Helicobacter pylori promote inflammation and host defense through the cagA-dependent activation of mTORC1
Guang-Jing Feng and Yi Chen contributed equally to this study.
Abstract
Mechanistic target of rapamycin complex 1 (mTORC1) functions as regulating different cellular processes, including cell growth, proliferation, motility, survival, metabolism, autophagy, and protein transcription. Recently, it also found to be associated with many infections and inflammatory diseases, playing complex roles in pathogens growth and inflammation regulation. However, the regulation mechanism of mTORC1 in gastric epithelial cells and its role in Helicobacter pylori (H. pylori) infection and related gastritis remain unclear. Here, we identified that the phosphorylation of mechanistic target of rapamycin (mTOR) and the expression of DEP domain-containing mTOR-interacting protein (DEPTOR) was increased in gastric mucosa of H. pylori-infected patients and mice, as well as in H. pylori-infected gastric epithelial cells, which were largely depended on H. pylori cagA. The expression of DEPTOR was regulated via mTORC1, but, in turn, inhibited mTORC1. Knockdown mTOR significantly decreased expression and secretion of cytokines tumor necrosis factor-α, interleukin-1β, and interleukin-6, chemokines CCL7 and CXCL16, and antimicrobial peptide LL37 in vitro, while knockdown DEPTOR had the opposite effect. Similar observations were made using mTOR knockout (KO) mice in vivo, moreover. The gastric inflammation was attenuated, while the bacterial burden was increased in mTOR KO mice during H. pylori infection. These findings supported H. pylori promote gastritis and inhibit bacterial colonization through the cagA-dependent activation of mTORC1.
Abbreviations
-
- Abs
-
- antibodies
-
- BM
-
- bone marrow
-
- DEPTOR
-
- DEP domain-containing mTOR-interacting protein
-
- EpCAM
-
- epithelial cell adhesion molecule
-
- H. pylori
-
- Helicobacter pylori
-
- IL
-
- interleukin
-
- KO
-
- knockout
-
- mTOR
-
- mechanistic target of rapamycin
-
- mTORC1
-
- mTOR complex 1
-
- mTORC2
-
- mTOR complex 2
-
- OMVs
-
- outer membrane vesicles
-
- Rictor
-
- rapamycin-insensitive companion of mTOR
-
- S6K
-
- ribosomal protein s6 kinase beta-1
-
- siRNA
-
- small interfering RNA
-
- T4SS
-
- type IV secretion system
-
- TNF-α
-
- tumor necrosis factor-α
1 INTRODUCTION
Helicobacter pylori (H. pylori), a Gram-negative bacterium, widely colonizes in the stomach of nearly half the world's population (Hooi et al., 2017). Persistent H. pylori infection can result in many gastric diseases, including chronic gastritis, peptic ulceration (Mccoll, 2010), and most seriously, increase the risk of gastric adenocarcinoma (Polk, & Peek, 2010). The mechanism of diverse outcomes in H. pylori infection is still not fully understood, and it seems to be multifactorial. Bacterial virulence factors (Posselt, Backert, & Wessler, 2013; Yamaoka, 2010), environmental factors (Bleich, & Mahler, 2005), and gene susceptibility (El-Omar et al., 2003) play important role in this pathological process. Especially cagA is injected into the host cell by type IV secretion system (T4SS), resulting in severe tissue inflammation and carcinogenesis (Amieva, & Peek, 2016; Yong et al., 2015). Gastric epithelial cells are major cells of H. pylori attachment and cagA injection and maybe the first line of host defense.
The mechanistic target of rapamycin (mTOR) is a conserved protein kinase that links with other proteins and serves as a core component of two distinct protein complexes, mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2). mTORC1, sensitive to rapamycin, regulates protein synthesis through the activation of ribosomal protein S6 kinase (S6K) and inhibition of the eukaryotic transcriptional initiation factor 4E binding protein 1, while mTORC2 is known to be insensitive to rapamycin and regulates the actin cytoskeleton of cell growth (Laplante, & Sabatini, 2012; Saxton, & Sabatini, 2017; Sokolova, Vieth, Gnad, Bozko, & Naumann, 2014). mTORC1 is reported to be associated with many inflammatory diseases, such as rheumatoid arthritis (Meng et al., 2017), inflammatory bowel disease (Cosin-Roger et al., 2017), allergy (Sinclair et al., 2017), acute lung injury (Hu et al., 2016), playing proinflammation and immune regulation role. Moreover, mTORC1 has a biphasic effect in infection, on one hand, it suppresses virus and bacterial growth (Liu et al., 2016; Zhao et al., 2018), while on the other hand, it promotes bacterial survival (Owen, Meyer, Bouton, & Casanova, 2014). However, the full extent to which cagA-dependent mTORC1 activity affects gastric inflammation and H. pylori survival is poorly understood.
In the current study, we have demonstrated that H. pylori infection of gastric cells results in cagA-dependent activation of the mTORC1, which is feedback inhibited by DEP domain-containing mTOR-interacting protein (DEPTOR). And, our studies, for the first time, it showed a proinflammation and bactericidal role of mTOR in H. pylori infection in vivo. We further demonstrated that mTOR promoted CCL7, CXCL16, and LL37 production, which might contribute to inflammation and host defense. Collectively, these data highlight a pathological role for mTORC1 in H. pylori-related gastritis.
2 MATERIALS AND METHODS
2.1 Patients and specimens
The gastric biopsy specimens were collected from 43 H. pylori-infected and 10 uninfected patients who underwent upper esophagogastroduodenoscopy for dyspeptic symptoms at Chongqing Traditional Chinese Medicine Hospital. All H. pylori strains from patients carry the vacA gene. In consideration of the VacA can inhibit mTORC1 (I. J. Kim et al., 2018), 20 cases with vacAs1m1 genotype, expressing a functional VacA toxic, were excluded from the study. The rest of the cases with vacAs1m2 genotype, including 13 cagA+ and 10 cagA−. The clinical characteristics of the patients were listed in Table S1. H. pylori infection was determined by 14C urea breath test and rapid urease test of biopsy specimens taken from the antrum, and subsequently confirmed by real-time polymerase chain reaction (PCR) for 16S ribosomal DNA (rDNA) and serology test for specific anti-H. pylori antibodies (Abs). For the isolation of human primary gastric epithelial cells, fresh nontumor gastric tissues (at least 5 cm distant from the tumor site) were obtained from gastric cancer patients who underwent surgical resection and were identified as H. pylori-negative individuals as above at Chongqing Traditional Chinese Medicine Hospital. None of these patients had received chemotherapy or radiotherapy before sampling. Individuals with atrophic gastritis, hypochlorhydria, antibiotics treatment, autoimmune disease, infectious diseases, and multiprimary cancer were excluded.
2.2 Bacteria culture
H. pylori NCTC 11637 (cagA positive; wild-type [WT] H. pylori) and cagA-knockout (KO) mutant H. pylori NCTC 11637 (ΔcagA) were grown in brain–heart infusion plates containing 10% rabbit blood at 37°C under microaerophilic conditions. For infecting mice, bacteria were propagated in Brucella broth with 5% fetal bovine serum (FBS) with gentle shaking at 37°C under microaerobic conditions for 1 day.
2.3 Generation of bone marrow chimera mice
On the basis of C57BL/6 mTOR KO mice, the following bone marrow (BM) chimeric mice were created: male WT BM→female WT mice, male WT BM→female mTOR KO mice, male mTOR KO BM→female WT mice, and male mTOR KO BM→female mTOR KO mice. BM cells were collected from the femurs and tibia of donor mice by aspiration and flushing and were suspended in phosphate-buffered saline (PBS) at the concentration of 5 × 107/ml. The BM in recipient mice was ablated with lethal irradiation (8 Gy). Then, the animals received intravenously 1.5 × 107 BM cells from donor mice in a volume of 300 µl sterile PBS under the anesthesia. Thereafter, the transplanted BM was allowed to regenerate for 8 weeks before subsequent experimental procedures. To verify the successful engraftment and reconstitution of the BM in the host mice, genomic DNA was isolated from tail tissues of each chimera mouse 8 weeks after BM transplantation. Quantitative PCR was performed to detect the Sry gene present in the Y chromosome and mouse β2-microglobulin gene as an internal control. The primers were listed in Table S2. The chimeric rates were calculated on the assumption that the ratio of the Sry to β2-microglobulin gene was 100% in male mice. We confirmed that the chimeric rates were consistently higher than 90%.
2.4 Animal experiments
Specific-pathogen-free (SPF) female C57BL/6 WT mice and mTOR KO mice were purchased from the Jackson Laboratory, and BM chimeric mice were generated as described. All mice were negative for pathogenic bacteria, parasites, and viruses, and were maintained under SPF conditions in a barrier sustained facility, and provided with sterile food and water. To induce the H. pylori infection, mice (6–8 weeks old) were fasted overnight and oralgastrically inoculated twice at a 1-day interval with 3 × 108 colony-forming unit bacteria.
Mice were killed at the indicated times. The greater curvature of the stomach was cut to perform hematoxylin and eosin staining. The intensity of inflammation was evaluated independently by two pathologists according to previously established criteria (Ferrero et al., 2008). DNA of the biopsy specimens were extracted with QIAamp DNA Mini Kit. As previously described (Roussel, Wilks, Harris, Mein, & Tabaqchali, 2005), H. pylori colonization was quantified by measuring H. pylori-specific 16S rDNA using specific primer and probe by TaqMan method. In the same specimen, the amount of mouse β2-microglobulin DNA was used to normalize data. The primers and probes were listed in Table S2. According to a previous study (Mikula, Dzwonek, Jagusztynkrynicka, & Ostrowski, 2003), the density of H. pylori was shown as the numbers of bacterial genomes per nanogram of host genomic DNA. All animal experiments were performed in strict accordance with the Guide for the Care and Use of Laboratory Animals issued by the Ministry of Science and Technology of the People's Republic of China.
2.5 Cell isolation
Fresh nontumor gastric tissues were washed three times with Hank's solution containing 1% FBS (Cat. No. 10099133; Gibco) before being cut into small pieces. The specimens were then collected in Roswell Park Memorial Institute 1640 (Cat. No. 22400071; Gibco) containing 1 mg/ml Collagenase IV and 10 mg/ml DNase I (Cat. Nos. C5138 and D5025; Sigma-Aldrich), and mechanically dissociated by using the gentleMACS Dissociator (Miltenyi Biotec). Dissociated cell suspensions were further incubated 0.5–1 hr at 37°C under continuous rotation. The cell suspensions were then filtered through a 70-μm cell strainer (Cat. No. 431751; BD Biosciences). Human primary gastric epithelial cells were purified from gastric tissue single-cell suspensions using a MACS column purification system and anti-epithelial cell adhesion molecule (EpCam) magnetic beads (Cat. No. 130-061-101; Miltenyi Biotec). The sorted cells were not used unless their viability was determined >90% and their purity was determined >95%.
2.6 Cell culture and stimulation
Human gastric epithelial cell lines AGS, GES-1, and HGC-27 and primary gastric epithelial cells (2.5 × 105 cells) were stimulated with WT H. pylori or ΔcagA at a multiplicity of infection (MOI) of 100 for 3 or 24 hr. AGS cells were also stimulated with WT H. pylori at different MOI at the indicated time points, or at different time points at the indicated MOI. For mTOR or DEPTOR inhibition experiments, AGS was pretreated with nonspecific control small interfering RNA (siRNA), mTOR, or DEPTOR siRNA (40 nM) for 24 hr by Lipofectamine 3000 (Cat. No. L3000001; Thermo Fisher Scientific). After coculture, cells were collected for real-time PCR and western blot, and the culture supernatants were harvested for enzyme-linked immunosorbent assay (ELISA). Control siRNA, mTOR siRNA, and DEPTOR siRNA were from GenePharma.
2.7 Immunohistochemistry and immunofluorescence
Human gastritis tissue and H. pylori-infected mouse gastric tissue samples were fixed with paraformaldehyde and embedded with paraffin, and then were cut into 5-µm sections. For immunohistochemical staining, rabbit anti-human/mouse p-mTOR (Ser2448), rabbit anti-human mTOR (Cat. Nos. ab109268 and ab134903; Abcam), and mouse anti-human DEPTOR (Cat. No. SC-398169; Santa Cruz Biotechnology) were used as the primary Abs, and goat anti-rabbit-horseradish peroxidase (HRP) and goat anti-mouse-HRP (Cat. No. ZDR-5306 and ZDR-5307; Zhongshan Biotechnology) as the second Abs, and then sections were stained by 3,3′-diaminobenzidine reagent (Cat. No. ZLI-9017; Zhongshan Biotechnology). After that all the sections were counterstained with hematoxylin and reviewed using a microscope (Eclipse 80i; Nikon).
For immunofluorescence staining of tissue sections or AGS cells, rabbit anti-human p-mTOR (Ser2448; Cat. No. ab109268; Abcam) and/or mouse anti-human EpCam (Cat. No. 14-9326-82; Invitrogen) were used as the primary Abs, and goat anti-mouse FITC/goat anti-rabbit TRITC (Cat. Nos. ZF-0312 and ZF-0316; Zhongshan Biotechnology) as the second Abs, and then nucleus was stained by 4′,6-diamidino-2-phenylindole (Cat. No. ZLI-9557; Zhongshan Biotechnology). Slides were examined with a confocal fluorescence microscope (TCS SP8; Leica Camera AG).
2.8 Real-time PCR
Extracted RNA from biopsy specimens and cultured cells was reverse-transcribed to complementary DNA by PrimeScript RT Reagent Kit (Cat. No. RR037A; Takara Bio). Real-time PCR was measured with the Real-Time PCR Master Mix in accordance with the manufacturer's specifications on the IQ5 (Bio-Rad Laboratories). Expression of mTOR, DEPTOR, tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), interleukin-6 (IL-6), CCL7, CXCL16, BD1, BD2, Reg3a, Reg3g, and LL37 were detected using the SYBR Green (Cat. No. QPK-201; Toyobo) with the respective primers. For mouse samples, mouse β-actin served as the normalizer, and an uninfected stomach served as a calibrator. For human samples, uninfected control cells served as a calibrator, and human glyceraldehyde 3-phosphate dehydrogenase (GAPDH) served as the normalizer. The primers were listed in Table S2. The relative gene expression was shown as fold change calculated by the method.
2.9 Western blot analysis
The cells and tissues were lysed with lysis buffer in the presence of protease and phosphorylation inhibitor mixture. Total cell/tissue lysates were eluted in sodium dodecyl sulfate (SDS) sample buffer and resolved in 10% SDS-polyacrylamide gel electrophoresis gels and blotted on polyvinylidene fluoride membranes (Cat. No. 1620177; Bio-Rad Laboratories). Human/mouse phospho-mTOR (p-mTOR) and mTOR were detected with rabbit anti-p-mTOR (Ser2448) and rabbit anti-mTOR Abs (Cat. Nos. ab109268 and ab134903; Abcam); human phospho-protein kinase B (p-AKT), protein kinase B (AKT), phospho-S6K (p-S6K), S6K, phospho-rapamycin-insensitive companion of mTOR (p-Rictor), rapamycin-insensitive companion of mTOR (Rictor), and GAPDH were detected, respectively, with rabbit anti-p-AKT (Ser473), mouse anti-AKT, rabbit anti-p-S6K (Thr421/Ser424), rabbit anti-S6K, rabbit anti-p-Rictor, rabbit anti-Rictor, and rabbit anti-GAPDH Abs (Cat. Nos. 4060S, 2920S, 9204S, 2708S, 3806S, 2114S, and 5174S; Cell Signaling Technology); Human/mouse DEPTOR and cagA were detected with mouse anti-DEPTOR and mouse anti-cagA Abs (Cat. Nos. SC-398169 and SC-28368; Santa Cruz Biotechnology); human/mouse GAPDH were detected with rabbit anti-GAPDH Abs (Cat. No. 5174S; Cell Signaling Technology). The blots were then incubated with secondary Abs labeled with HRP (Cat. Nos. ZDR-5306 and ZDR-5307; Zhongshan Biotechnology). Detected proteins were shown with a SuperSignal Extended Duration Substrate kit (Cat. No. 37071; Thermo Fisher Scientific).
2.10 Enzyme-linked immunosorbent assay
Mouse gastric tissues from specimens were collected, homogenized in 1-ml sterile protein extraction reagent, centrifuged, and harvested. The media of AGS cells were collected and centrifuged at 5,000 rpm for 5 min to remove floating cells and bacteria, and the supernatants were harvested. The supernatants of mouse gastric tissue lysates or AGS cells were assayed using the mouse or human TNF-α, IL-1β, IL-6, CCL7, CXCL16, and LL37 ELISA Kit (Cat. Nos. MTA00B, DTA00D, MLB00C, DLB50, M6000B, and D6050; R&D Systems; Cat. Nos. ab205571 and ab193769; Abcam; Cat. Nos. ELM-CXCL16-1 and ELH-CXCL16-1; RayBiotech; and Cat. Nos. LS-F32286-1 and LS-F10996-1; LSBio.). All experiments were according to the manufacturer's instructions.
2.11 Statistical analysis
Results are showed as mean ± standard error. Student's t test and analysis of variance were used in the comparison of two groups or multiple groups, respectively, but when the variances differed, the Mann–Whitney U test or Kruskal–Wallis test was used. SPSS statistical software (version 13.0) was used for all statistical analyses. All data were analyzed using two-tailed tests, and p < .05 was considered statistically significant.
3 RESULTS
3.1 p-mTOR is increased in gastric mucosa of H. pylori-infected patients and mice
First, we analyzed the p-mTOR levels in H. pylori-infected and uninfected gastric mucosa to evaluate the potential role of p-mTOR in H. pylori-associated immunopathogenesis, and found that compared to uninfected donors, the levels of p-mTOR (Figure 1a,b), but not total mTOR–messenger RNA (mRNA) and protein (Figure S1a,b), in gastric mucosa of H. pylori-infected patients were significantly higher, suggesting that H. pylori infection could induce the increase of p-mTOR.
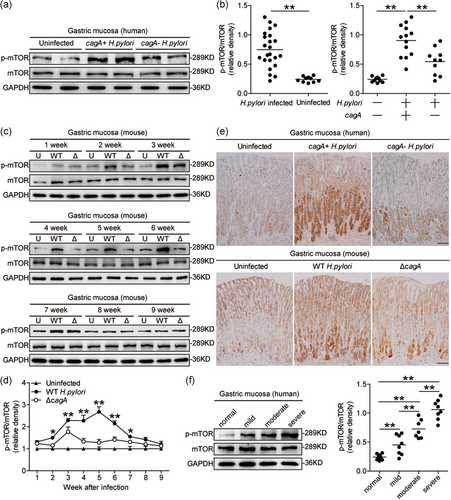
The presence of cagA is closely related to the development of H. pylori-associated gastritis and adenocarcinoma. Interestingly, compared to cagA-negative individuals, p-mTOR was significantly higher in cagA-positive patients (Figures 1a,b and 1e). However, p-Rictor and Rictor did not change (Figure S1c). Consistent with our findings in humans, compared to uninfected mice or ΔcagA-infected mice, p-mTOR was also significantly higher in WT H. pylori-infected mice, from 2- to 7-week postinfection (pi; Figure 1c–e), indicating a key role for cagA to induce mTOR phosphorylation during H. pylori infection in vivo. Furthermore, we found that p-mTOR was positively correlated with the severity of gastritis (Figure 1f), suggesting that H. pylori-induced mTOR phosphorylation might contribute to H. pylori-associated gastritis. Taken together, these findings suggested that p-mTOR is increased in H. pylori-infected gastric mucosa of patients and mice.
3.2 H. pylori activate mTORC1 (PI3K/AKT/mTOR/S6K) pathway in gastric epithelial cells in a cagA-dependent manner
Gastric epithelial cells are known to be the first-contacting cell type in the gastric mucosa during H. pylori infection. We, therefore, sought to determine whether gastric epithelial cells were responsible for mTOR phosphorylation during H. pylori infection. Interestingly, within gastric mucosa of H. pylori-infected donors, p-mTOR was detected in CD326+ gastric epithelial cells (Figure 2a), and immunofluorescence staining showed that p-mTOR was more noticeable in AGS cells, an immortalized human gastric epithelial cell line when infected with WT H. pylori compared to either no infection or infection with ΔcagA (Figure 2b). Next, we found that H. pylori-infected AGS cells were able to potently increase the levels of p-mTOR in a time-dependent as well as a dose-dependent manner (Figure 2c,d). And p-mTOR was more noticeable when using WT H. pylori compared to ΔcagA in human gastric epithelial cell lines such as AGS cells (Figure 2e), GES-1 cells, and HGC-27 cells (Figure S2b,c). These data altogether suggested that H. pylori infection induces gastric epithelial cells to increase mTOR phosphorylation.
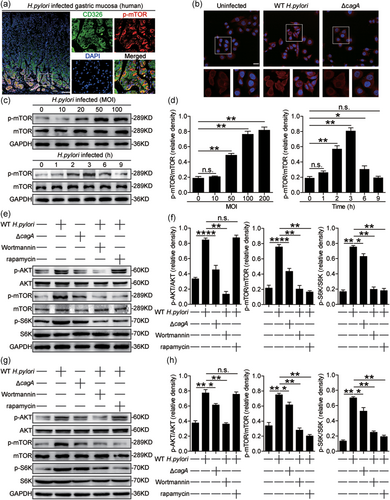
It is reported that activation of phosphatidylinositol-3-kinase (PI3K)/AKT could induce mTOR phosphorylation and subsequently induce S6K phosphorylation (Canales et al., 2017). To see whether PI3K/AKT/mTOR/S6K pathway activated in gastric epithelial cells upon H. pylori infection, we blocked PI3K/AKT or mTOR pathway, and then stimulated AGS cells with H. pylori. First, we found that AKT was predominantly phosphorylated in AGS cells after stimulated with H. pylori, and this was more noticeable when infected with WT H. pylori compared to ΔcagA (Figure 2e,f). Similar observations were made when analyzing p-mTOR induction as well as p-S6K, a downstream substrate of p-mTOR (Figure 2e,f). However, the expression and the phosphorylation of Rictor, an important component of mTORC2 complex, did not change (Figure 2d). Next, blocking PI3K/AKT pathway with Wortmannin effectively suppressed the induction of p-AKT, p-mTOR, and p-S6K in H. pylori-infected AGS cells (Figure 2e,f). Furthermore, blocking mTOR phosphorylation with rapamycin also effectively suppressed the induction of p-S6K but not p-AKT in H. pylori-infected AGS cells (Figure 2e,f). Similar observations were made when human primary gastric epithelial cells were infected with H. pylori (Figure 2g,h). Collectively, these results implied that activation of mTORC1 (PI3K/AKT/mTOR/S6K) pathway is involved in H. pylori-infected gastric epithelial cells.
3.3 DEPTOR negatively regulates mTORC1 in gastric epithelial cells during H. pylori infection
DEPTOR is involved in the mTOR signaling pathway as an endogenous regulator (Peterson et al., 2009). First, we analyzed the DEPTOR levels in H. pylori-infected and uninfected gastric mucosa and found that, compared to uninfected donors, the levels of DEPTOR mRNA and protein in gastric mucosa of H. pylori-infected patients were significantly higher, and that compared to cagA-negative individuals, the levels of DEPTOR mRNA and protein were significantly higher in cagA-positive patients (Figure 3a), a result that was also supported by immunohistochemical staining (Figure 3b). Consistent with our findings in humans, compared to uninfected mice or ΔcagA-infected mice, DEPTOR was also significantly higher in WT H. pylori-infected mice at 6- and 7-week pi (Figures 3c and S3a), indicating a key role for cagA to induce DEPTOR during H. pylori infection in vivo. Furthermore, the immunofluorescence staining showed that DEPTOR protein was most noticeable in AGS cells when infected with WT H. pylori compared to either no infection or infection with ΔcagA (Figure 3d). Similar observations were made when other human gastric epithelial cell lines such as GES-1 cells and HGC-17 cells were infected with H. pylori (Figure S3b,c). Taken together, these findings suggested that H. pylori infection induced DEPTOR expression.
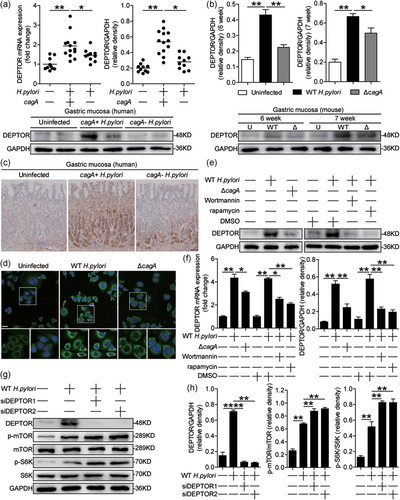
We also blocked the PI3K/AKT or mTORC1 pathway, and then stimulated AGS cells with H. pylori to see whether these pathways regulated DEPTOR in gastric epithelial cells upon H. pylori infection. First, we found that DEPTOR was predominantly expressed in AGS cells after stimulated with H. pylori, and this was more noticeable when infected with WT H. pylori compared to ΔcagA (Figure 3e,f), which was abolished by the PI3K/AKT or mTORC1 pathway inhibitor (Figure 3e,f). These results implied that activation of mTORC1 pathway was crucial for DEPTOR expression in H. pylori-infected gastric epithelial cells.
It is reported that overexpression of DEPTOR downregulated the activity of mTOR in vitro (Peterson et al., 2009). To see whether DEPTOR negatively regulates p-mTOR in H. pylori-infected gastric epithelial cells, we knocked down DEPTOR expression in AGS cells and stimulated AGS cells with WT H. pylori, and found that p-mTOR and p-S6K proteins were increased in H. pylori-infected AGS cells (Figure 3g,h), implying that DEPTOR negatively regulates mTORC1 in gastric epithelial cells during H. pylori infection.
3.4 H. pylori-induced mTORC1 promotes proinflammatory cytokines, chemokines, and antimicrobial peptide from gastric epithelial cells
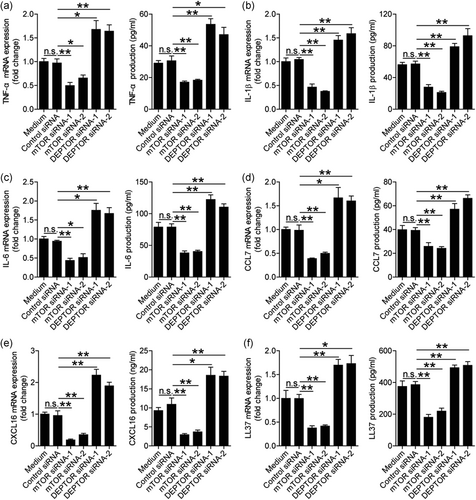
To evaluate the possible biological effects of DEPTOR and mTORC1 in H. pylori-infected gastric epithelial cells in vitro, we first compared the levels of proinflammatory cytokine expression in H. pylori-infected AGS cells pretreated with or without mTOR siRNAs, and found that compared to control siRNA, the expression and production of cytokines TNF-α (Figure 4a), IL-1β (Figure 4b), and IL-6 (Figure 4c) were significantly decreased in H. pylori-infected AGS cells pretreated with mTOR siRNAs. As for the negative regulation of DEPTOR on mTORC1 activation in H. pylori-infected AGS cells (Figure 3g,h), the expression and production of TNF-α (Figure 4a), IL-1β (Figure 4b), and IL-6 (Figure 4c) were significantly increased in H. pylori-infected AGS cells pretreated with DEPTOR siRNAs compared to those pretreated with control siRNA. Moreover, similar observations were made when analyzing the expression and production of chemokines CCL7 (Figure 4d) and CXCL16 (Figure 4e), and antimicrobial peptide LL37 (Figure 4f) but not antimicrobial peptides BD1, BD2, BD3, BD4, Reg3a, and Reg3g (Figure S4b). Taken together, these findings suggested that H. pylori-induced mTORC1 promoted proinflammatory cytokines, chemokines, and antimicrobial peptide from gastric epithelial cells.
3.5 The mTORC1 promotes proinflammatory cytokines, chemokines, and inflammation in mice gastric mucosa during H. pylori infection
H. pylori-associated gastritis is characterized as inflammatory cell infiltration and inflammatory factor increase under chronic inflammatory conditions. We found that the productions of inflammatory cytokines TNF-α, IL-1β, and IL-6, chemokines CCL7 and CXCL16, and the histological scores of inflammation at 7-week pi were lower than those at 5-week pi (Figure S5a–c). Next, we also evaluated the inflammatory response in gastric mucosa at 5-week pi between WT and mTOR KO mice and found that less gastric inflammation was observed in mTOR KO mice compared to in WT mice during H. pylori infection (Figure 5a). Our previous data indicated that gastric epithelial cells are the source cells that express mTORC1 activation (Figure 2). Therefore, we generated BM chimera mice to determine the contribution of BM-derived or non-BM-derived mTOR-expressing cells to inflammation. In BM chimera mice, we found that non-BM-derived mTOR-expressing cells were largely responsible for gastric inflammation in the gastric mucosa during H. pylori infection (Figure 5b). Furthermore, we also detected the expression and production of proinflammatory cytokines and chemokines in these KO mice and BM chimera mice described above and found that the expression (Figure S5d) and production (Figure 5c) of TNF-α, IL-1β, and IL-6 were significantly decreased in gastric mucosa of mTOR KO mice. Again, BM chimera experiments confirmed that non-BM-derived mTOR-expressing cells were largely responsible for TNF-α, IL-1β, and IL-6 expression (Figure S5e) and production (Figure 4d) in the gastric mucosa during H. pylori infection. Moreover, similar observations were made when analyzing the expression (Figure S5f,g) and production (Figure 5e,f) of chemokine CCL7 and CXCL16. Collectively, these results suggested that mTOR had effects of promoting inflammation, proinflammatory cytokines, and chemokines during H. pylori infection in vivo.
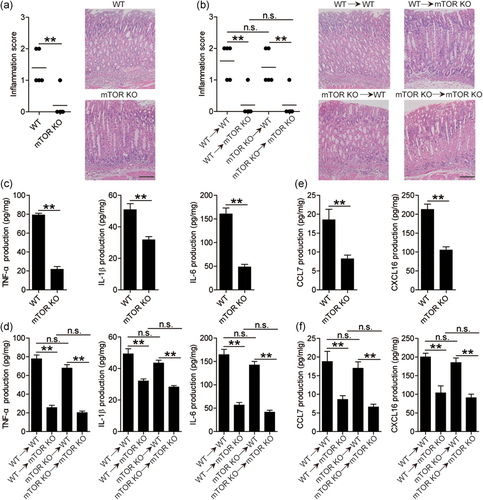
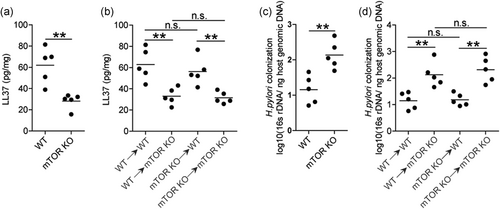
3.6 The mTORC1 promotes antimicrobial peptide and decreases bacterial burden in gastric mucosa during H. pylori infection
To evaluate the possible biological effects of mTORC1 in H. pylori-associated immunopathogenesis in vivo, we finally evaluated the levels of antimicrobial peptide LL37 and bacterial colonization in gastric mucosa at 5-week pi between WT and mTOR KO mice and found that lower expression (Figure S6a) and production (Figure 6a) of antimicrobial peptide LL37 was observed in gastric mucosa of mTOR KO mice compared to that in WT mice during H. pylori infection. In BM chimera mice, we found that non-BM-derived mTOR-expressing cells were largely responsible for the increased antimicrobial peptide LL37 expression (Figure S6b) and production (Figure 6b) in gastric mucosal. In turn, more H. pylori colonization was shown in mTOR KO mice compared to WT mice (Figure 6c), and in BM-derived compared to non-BM-derived mTOR-expressing cells (Figure 6d). Collectively, these results suggested that mTOR had effects of promoting antimicrobial peptide, inhibiting bacterial colonization during H. pylori infection in vivo.
4 DISCUSSION
mTOR is well known for its crucial role in regulating autophagy, and considered as a pharmacologic target for autophagy regulation in several disease treatments (Y. C. Kim, & Guan, 2015). Autophagy has been associated with infection, inflammation, and immunity, and can control the elimination of microorganisms (Deretic, Saitoh, & Akira, 2013; Netea-Maier, Plantinga, van de Veerdonk, Smit, & Netea, 2016). A previous study reported cagA protein negatively regulates autophagy via c-Met-PI3K/Akt-mTOR signaling pathway (Li et al., 2017); moreover, Thiem et al. (2013) mentioned mTORC1 inhibition restricts inflammation-associated gastrointestinal tumorigenesis in mice. These suggested mTORC1 may be involved in the whole process of gastritis and followed gastric cancer caused by H. pylori infection. In this study, we uncovered a multistep mechanism of gastritis during H. pylori infection involving interactions between virulence factor cagA and gastric epithelial cells via the mTORC1 pathway (Figure 7). We found that cagA contributed to activated mTORC1 in gastric epithelial cells, and then promoted inflammation and inhibited H. pylori colonization in vivo and in vitro. Moreover, the upregulation of DEPTOR expression was downstream of the mTORC1 pathway, which, in turn, inhibited the activation of mTORC1, forming a negative feedback loop.
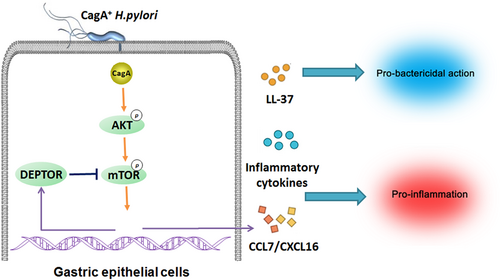
Gastric epithelial cells provide the first and major point of contact between H. pylori and the host. When cagA enters the gastric epithelial cells, it is able to interact and activates several intracellular signaling pathways, such as Wnt/β-catenin, PI3K/Akt, nuclear factor-κB, AMP-activated protein kinase, Janus kinase/signal transducer and activator of transcription pathways (Yong et al., 2015), and play a critical role in chronic gastritis and cancer development. However, the past reports about the mTORC1 pathway in H. pylori infection are rare, especially in cagA-dependent manner. H. pylori outer membrane vesicles (OMVs) induce upregulation of Heme oxygenase-1 requires mTORC1 positive regulation in dendritic cells (Ko et al., 2016), while G0/G1 cell cycle arrest caused by H. pylori in human gastric cells is also through mTORC1 activation (Canales et al., 2017). Interestingly, H. pylori virulence factor VacA seems to have the opposite effect to the OMVs and cagA on mTORC1 and autophagy (I. J. Kim et al., 2018; Ko et al., 2016; Li et al., 2017), which demonstrates there exist a complex signaling network around mTORC1 in the gastric epithelial cells during H. pylori infection. Several studies have reported H. pylori activates PI3K/AKT pathway via cagA (Dibble, & Cantley, 2015; Yong et al., 2015); moreover, PI3K/AKT signaling can activate mTORC1 and their downstream S6K through the tuberous sclerosis complex and Rheb (Canales et al., 2017), which are in keeping with findings from our study. DEPTOR, as an endogenous mTOR inhibitor, is able to directly bind mTOR kinase and inhibits mTORC1 and mTORC2 activities (Zhao, Xiong, Jia, & Sun, 2012). Following mTOR activation, DEPTOR is phosphorylated and degraded by mTOR, which generates an autoamplification loop (Duan et al., 2011). However, this study shows a novel regulation mechanism that mTORC1 promotes the inhibitor DEPTOR expression in H. pylori infection, which generates a negative feedback loop. These data suggest mTORC1 may avoid side effects caused by overactivation through DEPTOR.
Given the apparent relationship between phosphorylated mTOR and the severity of gastritis in H. pylori-infected patients is observed, and the trend of gastritis reduction is consistent with the decrease of mTOR phosphorylation and the increase of DEPTOR in the mice model, which demonstrates the inhibitory role of DEPTOR on p-mTOR may be one of the reasons for reduction of gastric inflammation. We have further confirmed the proinflammation role of mTORC1 in H. pylori infection using mTOR KO and BM chimera mice. mTOR plays an important proinflammation role in several bacterial or viral infections through secreting various cytokines and chemokines (Hu et al., 2016; X. Jia et al., 2018; Lin et al., 2014). It is clear that H. pylori infection is accompanied with increased infiltration of many types of immune cells (Wei, Wang, & Liu, 2015; Whitmore, Weems, & Allen, 2017), and chemokines can attract the migration and residence of immune cells, and have crucial proinflammation and immunity regulation role in inflammatory diseases, especially in bacterial infection (Palomino, & Marti, 2015). Previous studies have showed the increase of chemokine CCL7 and CXCL16 expression in H. pylori-infected gastric epithelial cells (Kuzuhara, Suganuma, Kurusu, & Fujiki, 2007; Palomino, & Marti, 2015), and our results further demonstrate these effects are through the mTOR signaling pathway. CCL7 specifically attracts neutrophils and monocytes by interacting with CCR2 (T. Jia et al., 2008; Mercer et al., 2014), while several subsets of T cells and natural killer T cells migrate in response to CXCL16 via their surface CXCR6 (Lv et al., 2019). Therefore, the inflammatory cells infiltrating the site of gastritis may be attracted by CCL7 and CXCL16.
LL37, also known as cathelicidin antimicrobial peptide 18 kDa, serves a critical role in innate immune defense against invasive bacterial infection (Zanetti, 2004). LL37 is expressed in a distinct distribution by surface epithelial cells as well as chief and parietal cells in the fundic glands of the normal gastric mucosa. LL37 is significantly increased in the epithelium and gastric secretions of H. pylori-infected patients than in those of individuals with non-H. pylori-induced gastric inflammation, which is in a T4SS-dependent manner (Hase et al., 2003). Moreover, LL37 has anti-H. pylori activity in the gastric juice (Leszczyńska et al., 2009). However, it has not been reported that the expression of LL37 is regulated by the mTOR signaling pathway. We observe the decrease of LL37 and the increase of H. pylori colonization using mTOR siRNA and mTOR KO mice. Thus, our results demonstrate that gastric epithelial cells recognize H. pylori cagA, then produce more LL37 through the mTORC1 pathway, participating in host defense effect.
In this study, we first explain how H. pylori regulate the inflammation and bactericidal action through the mTORC1 signaling pathway. Future studies are necessary to investigate the feedback effect of DEPTOR in vivo, once we obtain the DEPTOR KO mice. In addition, the mechanisms of action of chemokines and antimicrobial peptides in H. pylori infection are also needed deeper.
Taken together, gastric epithelial cells can produce various cytokines, chemokines, and antimicrobial peptides to promote gastritis and inhibit bacterial colonization when H. pylori attach to the cells and inject cagA into the cells. The cagA-dependent activation of the mTORC1 pathway plays a central role in regulating inflammation and host defense. This model suggests host identify the presence of a pathogen by detection of cagA, and intend inflammatory response to deal with this intruder, followed by damage limitation of excessive inflammation through feedback inhibit of DEPTOR. Although this idea remains to be evaluated experimentally, our finding described here provide new insight for further studies to understand how regulatory network around mTORC1 influence interaction between bacterial virulence factor and host defense during H. pylori infection.
ACKNOWLEDGMENTS
The authors are grateful to Chihiro Sasakawa (Department of Microbiology and Immunology, Institute of Medical Science, University of Tokyo) for providing cagA-KO mutant H. pylori 11637 (ΔcagA) strain. This study was supported by the grant of Chongqing Health and Family Planning Commission (Grant number: ZY201802022).
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Conception and design, data analysis, and drafting the manuscript: K. L. Statistical analysis: G.-J. F. and Y. C. Obtained funding: K. L. Technical support: G.-J. F. and Y. C. Final approval of submitted version: K. L.
Open Research
DATA AVAILABILITY STATEMENT
All data in this study are available if requested.




