 2004). Besides NKG2D, CIK cells can also exert their antitumor activity in a TCR-mediated lytic manner, which is the so-called “dual-functional capability” (Pievani et al., 2011). Both pathways above could be blocked concurrently by the antibodies against lymphocyte function-associated antigen (LFA-1) and DNAM-1. Moreover, NKp30, which is usually expressed on NK cells but also on CIK cells with a low density, was indicated to take part in CIK cell-mediated tumor eradication. Some groups also reported that the CD3+CD56+CD16+ subset demonstrated a dramatic antitumor response after therapeutic monoclonal antibodies, such as trastuzumab or cetuximab administration via antibody-dependent cell-mediated cytotoxicity (ADCC) with the expression of FcyRIII receptor (CD16) in a donor-dependent manner (Cappuzzello, Tosi, Zanovello, Sommaggio, & Rosato, 2016).
Novel combinational immunotherapies based on CIK cells are emerging so as to achieve a higher efficacy and specific recognition against malignancies. CIK cells in combination with dendritic cells have been shown to improve antitumor effectiveness for cancer preclinically and clinically as DCs possess high ability of antigen recognition and presentation (Anguille et al., 2015). For the purpose of reversing the inhibition of T lymphocytes signaling in the tumor microenvironment, blockades of immune checkpoints including PD-1, PD-L1, KIR, LAG-3, or TIM-3 were adopted and suggested to increase the antitumor potency of CIK cells against myeloid leukemia, gastrointestinal cancer and advanced non-small cell lung cancer (NSCLC; Poh & Linn, 2016). Apart from monoclonal antibodies against CD20 (rituximab or GA101) and CD30 (Brentuximab Vedotin), some studies revealed that bispecific antibodies (BsAbs) in combination with CIK cells exerted augmented antitumor efficacy (Fan, Wang, Hao, & Li, 2015). With the ability of recognizing two distinctive epitopes, BsAbs redirect CIK cells to tumor cells and strengthen their cytotoxicity in B cell lymphoma by CD19/CD5 BsAb, gastric cancer by EGFR/CD3 BsAb or CD133high cancer cells by CD3/CD133 BsAb (Tita-Nwa et al., 2007). With the development of gene-editing technology and clinical success achieved by second-generation CAR T cells targeting CD19, genetically engineered CIK cells to express CAR targeting tumor-associated antigens (TAAs) have become one of the favorable immunotherapeutic strategies (June, O'Connor, Kawalekar, Ghassemi, & Milone, 2018). CIK cells with engineered CAR targeting TAAs including carcinoembryonic antigen (CEA), CD123, and CD33 have demonstrated exciting results with significantly elevated killing activity of the modified CIK cells (Schlimper, Hombach, Abken, & Schmidt-Wolf, 2012).
Since the very first report describing the generation protocol and promising antitumor efficacy on B cell lymphoma by CIK cells in 1991, rapidly updated technologies and expanding clinical practice have been increasing our understanding about how to optimize the therapeutic regimen based on CIK cells to gain durable responses and ideal treatment effectiveness in patients (Schmidt-Wolf et al., 1991). To collect exhaustive information of clinical trials worldwide and standardize the CIK cell-based treatment system, we established the international registry on CIK cells (IRCC) in cooperation with the Stanford University School of Medicine in 2010 and have published two reports to provide an overview of the CIK cell therapy state (Hontscha, Borck, Zhou, Messmer, & Schmidt-Wolf, 2011; Schmeel, Schmeel, Coch, & Schmidt-Wolf, 2015). Quantities of clinical information of CIK cells have been included in our database and new trials can also be registered on our homepage: https://www.ciobonn.de/cik/.
In the present review, we update our former reports and provide a synopsis of the current clinical trials based on CIK cells. We also discussed the new strategies of combination therapies and offer insight into the future directions of CIK cell-based cancer immunotherapy.
2 MATERIALS AND METHODS
2.1 Search strategy and eligibility criteria
A systematic literature search was conducted on online databases including PubMed, Web of Science Core Collection, WHO International Clinical Trials Registry Platform (ICTRP), ClinicalTrials.gov, and online proceedings of the American Society of Clinical Oncology (ASCO) Annual Meetings and the European Cancer Conference (ECCO). We searched articles before February 2020 with the following keywords: “cytokine-induced killer cells,” “CIK cells,” “clinical trials,” “cancer,” “tumor,” and “carcinoma.” Only papers with the full text published in English were reviewed.
We included clinical studies that involved CIK cells for the treatment of malignancies alone or in combination with conventional and other immunotherapeutic strategies. We investigated the feasibility and efficacy of CIK cell therapy by survival data like overall survival (OS), progression-free survival (PFS), and so forth. Studies only focusing on ex vivo or animal studies were excluded.
2.2 Data collection
The following information in the included clinical trials was gathered: general information including the authors’ names, addresses, e-mail, article title, journal and the year of publication; participants’ information including the number of enrolled subjects (total and individual groups), baseline characteristics, median- and age range, gender, diagnostic criteria, tumor entity, stage of the disease, inclusion as well as exclusion criteria; treatment information including phase, cell type (autologous or allogeneic), therapeutic regime, dose, infusion cycles, storage conditions (fresh or frozen), clinical outcomes, immunologic responses, hematologic and non-hematologic toxicity, and follow-up (the time period of follow-up and duration of responses).
2.3 Evaluation of studies
We analyzed the clinical data with the above indices. The primary outcome measures are OS, PFS, complete response, partial response (PR), and overall response rate (ORR). Immunologic responses, quality of life (QoL) assessment and other indices such as stable disease, and progressive disease (PD) were also taken fully into account.
3 RESULTS
In this paper, we investigated the considerable clinical information from the year of 1999–2019 with a total of 106 clinical trials and mainly kept our attention on the clinical responses of the integrated therapies with CIK cells in recent 5 years.
3.1 Patient characteristics
A total of 10,225 patients were enrolled in the estimated clinical trials, of which 4,889 patients representing 47.8% of the total were treated with CIK cell therapy alone or in combination with other conventional or novel therapeutic strategies. The enrollment criteria commonly used in the clinical trials are listed in Table 1. In the evaluated 106 clinical trials, specific gender information of the patients was given in 98 studies with a male percentage of 68.2% and a female percentage of 31.8%. A total of 69 studies indicated the extent of patients’ age, which ranged from 19 to 93 with 18–80 as the inclusion criteria in most studies. It is noteworthy that six studies only included elderly patients with the age from 57 to 93 (Y. Liu et al., 2016; Lu et al., 2012; Y. Wang, Bo, et al., 2013; Z. Wang et al., 2018; B. Yang et al., 2012; X. Zhou et al., 2013). Besides this, more than 30 kinds of cancers based on CIK cell therapy were included in the enrolled studies and clinical information from part of the prominent studies is listed in Table 2. The tumor entities investigated in most studies were lung cancer, hepatocellular carcinoma, gastric cancer, renal cell carcinoma and lymphoma, accounting for 26.4%, 18.9%, 16.0%, 15.1% and 15.1% of the total, respectively.
| Inclusion criteria | Exclusion criteria |
|---|---|
| Pathologically confirmed relapse or progression of disease | Immunosuppressive therapy |
| No active GvHD | Pre-existing autoimmune diseases |
| Karnofsky performance score of >40 | Decompensated heart insufficiency |
| Eastern Cooperative Oncology Group performance status (ECOG-PS) of 0–2 | Ventricular rhythm disorders |
| Child-pugh class A or B | Severe coronary artery diseases |
| No metastases/metastatic stage (depending on study conditions) | Active uncontrolled acute GvHD |
| Solitary tumor | Evidence of another active malignancy |
| No preoperative CIK cell transfusion | Life expectancy <3 months |
| Maximal cytoreductive surgery as the initial treatment | Chronic infection disease or concomitant serious ongoing infection |
| Serum creatinine of <2 mg/dl | Alcohol abuse |
| Direct bilirubin of <3 mg/dl | Drug addiction |
| Transaminases <3 times the upper limit or normal | Severe psychiatric disease |
| Leukocyte count >3,000/μl | Pregnant or lactating females |
| Platelet count >100,000/μl | Refused to participate |
| 18–80-year old |
- Abbreviations: CIK, cytokine-induced killer; GvHD, graft-versus-host diseases.
| Tumor entity | Reference | Phase | Treatment regime | Enrolled patients in Total (n) | Patients with CIK therapy (n) | Type of CIK cells | Median PFS/month (CIK vs. control) | Median OS/month (CIK vs. control) |
|---|---|---|---|---|---|---|---|---|
| Lung cancer | Wu, Jiang, Shi, and Xu (2008) | Prospective | CIK + chemotherapy | 59 | 29 | Autologous | Longer* | 15.0 vs. 11.0* |
| Niu et al. (2011) | – | CB-CIK + chemotherapy | 8 | 8 | Allogeneic | 3.5 vs. 2.0* | 11.7 vs. 7.5* | |
| R. Li et al. (2012) | II | CIK + chemotherapy | 174 | 87 | Autologous | 13 vs. 6* | 24 vs.10* | |
| Jin et al. (2014) | – | CIK | 943 | 411 | Autologous | – | 48 vs. 36 | |
| J. Zhang et al. (2015) | Retrospective | CIK + chemotherapy | 120 | 60 | Autologous | 24 vs. 14* | 72 vs. 44* | |
| H. Luo et al. (2016) | Retrospective | CIK | 140 | 70 | Autologous | 6 vs. 4* | 28 vs. 22 | |
| L. Zhang, Xu, et al. (2016) | – | DC-CIK + thoracic radiotherapy | 82 | 21 | Autologous | 11 vs. 7.8* | 13.3 vs. 15.3 | |
| Gu et al. (2017) | – | CIK + conventional therapya | 64 | 32 | Autologous | 15.5 vs. 6.5* | Longer* | |
| Huang et al. (2017) | Retrospective | DC-CIK + chemotherapy | 44 | 22 | Autologous | 8 vs. 4* | 13 vs. 11 | |
| (Gu et al. (2017) | – | CIK + conventional therapya | 64 | 32 | Autologous | 15.5 vs. 6.5* | Longer* | |
| D. Chen, Sha, et al. (2018) | – | CB-CIK + chemotherapy | 134 | 68 | Allogeneic | – | 38 vs. 30* | |
| L. Zhang et al. (2019) | II | Group 1: Livin pulsed-DC-CIK | 79 | Group 1: 13 | Autologous | Group 1: 6.5 vs. 4.6* | Group 1: 9.7 vs. 13.7 | |
| Group 2: MUC-1 pulsed-DC-CIK | Group 2: 27 | Group 2: 7.0 vs. 4.6* | roup 2: 13.3 vs. 13.7 | |||||
| Hepatocellular carcinoma | Hao et al. (2010) | – | CIK + transcatheter arterial chemoembolization (TACE) | 146 | 72 | Autologous | – | 31 vs. 10* |
| Qiu et al. (2011) | – | α1,3- galactosyl epitope-pulsed DC-CIK | 18 | 9 | Autologous | – | 17.1 vs. 10.1* | |
| J. Zhang et al. (2018) | – | DC-CIK + postoperative transcatheter arterial chemoembolization (POTACE) | 52 | 20 | Autologous | – | 36.8 vs. 28.7 | |
| Z. Zhou, Qin, et al. (2019) | Retrospective | DC-CIK + sorafenib | 71 | 35 | – | – | 18.6 vs. 13.8* | |
| Pancreatic cancer | M. Wang, Shi, et al. (2013) | Prospective | CIK + S-1 (Tegafur, Gimeracil, and Oteracil Potassium Capsules) | 58 | 28 | – | 2.9 vs. 2.5 | 6.6 vs. 6.1* |
| Z. Wang, Liu, et al. (2016) | Retrospective | CIK | 82 | 25 | Autologous | – | 13.6 vs. 6.6* | |
| N. Jiang et al. (2017) | Prospective | Group 1: DC-CIK + S-1 | 47 | Group 1: 25 | Autologous | Group 1: 4.5 vs. 1.4* | Group 1: 7.1 vs. 1.7* | |
| Group 2: DC-CIK | Group 2: 11 | Group 2: 2.8 vs. 1.4 | Group 2: 4.3 vs. 1.7 | |||||
| Colorectal cancer | J. Zhang et al. (2014) | – | CIK + chemotherapy | 60 | 30 | Autologous | 25.8 vs. 12* | 41.3 vs. 30.8* |
| T. Lin, Song, Chuo, Zhang, and Zhao (2016) | Prospective | DC-CIK + chemotherapy | 255 | 134 | Autologous | 8.8 vs. 5.8 | 14.7 vs. 10.8 | |
| H. Zhao et al. (2016) | II | CIK + chemotherapy | 122 | 61 | Autologous | 16 vs. 10 | 36 vs. 16* | |
| Xie et al. (2017) | Retrospective | DC-CIK + first-line treatment (surgery + postoperative FOLFIRI chemotherapy ± radiotherapy) | 142 | 71 | Autologous | - | 32 vs. 17* | |
| Gastric cancer | Shi et al. (2012) | – | CIK + chemotherapy | 151 | 74 | Autologous | 40.4 vs. 34.1* | 48.1 vs. 42.1* |
| H. Liu, Song, Yang, and Zhang (2013) | – | CIK + chemotherapy | 98 | 51 | Autologous | – | 21 vs. 29 | |
| H. Zhao et al. (2013) | Retrospective | CIK + chemotherapy | 165 | 53 | Autologous | 36 vs. 23* | 96 vs. 32* | |
| Nasopharyngeal carcinoma | Y. Li et al. (2015) | Retrospective | CIK + chemotherapy | 222 | 112 | Autologous | 21 vs. 15* | 32 vs. 23* |
| J.-J. Zhao et al. (2018) | Retrospective | CIK + conventional therapya | 170 | 85 | Autologous | 76.6 vs. 61.3* | 81.2 vs. 68.6* | |
| Glioblastoma | Kong et al. (2017) | III | CIK + temozolomide (TMZ) | 180 | 91 | Autologous | 8.1 vs. 5.4* | 22.47 vs. 16.68 |
| Melanoma | H. Li et al. (2017) | – | CIK + conventional therapya | 104 | 55 | Autologous | – | 40 vs. 23* |
| Renal cell carcinoma | X. Zhao et al. (2015) | – | DC-CIK | 122 | 61 | Autologous | Longer* | 28 vs. 11* |
| Zhan et al. (2012) | – | DC-CIK | 137 | 46 | Autologous | – | Longer* | |
| L. Liu et al. (2012) | – | CIK | 148 | 74 | Autologous | 12 vs. 8* | 46 vs. 19* | |
| Y. Zhang et al. (2013) | – | CIK + IL-2 | 20 | 10 | Autologous | 32.2 vs. 21.6* | 35 vs. 33.6 | |
| Ovarian cancer | J. Liu et al. (2014) | – | CIK + chemotherapy | 92 | 46 | Autologous | 37.7 vs. 22.2* | 61.5 vs. 55.9 |
| Y. Zhou, Chen, et al. (2019) | Retrospective | CIK | 646 | 72 | Autologous | 41.6 vs. 26.1 | 63.6 vs. 39.6* | |
| Breast cancer | Ren et al. (2013) | – | DC-CIK + chemotherapy | 166 | 87 | Autologous | 10.2 vs. 3.7* | 33.1 vs. 15.2* |
- Abbreviations: CB-CIK, cord blood-derived cytokine-induced killer cells; CR, complete response; DC-CIK, dendritic cell-activated cytokine-induced killer cells; FPS, progression-free survival; OS, overall survival; PR, partial response.
- a Conventional therapy: surgery, chemotherapy, radiotherapy and biological therapy.
- * p < .05.
3.2 Cells, infusions, and expansion
In the evaluated studies, the majority adopted autologous CIK cells in a total of 85 studies, equal to 4,039 patients, while 8 studies used allogeneic CIK cells and 5 studies used cord blood-derived CIK (CB-CIK) cells for infusion.
The CIK cell dose and infusion number varied between different studies due to the distinct therapeutic regimes and patients’ health conditions. More detailed information of the number of CIK cells per infusion was achieved in 54 studies with a wide range of 7.9 × 108 to 7.9 × 1010. In some studies, the cell dose per infusion was calculated according to the weight of the patients and an escalating dose was adopted according to the study design. In 37 studies with DC-CIK cell treatment, the number of CIK cells used per fusion varied from 7.9 × 108 to 2.2 × 1010 while the number of dendritic cells in each infusion ranged from 2 × 106 to 4.2 × 109. The number of infusions also varied according to the treatment efficiency or adverse effects. Ten studies reported that more cycles of CIK cell infusion were significantly related to the prolonged OS, PFS, or the decreasing risk of death (F. Chen et al., 2017; Gu et al., 2017; J.-T. Jiang et al., 2010; R. Li et al., 2012; Pan et al., 2013; D. Wang, Zhang, et al., 2014; J. Zhang et al., 2015; H. Zhao et al., 2016; Y. Zhao et al., 2019; Zhong, Han, & Zhong, 2014).
The common qualified CIK cell criteria for infusion was the viability of CIK cells exceeded 90%-95% with CD3+ cells over 70–80%, CD3+CD8+ cells over 60–80% and CD3+CD56+cells over 20–30%, respectively; for dendritic cells, the proportion of CD80+ and CD86+ cells reached more than 80%; all products were free of bacterial, fungal or mycoplasma contamination, and contained < 5 Eu endotoxin.
When comparing PBMCs to CIK cells on the last culture day, the number of subsets varied greatly depending on the individual. CD3+ cells increased from over 1-fold to 150-fold, and CD3+CD56+cells expanded from 20-fold to even more than 1400-fold. A study also demonstrated that after the 14-day culture, the expression of the inhibitory receptor LAG-3 by CIK cells was upregulated on the CD3+CD8+ and CD3+CD56+ cells, and the expression of PD-L1 on CD3+CD4+ and Tim-3 on CD3+CD8+ cells were also elevated significantly (Huang et al., 2017).
3.3 Clinical outcome, therapeutic regime, and quality of life
In the enrolled studies based on CIK cell immunotherapy, significantly improved mPFS after treatment was reported in a total of 27 studies including 1,396 patients in the treatment groups and 1,512 patients in the control groups, while significantly improved median overall survival (mOS) was reported also in 27 studies including 1,719 patients in the treatment groups and 2,585 patients in the control groups. According to the detailed information of clinical response given in 31 trials, the ORR was 340/897 based on these studies. Besides this, 10 studies reported a significantly increased 1-year survival rate, and 9 studies reported a significantly increased 5-year survival rate.
CIK cell immunotherapy has been applied in the treatment of patients in a variety of tumor entities. To be specific, a multicenter, Phase III trial on 230 patients with hepatocellular carcinoma indicated a prolonged recurrence-free survival of 44 months in the CIK group and 30 months in the control group (Lee et al., 2015). In a total of 122 metastatic colorectal cancer patients, 61 patients in the CIK cell therapy group showed significantly increased mOS of 36 months compared to 16 months in the control group and also remarkably prolonged 3-year PFS and OS (H. Zhao et al., 2016). The effect of the combination of CIK cells and chemotherapy on NSCLC after surgery was investigated in a study with 68 patients in each group. The mOS of patients that received autologous CIK cell immunotherapy was significantly prolonged by 8 months with a significantly increased 3-year OS rate (D. Chen, Sha, et al., 2018). A study on 82 patients with advanced pancreatic cancer demonstrated significantly increased mOS, disease control rate, and 6-, 12-, 18-month OS rate (Z. Wang, Liu, et al., 2016). Another study including 646 postoperative epithelial ovarian cancer patients showed a significantly higher OS rate and a favorable PFS in the CIK cell plus chemotherapy group (Y. Zhou, Chen, et al., 2019). Moreover, a recent Phase II study targeting myeloid neoplasms after nonmyeloablative allogeneic transplantation implied that one-time CIK infusion did not increase the rate of full donor chimerism (FDC) by day + 90, but the favorable safety profile of CIK cells made it possible to perform further strategies such as repeat dosing or genetic modification (Narayan et al., 2019).
Besides the 63 studies that adopted CIK cell therapy as an adjuvant approach in addition to standard therapies, other studies used CIK cells with improved clinical manufacturing, pulsed with dendritic cells, or in combination with novel treatment methods. Two studies used RetroNectin (Recombinant Human Fibronectin CH-296) in CIK cell culture to promote cell proliferation and enhance the cytotoxicity. RetroNectin is used to enhance lentiviral-mediated gene transfer and has been shown to improve cell conglutination and stimulate T-cell proliferation with the presence of T-cell receptor (TCR)-stimulating signals. 16 patients receiving CH-296 pulsing CIK cells experienced relief of clinical symptoms with the mOS of 16.95 ± 6.10 months (Yu et al., 2011). To enhance the tumor antigen specificity of dendritic cells, Livin peptide-loaded DC-CIK cells was used on advanced NSCLC (L. Zhang et al., 2019). Livin, as a member of inhibitors of apoptosis protein (IAP), inhibits apoptosis and is highly expressed in various tumor tissues. The group treated with Livin peptide-loaded DC-CIK cells showed a longer mPFS by 1.9 months and a higher ORR by 18%, significantly. The tyrosine kinase inhibitors sunitinib and sorafenib were also applied in two CIK cell-based studies (Mai et al., 2018; Z. Zhou, Qin, Weng, & Ni, 2019). In the study targeting advanced hepatocellular carcinoma, the combination of sorafenib and DC-CIK cells decreased the alanine transaminase and total bilirubin levels and improved the clinical benefit rate (88.6% vs. 41.9%) and mOS (18.6 vs. 13.8) statistically when compared with the control group. Notably, a Phase I study evaluated the safety and clinical activity of DC-CIK cells in combination with a PD-1 blockade pembrolizumab in hepatocellular carcinoma, renal cell carcinoma and other solid tumors (C.-L. Chen, Pan, et al., 2018). Even though controllable grade 3 or 4 toxicities were observed in two patients, the antitumor activity was encouraging with a disease control rate of 64.5%, mOS of 270 days, and mPFS of 162 days.
Clinical studies paid more attention to the patients’ quality of life after treatment. Ten studies calculated the Karnofsky score (KPS) to quantify patients' general well-being and quality of life and 8 of them indicated higher KPS after CIK cell therapy (N. Li et al., 2019; H. Luo et al., 2016; Y. Liu et al., 2016; Lu et al., 2012; Qiu et al., 2011; H. Wang, Cui, Wang, Zhao, & Sun, 2016; Yan et al., 2015; Yu et al., 2011). Patients in other studies also achieved an improvement of the general condition in different degrees including better appetite, improved sleep, weight gain, pain relief, and so forth.
3.4 Immunologic response
Patients’ immune state before and after CIK cell therapy was detected by phenotype analysis in most of the studies. A significantly elevated number of CD3+CD56+, CD3+CD8+ cells or CD4+/CD8+ ratio in peripheral blood after CIK cell infusion was detected in 24 studies, while decreased CD4+CD25+CD127dim Treg cells was shown in 5 studies. Moreover, increased Th1 cytokines including IFN-γ, TNF-α, and IL-2 or release of granzyme-B was observed in 13 studies. The serum level of AFP after CIK cell therapy decreased in 4 studies and that of CA199 and CEA decreased in 5 studies. It is also indicated that the level of other tumor markers such as the level of CA125, CA724, AFP, β2-mikroglobulin, and LDH were ameliorated in some patients treated with CIK cell therapy.
3.5 Treatment toxicity and adverse effects
The common side effects were mainly grade I–II toxicities like fever, chills, fatigue, headache, and skin rash (Table 3). Low-grade fever occurring in 65% of the studies was the most common one which usually ranged from 37.5 to 40℃ and recovered without any treatment. Grade III and IV toxicities including leukopenia, neutropenia, or thrombocytopenia were considerably rare during CIK cell treatment and tended to be lower in the CIK group compared to the control group (Cui et al., 2015).
| Adverse reaction | Relative frequency (%) |
|---|---|
| Low-grade fever | 65.1 |
| Fatigue | 22.6 |
| Chills | 21.7 |
| Nausea and vomiting | 16.0 |
| Rash | 11.3 |
| Headache | 10.4 |
| Diarrhea | 9.4 |
| Anorexia | 7.5 |
| Arrhythmia and chest distress | 5.7 |
| Myalgia | 4.7 |
| Mild hypotension | 2.8 |
| Arthralgia | 2.8 |
| Insomnia | 2.8 |
Nine studies reported acute or chronic graft-versus-host diseases (GvHD), which were related to infusion of allogeneic CIK cells. A total of 17 patients developed grade I-II GvHD and 11 patients developed grade III-IV GvHD after CIK cell infusion. Most of them were controlled by the administration of immunosuppressive corticosteroids (Introna et al., 2007, 2017, 2010; Laport et al., 2011; Y. Linn et al., 2012; Narayan et al., 2019; Niu et al., 2011; Pfeffermann et al., 2018; Y. Luo et al., 2016).
4 DISCUSSION
CIK cells, as a remarkable tool of adoptive cell-based immunotherapy, exert MHC-unrestricted cytotoxicity against both hematological and solid malignancies without treatment-related severe adverse effects. After sequential application of cytokines like IFN-γ, anti-CD3 antibody, and IL-2 into PBMCs obtained from normal donors or patients, CIK cells are achieved with high availability and ease of expansion, which is another reason that CIK cells translate from bench to bedside.
Since our previous two reports in years 2010 and 2014, there has been an increasing number of studies evaluating the clinical efficacy of various CIK cell-based therapeutic regimes. CIK cell treatment not only increases short-term antitumor efficiency but also improves the long-term clinical benefits. There are nine studies reviewed above reporting a significantly increased 5-year survival rate and a quarter of the studies demonstrate improved mFPS and mOS after CIK cell therapy. Based on the advantages, several Phase III and Phase IV clinical trials have recently been performed to get a more precise evaluation of their safety and efficacy. In a multi-center, Phase III trial on a total of 180 patients with newly diagnosed glioblastoma, autologous CIK cells infusion combined with standard radiotherapy-temozolomide chemoradiotherapy significantly prolonged the mPFS of 8.1 months in the CIK group compared with 5.8 months in the control group. Grade 3 or higher adverse events, health-related quality of life and performance status did not differ between the two groups (Kong et al., 2017). In another Phase IV study targeting hepatocellular carcinoma, with a median follow-up duration of 28.0 months, the CIK immunotherapy group also indicated significantly longer recurrence-free survival than the control group without severe Grade 3 or 4 adverse events (Yoon et al., 2019). Besides clinical outcomes, more emphasis was laid on the QoL of patients with cancer. Several studies indicated that CIK cells did not induce additional deterioration of QoL when in combination with conventional therapy or increased the KPS score of the first-line treatment (Kong et al., 2017).
Another pivotal advantage of CIK cells is their reduced GvHD potential. GvHD represents the most frequent and severe complication related to the allogeneic lymphocyte transfusion. Studies demonstrated that the main effector subpopulation CD3+CD56+ cells present a terminally differentiated late effector phenotype with a limited proliferative potential (Y. C. Linn et al., 2009). The slower division rate and certain cytokines protective against GvHD released by CIK cells, such as IFN-γ, can both explain their minimal alloreactive capacity (Y.-G. Yang, Dey, Sergio, Pearson, & Sykes, 1998). In several clinical studies, allogeneic, haploidentical donor-derived, or totally mismatched cord blood-derived CIK cells were administered to patients with distinct tumor entities and mild GvHD activity and low toxicity were observed (Introna, 2017). Even though the molecular mechanism of CIK cells’ GvHD specificity remains not entirely clear, it is revealed that the CD3+CD56− subpopulation in CIK cells retains its alloreactive potential and accounts for the majority of GvHD reactions. Therefore, depletion of CD3+/CD56− cells might help reduce the risk of GvHD from CIK cells in the non-autologous setting.
The promising antitumoral activity and lack of severe side effects make CIK cells an ideal candidate for immunotherapy on both hematological and solid tumors. Nevertheless, the heterogeneity in study design and clinical response evaluation of ever-growing clinical studies makes it challenging to assess and compare the clinical efficacy comprehensively. Thus, the first international platform for the registration of clinical trials on CIK cells, IRCC, was established in the year of 2010, with the aim of clinical data collection, clinical protocol standardization and evaluation criteria normalization. We hope the increasing enrollment of more precise and standardized clinical studies can expand our knowledge in the efficacy and safety profile of CIK cell therapy and reach solid conclusions on CIK administration.
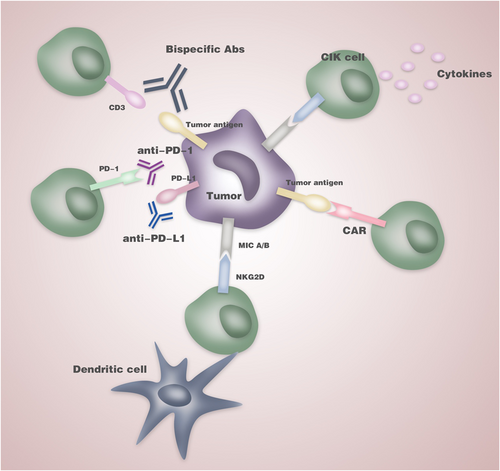
Notably, many attempts have been made to improve the antitumoral activity by CIK cells recent years (Figure 1). CIK cells are usually generated from PBMCs in the presence of IFN-γ, anti-CD3 mAbs, and IL-2. However, an ex vivo study revealed that addition of IL-15 from Day 4 of culture after 3 days’ generation based on the standard protocol significantly enhanced CIK cell-mediated cytotoxicity against acute lymphoblastic leukemia (AML)/lymphoma cell lines, primary acute myeloid and defined lymphoblastic leukemia cells. The significantly increased CD3+CD8+CD25+CD56– subpopulations of CIKIL-15 showed more effective killing ability of AML cells than that of the CD3+CD56+ subpopulation (Rettinger et al., 2012). The same group also demonstrated the safety and feasibility of IL-15 activated CIK cells on two AML patients with minimal GvHD (Rettinger et al., 2013). Immune checkpoints are primarily negative immunologic regulators involving in tumor escape of immune surveillance. The expression profile of eight major checkpoint molecules on CIK cells was detected and the high expression of PD-L1, LAG-3, TIM-3, and CEACAM-1 during long-term culture revealed that CIK cells might be partly exhausted before clinical transfusion (L. Zhang, Wang, et al., 2016). It was also suggested that the combined therapy of CIK cells and PD-L1/PD-1 blockade delayed tumor growth and exhibited a survival advantage in the murine gastric cancer model. The first clinical evidence came from a male patient with advanced NSCLC. After five courses of pembrolizumab (2 mg/kg every 3 weeks) and three courses of CIK cell therapy (every month), a PET-CT scan suggested significantly decreased tumor burden and radioactivity (Hui, Zhang, Ren, Li, & Ren, 2015). Recently, CIK has been used as an intriguing platform for genetic modification with CAR. CARs are constructed by fusing the single-chain variable fragment (scFv) of a tumor surface antigen-specific monoclonal antibody to T-cell intracellular signaling domains (Houot, Schultz, Marabelle, & Kohrt, 2015). CAR-modified CIK would exert antitumor activity both in a CAR-specific manner and by intrinsic NKG2D-mediated capability. A recent study transfected CIK precursors with a lentiviral vector encoding for anti-CD44v6-CAR. CD44v6 is broadly expressed in hematologic and some solid tumors. The cytotoxicity of CAR+CIK against the soft tissue sarcoma was significantly higher compared with the unmodified CIK cells (Leuci et al., 2018). In conclusion, CIK cell treatment might greatly benefit from these new developing strategies and become an important part of the future integrated antitumor therapeutic regimes.
All in all, CIK cell-based immunotherapy has demonstrated remarkable advantages of ease of preparation, safety, and tolerability during administration and considerable cytotoxicity against various malignancies. We are very glad to receive many responses in the past few years and would like to invite the reader again to contact us or visit our homepage (https://www.ciobonn.de/cik/) to register new clinical studies based on CIK cells. To obtain precise and detailed clinical data, the following parameters should be reported: publication details, title, journal, phase of clinical trial, CIK cell type (autologous or allogeneic), tumor entity, number of patients, gender of patients, median age, age range, stage of disease, inclusion and exclusion criteria, number of CIK cells per infusion, total number of infusions, number of cycles, HLA type of patients’ CIK cells, storage condition, hematologic and non-hematologic toxicity, clinical and immunologic response, time period of follow-up and survival status of patients.
CONFLICT OF INTERESTS
The authors declare that there are no conflicts of interests.




