The proinflammatory cytokines IL-1β and TNF-α modulate corneal epithelial wound healing through p16Ink4a suppressing STAT3 activity
Abstract
The proinflammatory cytokines interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α) are involved in the corneal inflammatory response and wound healing following corneal injuries. However, the mechanism by which proinflammatory cytokines modulate corneal epithelial wound healing remains unclear. In this study, we found that IL-1β or TNF-α was transiently elevated during corneal epithelial wound healing in mice. After corneal epithelial debridement, persistent treatment with IL-1β or TNF-α restrained the level of phosphorylated signal transducer and activator of transcription 3 (p-STAT3) and boosted the level of cell cycle inhibitor p16Ink4a, resulting in impaired corneal epithelial repair. When p16Ink4a was deleted, the p-STAT3 level in corneal epithelium was enhanced and corneal epithelial wound healing was clearly accelerated. In diabetic mice, IL-1β, TNF-α, and p16Ink4a appeared a sustained and strong expression in the corneal epithelium, and p16Ink4a knockdown partially reverted the defective diabetic corneal epithelial repair. Furthermore, immunoprecipitation proved that p16Ink4a interacted with p-STAT3 and thus possibly suppressed the STAT3 activity. Our findings revealed a novel mechanism that the proinflammatory cytokines modulate corneal epithelial wound healing via the p16Ink4a-STAT3 signaling.
1 INTRODUCTION
The corneal epithelium is the first protective barrier of eyes against physical trauma, pathogens, and chemicals, and it contributes to the maintenance of corneal transparency and rigidity. Corneal epithelial wound healing is a complex process involving cell apoptosis, proliferation, migration, senescence, and differentiation (Ljubimov & Saghizadeh, 2015; X. Wang et al., 2019). This process can be divided into three overlapping but functionally distinct phases—the inflammatory phase characterized by infiltration of neutrophils and macrophages, the proliferative phase marked by the homeostasis and regeneration of corneal epithelial stem cells (CESCs), and the maturation phase that involves the differentiation of CESCs and epithelial cells (Agrawal & Tsai, 2003; Gurtner, Werner, Barrandon, & Longaker, 2008; Mobaraki et al., 2019). Impairment of this process results in persistent corneal defect, and subsequently cause corneal neovascularization, loss of corneal transparency, even leading to blindness (Dua & Azuara-Blanco, 2000; Kaji, 2005).
Mounting evidence has shown that proinflammatory cytokines, particularly interleukin-1β (IL-1β), IL-6, and tumor necrosis factor-α (TNF-α), are involved in inflammation, immunity, tissue repair, and cancer (Aarreberg et al., 2019; Bachus et al., 2019; Balkwill, 2009; Gomez et al., 2018; Johnson, O'Keefe, & Grandis, 2018; Kang, Tanaka, Narazaki, & Kishimoto, 2019; Stock, Jama, Hansen, & Wicks, 2019). In the cornea, proinflammatory cytokines are pleiotropic, wherein they are associated with corneal immune response, angiogenesis, chemotaxis, wound healing, and corneal diseases (Torres & Kijlstra, 2001; Wilson et al., 2001; Yang et al., 2019). A recent study has reported that IL-1β is elevated and IL-1 receptor antagonist (IL-1Ra) is suppressed in diabetic corneas, and an imbalance in IL-1β and IL-1Ra levels causes delayed corneal epithelial wound healing (Yan et al., 2016). TNF-α has been proven to be upregulated in tear fluid in dry eyes or in eyes with allergic ocular surface disorders (Murdaca et al., 2015; Pflugfelder, Jones, Ji, Afonso, & Monroy, 1999; X. Zhang, Zhao, Deng, Sun, & Wang, 2016). Furthermore, TNF-α has been shown to not only disrupt the function of the corneal epithelial barrier, leading to the development of dry eyes (Tanaka et al., 2013), but also impair corneal epithelial wound healing by slowing the migration of corneal epithelial cells (Okada et al., 2007). However, the mechanism by which IL-1β and TNF-α modulate corneal epithelial wound healing after corneal debridement remains elusive.
Signal transducer and activator of transcription 3 (STAT3) is a transcription factor that is activated by a variety of cytokines, such as the IL-6 family of cytokines, epidermal growth factor, and leptin (Bates et al., 2003; Darnell, Kerr, & Stark, 1994; Johnson et al., 2018; Vaisse et al., 1996). Upon activation (phosphorylation of STAT3 at Tyr705, indicating its activation), STAT3 translocates into the nucleus, where it regulates the genes involved in immune function, cell proliferation, survival, and differentiation, depending on the cell type (Mertens & Darnell, 2007; Piperi, Papavassiliou, & Papavassiliou, 2019). Recently, several studies have found that the STAT3 signaling pathway plays a vital role in modulating corneal inflammation and CESCs proliferation, migration (Ebihara, Matsuda, Nakamura, Matsuda, & Murakami, 2011; Hsueh et al., 2011; Nakamura et al., 2014). For example, IL-6 promotes the proliferation and migration of CESCs by increasing the phosphorylated STAT3 (p-STAT3) level (Ebihara et al., 2011). Moreover, the ciliary neurotrophic factor promotes the activation and migration of CESCs and accelerates corneal epithelial wound healing by activating STAT3 signaling (Zhou et al., 2015). However, whether IL-1β and TNF-α function through the STAT3 signaling during corneal epithelial wound healing remains unknown.
Our previous study has demonstrated that IL-1β and TNF-α cause transient inhibition of CESCs, thus delaying corneal epithelial wound healing (Yang et al., 2019). However, the mechanism by which these proinflammatory cytokines modulate corneal epithelial wound healing remains elusive. Here, we showed that IL-1β or TNF-α modulated the expression of p16Ink4a (p16) and p-STAT3 after corneal epithelial debridement. We provided evidence suggesting that p16 suppresses STAT3 activity through binding p-STAT3. Our work indicated the vital role of the IL-1β/TNF-α-p16-STAT3 signaling axis in corneal epithelial wound healing.
2 MATERIALS AND METHODS
2.1 Animals
C57BL/6 mice (male, 8-week old) were purchased from the Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). Cyclin-dependent kinase 2 (Cdkn2a) knockout mice (01XB1: 129-Cdkn2atm1Rdp) were obtained from the Frederick National Laboratory for Cancer Research (FNL)/SAIC-Frederick, Inc. Type 1 diabetes was induced in the mice with streptozocin (STZ; 50 mg/kg; Sigma-Aldrich, St. Louis, MO) injections as described previously (Yang et al., 2014; Zhou et al., 2015). Blood from the tail vein of mice was prepared for blood glucose measurement. Blood glucose levels were monitored using a One-Touch Basic glucometer (LifeScan, Johnson & Johnson, Milpitas, CA). In this study, we used diabetic mice with a blood glucose level higher than 16.7 mmol/L (Jarrett, Keen, Fuller, & McCartney, 1979) after 12 weeks of final STZ injection. All animal experiments were conducted in accordance with the Committee guidelines of the Shandong Eye Institute and the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research.
For the corneal epithelial debridement model, C57BL/6 mice of the same age were anesthetized through intraperitoneal injection of 0.6% pentobarbital sodium followed by topical application of 2% xylocaine. Corneal central epithelium (2.5 mm in diameter for wild-type (WT) and diabetic mice, 2.0 mm in diameter for Cdkn2a knockout mice) was removed with an AlgerBrush II Rust Ring Remover (Alger, Lago Vista, TX) and subsequently applied with ofloxacin eye drops to avoid infection. One eye was wounded at a time in each mouse. All efforts were made to minimize animal suffering. IL-1β (10 ng/ml; Millipore, Temecula, CA) or TNF-α (10 ng/ml; R&D, Minneapolis, MN) in physiological saline (0.9% NaCl) was topically administered (5 μl, four times daily), whereas S3I-201 (50 μg per eye; Selleck Chemicals, Houston, TX; Zhou et al., 2015) was subconjunctivally injected into the eyes once daily for 2 days, starting on the day of corneal epithelial debridement; physiological saline was used in the control group. Defects in corneal epithelial healing were visualized in vivo at the indicated time points by instilling 0.25% fluorescein sodium and by photographed under a slit lamp (BQ900; Haag-Streit, Bern, Switzerland). The staining area was analyzed by using the ImageJ software (version 1.47, National Institutes of Health, Bethesda, MD) and then calculated as a percentage of the residual epithelial defect.
2.2 Cell culture, treatment, and cell scratch assay
Mouse corneal epithelial stem/progenitor cells (TKE2; 11033107 Public Health England, http://www.phe-culturecollections.org.uk/) were presented by Dr. Tetsuya Kawakita of Keio University (Tokyo, Japan). The TKE2 cells were cultured in a keratinocyte serum-free medium (KSFM; Gibco-Invitrogen, Carlsbad, CA) supplemented with 5 ng/ml human recombinant epithelial growth factor and 50 μg/ml bovine pituitary extract at 37°C and 5% CO2 (Kawakita, Shimmura, Hornia, Higa, & Tseng, 2008; Yang et al., 2019). The TKE2 cell line has been characterized in our group (Yang et al., 2019; Zhou et al., 2015). To induce persistent inflammation, we exposed the TKE2 cells to 10 ng/ml of IL-1β or TNF-α for 3 days.
Cell scratch experiments were carried out in six-well plates by creating a scratch in a confluent monolayer of TKE2 cells using a 1-ml tip at the center of the culture dish. After the scratch, cells were cultured with KSFM medium supplemented with 10 ng/ml of IL-1β or TNF-α for 48 hr. Digital images of wound closure were quantitatively assessed by using the ImageJ software. Each assay was conducted in at least triplicate.
2.3 Enzyme-linked immunosorbent assay
Total proteins were extracted from the mouse corneas in extraction reagent with protease inhibitors (Roche, Mannheim, Germany). After homogenization and centrifugation (10,000g for 15 min at 4°C), the supernatants were collected and calculated using the Bicinchoninic Acid Kit (Beyotime, Shanghai, China) for total protein concentrations, then analyzed with IL-1β and TNF-α enzyme-linked immunosorbent assay (ELISA) Kits (Proteintech, Wuhan, China) according to the manufacturer's procedures. The absorbance at 562 nm was measured using a multi-mode microplate reader (SpectraMax M2; Molecular Devices, Menlo Park, CA). The final concentrations were calculated with the total proteins measured by using the BCA Kit (Zhou et al., 2012).
2.4 Real-time quantitative polymerase chain reaction
The total RNA of mice corneal epithelia or of TKE2 cells was extracted using Nucleospin RNA kits (BD Biosciences, Palo Alto, CA). Reverse transcription was performed using Primescript RT Master Mix (Takara, Dalian, China) according to the manufacturer's protocol. Real-time quantitative polymerase chain reaction (qPCR) was carried out using SYBR Green reagents and the Applied Biosystems 7500 Real-Time PCR System (Applied Biosystems, Foster City, CA) according to the instructions of the manufacturer. The specific primers used in this assay were listed in Table 1. The cycling conditions were 10 min at 95°C followed by 40 two-step cycles (15 s at 95°C and 1 min at 60°C). The quantification data were analyzed with the Sequence Detection System Software (Applied Biosystems) using mouse β-actin as an internal control. Relative expression was calculated using the comparative method, and the values were expressed as (Livak & Schmittgen, 2001).
| Gene | Forward primer | Reverse primer | Product (bp) |
|---|---|---|---|
| IL-1β | CTTTCCCGTGGACCTTCCA | CTCGGAGCCTGTAGTGCAGTT | 150 |
| TNF-α | ATGAGAAGTTCCCAAATGGC | CTCCACTTGGTGGTTTGCTA | 125 |
| p16 | GGAGTCCGCTGCAGACAGA | GAATCGGGGTACGACCGAA | 122 |
| p19 | TCGCAGGTTCTTGGTCACTGT | ATCCTCTCTAGCCTCAACAACATG | 150 |
| p21 | GGGACAAGAGGCCCAGTACTT | CAATCTGCGCTTGGAGTGATAG | 191 |
| p53 | ACAGCGTGGTGGTACCTTATGA | GGTTCCCACTGGAGTCTTCCA | 150 |
| β-actin | ACTGCCGCATCCTCTTCCT | TCAACGTCACACTTCATGATGGA | 179 |
- Abbreviations: bp, base pair; IL-1β, interleukin-1β; qPCR, quantitative polymerase chain reaction; TNF-α, tumor necrosis factor-α.
2.5 Immunofluorescence staining
Mouse eyes were enucleated and embedded in Tissue-Tek optimum cutting temperature compound (Sakura Finetechnical, Tokyo, Japan) and then sectioned into 7 μm thickness. For immunofluorescence staining, corneal sections and TKE2 cells were fixed with 4% paraformaldehyde for 10 min at room temperature. After permeabilization with 0.1% Triton X-100 (Sigma-Aldrich) for 30 min, all samples were treated with 5% bovine serum albumin (Solarbio, Beijing, China) and incubated with the primary antibodies of p16, p-STAT3 (Y705), and Bmi1 (Abcam, Cambridge, UK) at 4°C overnight. The samples were subsequently washed and incubated with donkey Alexa Fluor 488-conjugated secondary antibodies (Invitrogen, Carlsbad, CA) for 1.5 hr at room temperature and then washed again with phosphate-buffered saline. All stained samples were counterstained with 4, 6-diamidino-2-phenylindole and examined under a Nikon (Eclipse e800, Tokyo, Japan) fluorescence microscope.
2.6 Immunoprecipitation and immunoblotting assays
Total proteins were extracted from the lysed samples of mouse corneal epithelia and cultured TKE2 cells in radioimmunoprecipitation assay (RIPA) buffer with protease inhibitors (Roche). Immunoblotting was carried out as previously described (X. Wang et al., 2019). The primary antibodies used were as follows: p16, p-STAT3, t-STAT3 (Abcam), β-actin, and GAPDH (Proteintech). After incubation with horseradish peroxidase (HRP)-conjugated secondary antibody (Proteintech), the blots were exposed to Immobilon Western chemiluminescent HRP substrate (Millipore, Billerica, MA) and visualized by ChemiDoc™ Touch imaging system (Bio-Rad, Hercules, CA). The quantification data were analyzed with ImageJ software. Comparative expression was calculated using the ratio of the interest protein to the internal reference protein. The values of comparative expression came from three independent experiments.
Immunoprecipitation was performed as previously described (Q. Wang et al., 2019). TKE2 cell lysates were prepared using RIPA buffer with protease inhibitors (Roche) and phenylmethylsulfonyl fluoride (Beyotime), and then used in immunoprecipitation assays using the appropriate antibodies. Immunoblotting was performed as above.
2.7 Small interfering RNAs in vivo
For the knockdown of p16 in mouse corneas, 10 μM (5 μl per cornea) p16 (p16-1 and p16-2) small interfering RNAs (siRNAs) or standard control (Sc) siRNA were subconjunctivally injected into diabetic mice eyes twice daily after corneal epithelial debridement. Defects in corneal epithelial healing were visualized at the indicated time points by instilling 0.25% fluorescein sodium and were photographed under a slit lamp. Corneal epithelia were harvested after 48 hr following epithelial debridement for western blot analysis. The siRNA target sites specific for mouse p16 were as follows: p16-1 siRNA: 5′-CCGAACTCTTTCGGTCGTA-3′ and p16-2 siRNA: 5′-ACTGAATCTCCGCGAGGAA-3′. The Sc siRNA was provided by RiboBio Company (Guangzhou, China).
2.8 Statistical analysis
All the experiments were performed three times. Data are presented as mean ± standard deviation. Statistical significance was evaluated through unpaired two-tailed Student's t test, through one-way analysis of variance (ANOVA; for comparison of more than two groups), and through two-way ANOVA (for comparison of two independent variables, such as control and diabetic, unwounded and wounded) by using the GraphPad Prism software (version 5.0, San Diego, CA). A p < .05 was considered to be statistically significant (*p < .05, **p < .01, ***p < .001).
3 RESULTS
3.1 IL-1β and TNF-α are transiently upregulated during corneal epithelial wound healing in mice
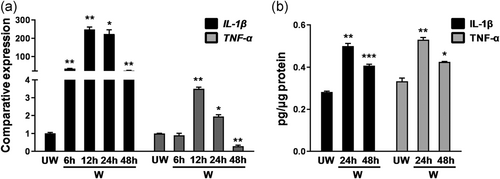
After corneal epithelial debridement in control mice, the messenger RNA (mRNA) expression levels of IL-1β (wound 12 hr 248.06 ± 23.79 vs. unwound 1.00 ± 0.09; **p < .01) and TNF-α (wound 12 hr 3.50 ± 0.15 vs. unwound 1.00 ± 0.02; **p < .01) in the corneal epithelium peaked at 12 hr and gradually declined thereafter (Figure 1a). ELISA assay for whole-mount corneas demonstrated that the secretion levels of IL-1β (wound 24 hr 0.50 ± 0.02 pg/μg vs. unwound 0.28 ± 0.01 pg/μg; **p < .01) and TNF-α (wound 24 hr 0.53 ± 0.02 pg/μg vs. unwound 0.33 ± 0.03 pg/μg; **p < .01) significantly increased after corneal epithelial debridement (Figure 1b). These results indicated that these proinflammatory cytokines were transiently elevated in corneal epithelia and in whole-mount corneas after corneal epithelial debridement.
3.2 Persistent treatment with IL-1β or TNF-α impairs corneal epithelial wound healing
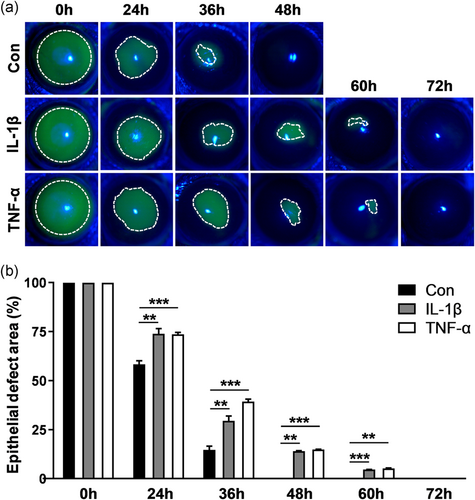
The prolonged elevation of IL-1β or TNF-α has been reported in diabetic conjunctiva, retinopathy, and skin, which compromises normal wound healing (Armstrong, Boulton, & Bus, 2017; Demircan, Safran, Soylu, Ozcan, & Sizmaz, 2006; C. Zhang et al., 2016). Persistent IL-1β or TNF-α treatment, which mimics persistent inflammation, the effect of IL-1β or TNF-α on corneal epithelial wound healing was evaluated. In control mice, the wound in the epithelium debrided cornea apparently shrank at 36 hr and then completely healed at 48 hr (Figure 2a,b). With the topical administration of IL-1β or TNF-α, wound healing was robustly delayed, and the wound remained incomplete healing at 48 hr (epithelia defect area [%]: IL-1β-48 hr 13.99 ± 0.13 vs. control [Con] 48 hr 0.00 ± 0.00; **p < .01; TNF-α-48 hr 14.81 ± 0.23 vs. Con 48 hr 0.00 ± 0.00; ***p < .001; Figure 2a,b). Similarly, the scratch assay for TKE2 cells showed that IL-1β or TNF-α treatment markedly impaired wound healing compared with the control treatment (Figure S1). These results demonstrated that persistent treatment with proinflammatory cytokines suppressed corneal epithelial wound healing, suggesting that these cytokines are an important cause of corneal epithelial repair defects in persistent inflammation.
3.3 IL-1β or TNF-α restrains STAT3 activation and boosts p16 expression both in vivo and in vitro
Emerging evidence has proven that STAT3 signaling modulates corneal inflammation and wound healing (Hsueh et al., 2011; Nakamura et al., 2014; Zhou et al., 2015). To investigate whether proinflammatory cytokines affect STAT3 signaling, we determined the p-STAT3 level in wounded corneal epithelium among the control and IL-1β and TNF-α treatment mice. At 48 hr following corneal epithelial debridement, immunostaining showed that the p-STAT3 staining in the IL-1β- or TNF-α-treated corneas was obviously attenuated compared with that in the control-treated corneas (Figure 3a), suggesting that proinflammatory cytokines IL-1β and TNF-α affect STAT3 signaling.
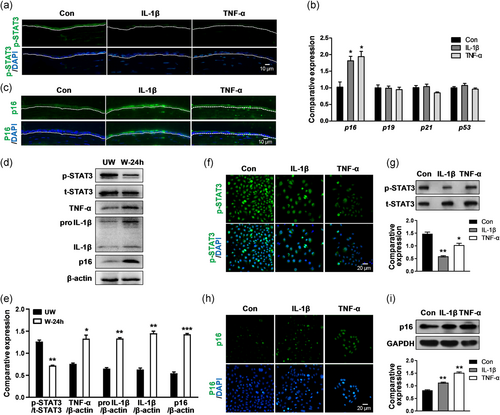
Cell cycle-associated proteins play a vital role in cell proliferation during corneal wound repair (Zieske, 2000), thus the expression levels of p16, p19, p21, and p53 were examined at 48 hr after epithelial debridement in the control and IL-1β and TNF-α treatment mice. qPCR results showed that the p16 expression (IL-1β: 1.80 ± 0.22 vs. Con: 1.02 ± 0.27; *p < .05, TNF-α: 1.93 ± 0.26 vs. Con: 1.02 ± 0.27; *p < .05) was enhanced after IL-1β or TNF-α treatment, whereas the p19, p21, and p53 expression levels remained unaltered (Figure 3b). This result suggested that p16 responds to IL-1β or TNF-α treatment during corneal epithelial wound healing. Immunofluorescence results also indicated that the staining intensity of p16 was obviously enhanced in the IL-1β- or TNF-α-treated corneas compared with that in the control corneas (Figure 3c). To further investigate the interaction of cytokines IL-1β and TNF-α with p-STAT3 and p16, we detected the protein levels of cytokines, p-STAT3, and p16 in untreated corneas at 24 hr following epithelial debridement. We observed that the expression levels of IL-1β (IL-1β precursor: wound 24 hr 1.33 ± 0.03 vs. unwound 0.65 ± 0.04; **p < .01, mature form of IL-1β: wound 24 hr 1.45 ± 0.08 vs. unwound 0.63 ± 0.06; **p < .01) and TNF-α (wound 24 hr 1.33 ± 0.14 vs. unwound 0.76 ± 0.04; *p < .05) were increased, and the expression level of p16 (wound 24 hr 1.43 ± 0.04 vs. unwound 0.54 ± 0.06; ***p < .001) was also increased, but the expression level of p-STAT3 (wound 24 hr 0.72 ± 0.02 vs. unwound 1.26 ± 0.06; **p < .01) was reduced in the wounded corneal epithelium compared with those in the unwounded corneal epithelium (Figure 3d,e). This result indicated that the transient elevation of IL-1β and TNF-α attenuated the p-STAT3 level and increased the p16 level. Consistently, both immunostaining and western blot showed the marked reduction of p-STAT3 level (IL-1β 0.59 ± 0.02 vs. Con 1.48 ± 0.07; **p < .01, TNF-α 1.03 ± 0.11 vs. Con 1.48 ± 0.07; *p < .05; Figure 3f,g) and a remarkable increase of p16 level (IL-1β 1.09 ± 0.04 vs. Con 0.81 ± 0.04; **p < .01, TNF-α 1.48 ± 0.10 vs. Con 0.81 ± 0.04; **p < .01; Figure 3h,i) in IL-1β- or TNF-α-treated TKE2 cells compared with that in control TKE2 cells. These results demonstrated that persistent IL-1β or TNF-α treatment attenuated the p-STAT3 level and promoted p16 upregulation, which may impede corneal epithelial wound healing.
3.4 The role of p16 in corneal epithelial wound healing depends on STAT3 activity
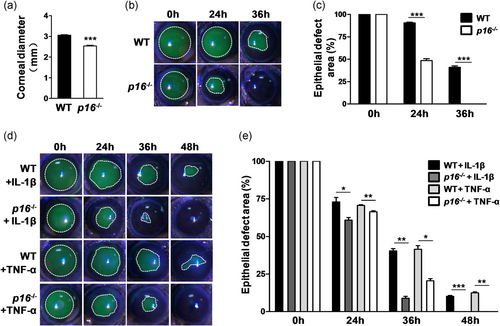
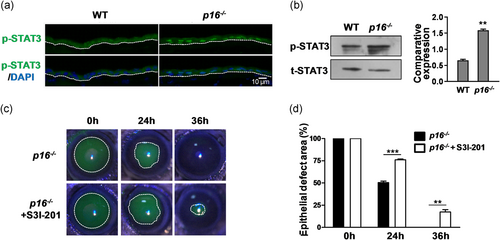
To investigate the role of p16 in corneal epithelial wound healing, we utilized Cdkn2a knockout mice (wherein both p16 and p19ARF were eliminated; since p19 expression did not change during corneal epithelial wound healing, the term p16 knockout (p16−/−) was used hereafter) and WT mice to observe corneal epithelial repair after epithelial debridement. By measurement, we observed that the p16−/− mice had low body weight (p16−/− 22.12 ± 0.76 g vs. WT 25.50 ± 0.85 g; **p < .01) and short body length (p16−/− 7.08 ± 0.04 cm vs. WT 7.62 ± 0.13 cm; **p < .01) compared with the WT mice of the same age (male, about 12-week old; Figure S2). Moreover, the eyes of the p16−/− mice were noticeably smaller (Cheong et al., 2006) than those of the WT mice of the same age. The corneal diameter of the WT mice was ∼3.05 ± 0.05 mm, whereas that of the knockout mice was 2.53 ± 0.09 mm at 12 weeks (Figure 4a). Due to the limitation of the trephine scale, 2.5- and 2-mm corneal central epithelium in WT mice and p16−/− mice, respectively, were removed. Sodium fluorescein labeling experiments showed that the p16−/− mice (healed at 36 hr; epithelial defect area [%] at 36 hr: p16−/− 0.00 ± 0.00 vs. WT 41.82 ± 1.62; ***p < .001) displayed a higher corneal epithelial repair rate than the WT mice (healed at 48 hr; Figure 4b,c). Topical treatment with IL-1β or TNF-α decreased the corneal epithelial healing rate (without complete healing at 48 hr), whereas p16 deletion improved the healing rate (with complete healing at 48 hr) with IL-1β or TNF-α treatment (epithelial defect area [%] at 48 hr: p16−/− + IL-1β 0.00 ± 0.00 vs. WT + IL-1β 10.22 ± 0.54; ***p < .001; p16−/− + TNF-α 0.00 ± 0.00 vs. WT + TNF-α 12.50 ± 1.10; **p < .01; Figure 4d,e).
To determine the mechanism by which p16 modulates corneal epithelial wound healing, we tested the association between p16 and p-STAT3. Immunostaining and western blot indicated that the p-STAT3 level (p16−/− 1.56 ± 0.06 vs. WT 0.66 ± 0.03; **p < .01) in the corneal epithelium of p16−/− mice was extremely enhanced compared with that of the WT mice at 48 hr following debridement (Figure 5a,b). Our results are consistent with those of a recent study, which found a significant increase in p-STAT3 level in p16 negative oropharyngeal squamous cell carcinoma (Lesinski et al., 2019). S3I-201 is a well-known STAT3 inhibitor (Pang et al., 2010), and we further evaluated the effect of the blockage of STAT3 signaling by S3I-201 on corneal epithelial wound healing. In p16−/− mice, topical application of S3I-201 (50 μg per eye) robustly delayed corneal epithelial repair compared with control treatment (epithelial defect area [%] at 36 hr: p16−/− + S3I-201 17.31 ± 2.68 vs. p16−/− 0.00 ± 0.00; **p < .01; Figure 5c,d), indicating that the promoted effect of p16 knockout on corneal wound repair is STAT3 activity-dependent.
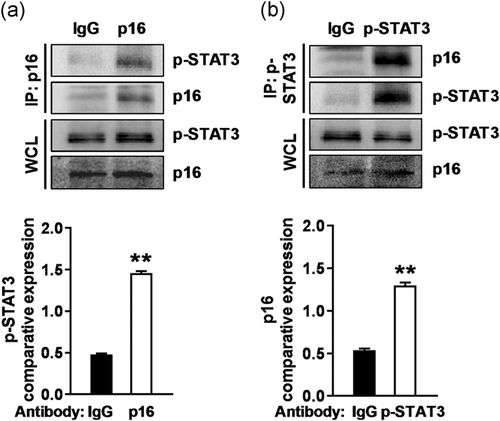
Considering that the total STAT3 level did not change (Figure 5b), we asked whether p16 interacts with p-STAT3. Immunoprecipitation assay in TKE2 cells showed that endogenous p-STAT3 was present in the p16 immunoprecipitates (Figure 6a) and that endogenous p16 was present in the p-STAT3 immunoprecipitates (Figure 6b), but neither of them was detected in the immunoglobulin G immunoprecipitate control (Figure 6). This finding suggested that p16 presumably function as a negative regulator of STAT3 activity at the protein level.
On the basis of the above findings, we conclude that the loss of p16 increased STAT3 activity, which in turn promoted corneal epithelial wound healing. This relationship suggested that p16 deletion relieves the inhibitory effect toward STAT3 activity.
3.5 Expression dynamics of IL-1β, TNF-α, p16, and p-STAT3 in corneal epithelial wound healing in control and diabetic mice
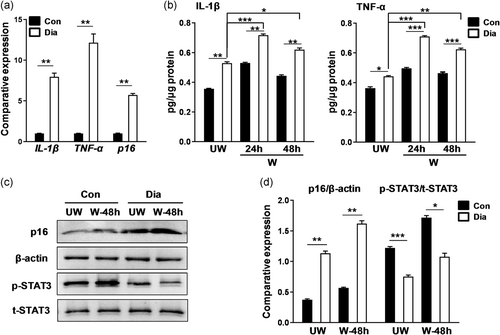
Patients with diabetes mellitus often develop corneal complications and delayed wound healing (Hyndiuk, Kazarian, Schultz, & Seideman, 1977; Ljubimov, 2017; Tsubota, Chiba, & Shimazaki, 1991; Xu, Li, Ljubimov, & Yu, 2009; Yan et al., 2016; L. Zhu, Titone, & Robertson, 2019). Considering that IL-1β and TNF-α affected corneal epithelial repair in WT mice, we determined whether IL-1β or TNF-α is upregulated and whether these cytokines influence corneal epithelial wound healing in diabetic mice. In the qPCR analysis, the mRNA levels of IL-1β (diabetic 7.95 ± 0.80 vs. Con 1.00 ± 0.02; **p < .01), TNF-α (diabetic 12.16 ± 1.85 vs. Con 1.00 ± 0.02; **p < .01), and p16 (diabetic 5.73 ± 0.32 vs. Con 1.00 ± 0.06; **p < .01) in the diabetic corneal epithelium were significantly upregulated compared to those in the control epithelium (Figure 7a). ELISA assay showed IL-1β (diabetic wound 24 hr 0.72 ± 0.01 pg/μg vs. Con wound 24 hr 0.53 ± 0.01 pg/μg; **p < .01) and TNF-α (diabetic wound 24 hr 0.71 ± 0.01 pg/μg vs. Con wound 24 hr 0.50 ± 0.01 pg/μg; ***p < .001) levels went up dramatically at 24 hr and then stabilized until at least at 48 hr after corneal epithelial debridement, indicating their higher and longer expression in diabetic corneas than in control corneas (Figure 7b). Similarly, diabetic corneal epithelium assumed a stronger p16 expression (diabetic wound 48 hr 1.62 ± 0.08 vs. Con wound 48 hr 0.57 ± 0.02; **p < .01) but a lower p-STAT3 level (diabetic wound 48 hr 1.08 ± 0.10 vs. Con wound 48 hr 1.72 ± 0.06; *p < .05) than the control corneal epithelium after corneal epithelial debridement (Figure 7c,d). These results confirmed the sustained and strong expression levels of IL-1β and TNF-α in corneas of the diabetic mice, causing defective healing of the corneal epithelium.
3.6 p16 deficiency partially restores corneal epithelial wound healing in diabetic mice
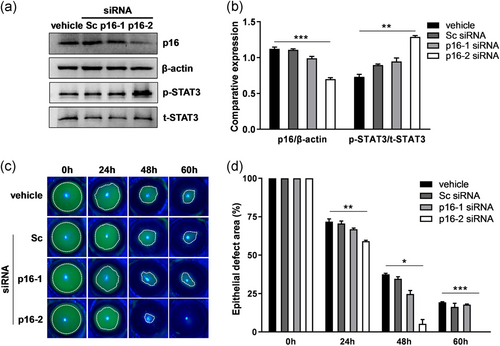
After proving that p16 upregulation impaired corneal epithelial wound healing, we further determined whether knockdown of p16 by siRNAs affects corneal epithelial wound healing in diabetic mice. The efficacy of p16 siRNAs was confirmed by western blot analysis in diabetic corneal epithelium. We found that p16-2 siRNA clearly inhibited p16 expression (p16-2 siRNA 0.70 ± 0.04 vs. vehicle 1.12 ± 0.04; ***p < .001) and reinforced p-STAT3 level (p16-2 siRNA 1.29 ± 0.03 vs. vehicle 0.73 ± 0.06; **p < .01), whereas Sc siRNA or p16-1 siRNA exerted no clear effects on p16 and p-STAT3 expression levels (Figure 8a,b). When subconjunctivally injected into diabetic mice eyes, Sc siRNA or p16-1 siRNA exerted no significant effect on corneal epithelial wound healing, wherein the wound remained unhealed at 60 hr; by contrast, p16-2 siRNA obviously facilitated corneal epithelial wound healing, wherein complete healing was achieved at 60 hr (epithelial defect area [%] at 60 hr: p16-2 siRNA 0.00 ± 0.00 vs. vehicle 19.25 ± 0.33; ***p < .001; Figure 8c,d). Thus, p16 depletion in the cornea partially recovered the delayed corneal epithelial wound healing in diabetic mice. This finding suggested that p16 is a potential therapeutic target for epithelial defects in diabetic corneas.
4 DISCUSSION
We previously demonstrated that transient inhibition of CESCs by IL-1β and TNF-α causes a delay in corneal epithelial wound healing (Yang et al., 2019). In this study, we revealed a novel mechanism by which IL-1β and TNF-α modulate corneal epithelial wound healing. Persistent treatment with IL-1β or TNF-α led to p-STAT3 depression and p16 elevation. Moreover, p16 bound p-STAT3 probably acting as a suppresser of STAT3 activity.
Extensive research has shown the vital role of STAT3 signaling during the wound healing in epithelial cells of the cornea, lung, intestine, and skin (Dauer et al., 2005; Gao et al., 2004; Neufert et al., 2010; Pickert et al., 2009; Zhou et al., 2015; B. M. Zhu et al., 2008). We observed that in the corneas with control treatment, the expression levels of IL-1β, TNF-α, and p16 were increased, but the expression level of p-STAT3 was reduced in the corneal epithelium at 24 hr following debridement (Figure 3d). We further found that IL-1β and TNF-α suppressed STAT3 signaling (including STAT3 activity and the expression of its downstream target Bmi1; Figure S3) probably by elevating p16 expression. Our result was consistent with a previous finding showing that TNF-α induces p16 upregulation in nucleus pulposus cells (Li et al., 2017). In corneal epithelium, we found for the first time that p16 interacted with p-STAT3 and it regulated STAT3 activity, inconsistent with the report that p16 is a downstream target of STAT3 signaling in human corneal endothelial cells (Lu et al., 2016). p16 has been reported to bind a cell death activator-GRIM-19 to block cell cycle progression (Sun et al., 2010); in turn, GRIM-19 interacts with STAT3 to repress STAT3-dependent target gene expression (Lufei et al., 2003; J. Zhang, Chu, Kawamura, Tanaka, & He, 2019; J. Zhang et al., 2003). Thus, we speculate that p16, GRIM-19, and p-STAT3 form a complex to suppress STAT3 activity. Our data thus suggest that p16 functions as a negative regulator of STAT3 activity at the protein level possibly via an indirect or transient interaction.
p16 is a product of Cdkn2a gene, as a cyclin-dependent kinase inhibitor, p16 frequently mediates the inhibition of cell cycle progression and the induction of cellular senescence, thus impairing the function and restricting the regenerative capacity of a tissue (Demaria et al., 2014; Janzen et al., 2006). p16 has been implicated as an upstream regulator of retinoblastoma protein for orchestrating reversible quiescence and irreversible senescence (Imai et al., 2014; Sousa-Victor et al., 2014). In our study, IL-1β and TNF-α repressed CESCs activity, probably driving CESCs to keep quiescent, consistent with the recent evidence showing that niche inflammatory signals keep stem cells in a quiescent state in the aging brain (Kalamakis et al., 2019). However, how these inflammatory cytokines, which are induced by corneal injuries, determine whether CESCs enter the quiescence or the senescence state remains largely unclear.
Dry eye disease and diabetic ocular surface diseases increase the susceptibility to epithelial defects and corneal ulcers, delaying corneal wound healing after epithelial injuries (Di et al., 2017; Kaji, 2005; Lutty, 2013). Growing evidence shows that IL-1β, TNF-α, and IL-6 are elevated in dry eyes (C. Zhang et al., 2016; X. Zhang et al., 2016). The IL-1 subfamily of cytokines, consisting of IL-1α, IL-1β, and IL-1Ra, has been reported to be associated with diabetic keratopathy (Yan et al., 2016). In the present study, we found that IL-1β and TNF-α were transiently increased in corneal epithelium following control corneal debridement, whereas they were sustainably increased in diabetic corneal epithelium following debridement. Accordingly, we hypothesize that the transient elevation of IL-1β and TNF-α is beneficial in maintaining the proper activity of CESCs to avoid their being hyperactivated; however, persistent elevation of IL-1β and TNF-α (such as diabetic keratopathy and dry eye) severely impairs CESCs activity, leading to persistent epithelial defects. Therefore, IL-1β and TNF-α not only affect the inflammatory phase of corneal epithelial wound healing but also influence the proliferative phase by modulating the cell cycle inhibitor p16 and STAT3 signaling.
Overall, our findings suggest that IL-1β and TNF-α function upstream of STAT3 signaling and participate in corneal epithelial repair by mediating p16 expression. This study reveals a novel mechanism through which proinflammatory cytokines modulate corneal epithelial wound healing, and it provides a potential therapeutic target for persistent epithelial defects in diabetic corneas.
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (81670828 and 81770904), Academic promotion programme of Shandong First Medical University (2019RC008), the Science and Technology Foundation of Shinan District, Qingdao, Shandong Province (2020-2-014-YY), Shandong Provincial Nature Science Fund (ZR2017PH065), and the Innovation Project of Shandong Academy of Medical Sciences. Lingling Yang and Qingjun Zhou are partially supported by the Taishan Scholar Program (20161059 and 20150215).
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
X. W. and S. Z. performed the experiments and wrote the main manuscript text. M. D. and Y. L. analyzed the data. L. Y. and Q. Z. checked and revised the manuscript, L. Y., Q. Z., X. W., and S. Z. designed the project and funded this study. All authors have reviewed the manuscript before submission.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




