Versatile role of curcumin and its derivatives in lung cancer therapy
Abstract
Lung cancer is a main cause of death all over the world with a high incidence rate. Metastasis into neighboring and distant tissues as well as resistance of cancer cells to chemotherapy demand novel strategies in lung cancer therapy. Curcumin is a naturally occurring nutraceutical compound derived from Curcuma longa (turmeric) that has great pharmacological effects, such as anti-inflammatory, neuroprotective, and antidiabetic. The excellent antitumor activity of curcumin has led to its extensive application in the treatment of various cancers. In the present review, we describe the antitumor activity of curcumin against lung cancer. Curcumin affects different molecular pathways such as vascular endothelial growth factors, nuclear factor-κB (NF-κB), mammalian target of rapamycin, PI3/Akt, microRNAs, and long noncoding RNAs in treatment of lung cancer. Curcumin also can induce autophagy, apoptosis, and cell cycle arrest to reduce the viability and proliferation of lung cancer cells. Notably, curcumin supplementation sensitizes cancer cells to chemotherapy and enhances chemotherapy-mediated apoptosis. Curcumin can elevate the efficacy of radiotherapy in lung cancer therapy by targeting various signaling pathways, such as epidermal growth factor receptor and NF-κB. Curcumin-loaded nanocarriers enhance the bioavailability, cellular uptake, and antitumor activity of curcumin. The aforementioned effects are comprehensively discussed in the current review to further direct studies for applying curcumin in lung cancer therapy.
Abbreviations
-
- AD
-
- Alzheimer's disease
-
- APC
-
- adenomatous polyposis coli
-
- B [a] p
-
- benzo [a] pyene
-
- BHBA
-
- Bis[2-hydroxybenzyldiene] acetone
-
- CK1
-
- casein kinase 1
-
- CP
-
- cyclodextrin
-
- CSCs
-
- cancer stem cells
-
- CT
-
- computed tomography
-
- CTX
-
- chlorotoxin
-
- DM
-
- diabetes mellitus
-
- DOX
-
- doxorubicin
-
- DTX
-
- docetaxel
-
- ECM
-
- extracellular matrix
-
- EGFR
-
- epidermal growth factor receptor
-
- elF2α
-
- eukaryotic initiation factor 2α
-
- EMT
-
- epithelial-to-mesenchymal transition
-
- ER
-
- endoplasmic reticulum
-
- EZH2
-
- enhancer of zeste homolog 2
-
- FOXP3
-
- forkhead box protein-3
-
- Fzd
-
- Frizzled
-
- GBA
-
- galbanic acid
-
- GSK-3β
-
- glycogen synthase kinase-3β
-
- GSLs
-
- glycosphingolipids
-
- HGF
-
- hepatocyte growth factor
-
- HIF-1α
-
- hypoxia-inducible factor-1α
-
- HMGB1
-
- high mobility group box 1
-
- HO-1
-
- heme oxygenease-1
-
- HSP90
-
- heat shock protein 90
-
- IFN-γ
-
- interferon-γ
-
- Keap1
-
- Kelch-like ECH-associated protein 1
-
- LCP
-
- liposomal curcumin dry
-
- lncRNAs
-
- long noncoding RNAs
-
- MMP
-
- mitochondrial membrane potential
-
- MMP-2
-
- matrix metalloproteinase-2
-
- MNPs
-
- magnetic nanoparticles
-
- MTA1
-
- metastasis-associated gene-1
-
- NAFLD
-
- nonalcoholic fatty liver disease
-
- NDs
-
- neurological disorders
-
- NF-κB
-
- nuclear factor-kappaB
-
- NIR
-
- near infrared light
-
- NQO1
-
- NADPH quinone reductase 1
-
- Nrf2
-
- nuclear factor erythroid 2-related factor 2
-
- NSCLC
-
- non-small-cell lung cancer
-
- OCT
-
- octreotide
-
- P-gp
-
- P-glycoprotein
-
- PD
-
- Parkinson's disease
-
- PECTIN-PVP-CUR
-
- pectin-PVP based curcumin particulates
-
- PEG
-
- polyethylene glycol
-
- PI3K
-
- phosphoinositide 3-kinase
-
- PPTT
-
- plasmonic photothermal therapy
-
- PSII
-
- paris saponin II
-
- PTX
-
- paclitaxel
-
- ROS
-
- reactive oxygen species
-
- SCLC
-
- small cell lung cancer
-
- Ser9
-
- serine9
-
- SOCS1
-
- suppressor of cytokine signaling 1
-
- SOCS3
-
- suppressor of cytokine signaling 3
-
- SOD
-
- superoxide dismutase
-
- STAT3
-
- signal transducer and activator of transcription 3
-
- TCF21
-
- transcription factor 21
-
- TGF-β
-
- transforming growth factor-β
-
- TKI
-
- tyrosine kinase inhibitor
-
- TLR4
-
- toll-like receptor 4
-
- Treg
-
- regulatory T cells
-
- TRIM26
-
- tripartite motif containing 26
-
- TrxR2
-
- thioredoxin reductase
-
- UCA1
-
- urothelial carcinoma-associated 1
-
- UPR
-
- unfolded protein response
-
- VEGFs
-
- vascular endothelial growth factors
-
- VM
-
- vasculogenic mimicry
1 PHARMACOLOGICAL PROFILE OF CURCUMIN
Curcumin (1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione) has a long history of being used in Chinese traditional medicine (Weng & Goel, 2020). For the first time, curcumin was used in the treatment of cholecystitis in 1937 (Hu, Carey, Lindor, & Tabibian, 2018). This naturally occurring nutraceutical compound is the major component of Curcuma longa (turmeric) and exclusively applied as a dietary spice (Willenbacher et al., 2019). It is held that the therapeutic impacts of curcumin emanate from its special structure. Structurally, curcumin has two similar-looking aromatic rings. Curcumin is capable of suppressing the production of reactive oxygen species (ROS) and this capability results from conjugated double bonds (Chikara et al., 2018). The extensive application of curcumin is because of its excellent pharmacological activities including antioxidant (Almatroodi et al., 2020; Mirzaei et al., 2018), anti-inflammatory (J. Xu, Jia, Chen, & Wang, 2020), antitumor (Hesari et al., 2019; Salehi et al., 2020; Shabaninejad et al., 2020), neuroprotective (J. Wang, 2020), antimicrobial (Pourhajibagher, Partoazar, Alaeddini, Etemad-Moghadam, & Bahador, 2020), lipid-modifier (Zhai et al., 2020), antianalgesic (Bulboaca, Bolboaca, Stanescu, Sfrangeu, & Bulboaca, 2017), hepatoprotective (Y. Lu, Wu, Xiang, Li, & Lin, 2020), cardioprotective (Ahmed, Khan, & Mirzaei, 2019; Y. Chen et al., 2020; Hallajzadeh et al., 2019), and osteoprotective (C. Yang et al., 2020). Curcumin has demonstrated great efficacy in the treatment of nonalcoholic fatty liver disease (NAFLD). While attenuating NAFLD, curcumin not only reduces hepatic steatosis but also inhibits insulin resistance (E. S. Lee et al., 2020). Curcumin decreases hepatic steatosis by inhibition of inflammation through nuclear factor-κB (NF-κB) downregulation (D. E. Lee, Lee, Kim, Lee, & Kwon, 2019).
In treatment of diabetes mellitus (DM), as a chronic metabolic disorder (Abbaszadeh-Goudarzi et al., 2020), curcumin affects various targets. Through stimulation of nuclear factor erythroid 2-related factor 2 (Nrf2), curcumin improves antioxidant defense system to protect the myocardium of diabetic rats against oxidative stress (Xiang et al., 2020). Notably, curcumin is able to reduce the levels of inflammatory cytokines to inhibit cardiac and renal dysfunction in diabetic mice (Biswas et al., 2019). It is said that the downregulation of high mobility group box 1 (HMGB1) contributes to the inhibitory effect of curcumin on inflammation in DM (Q. Yan et al., 2020).
It is worth mentioning that curcumin is beneficial in the treatment of neurological disorders (NDs) such as Alzheimer's disease (AD) and Parkinson's disease (PD). A newly published article has exhibited the effect of curcumin on the innate immune system in AD therapy: curcumin downregulates microRNA (miR)-155 to suppress the neurodegenerative phenotype of microglia. Besides, curcumin stimulates the migration of microglia to provide amyloid-beta phagocytosis (Teter et al., 2019), leading to the alleviation of AD. To diminish the stress on neurons, curcumin induces heat shock protein 90 (HSP90), which is a key chaperone involved in the correct conformation of proteins, resulting in inhibition of apoptosis in dopaminergic neurons (Sang et al., 2018).
Despite having great biological and therapeutic impacts, its potential in the treatment of diseases has been restricted by its poor bioavailability (Heidari, Mahdiani, Hashemi, & Kalalinia, 2020). Low water solubility, low absorption, and rapid excretion are involved in poor bioavailability of curcumin. Besides, curcumin is more stable in the acidic environment compared with neutral and alkaline environments (Goel, Kunnumakkara, & Aggarwal, 2008). Both animal and human experiments have demonstrated the poor bioavailability of curcumin as a result of rapid liver metabolism, low absorption in small intestine, and immediate excretion (Hsieh, 2001). A variety of studies have been performed to enhance the bioavailability of curcumin and some of them, particularly nanostrategies have been successful. Different nanocarriers such as liposomes (Ternullo, Schulte Werning, Holsaeter, & Skalko-Basnet, 2019), polymeric nanoparticles (Okagu, Verma, McClements, & Udenigwe, 2020), carbon dots (Pal, Mohiyuddin, & Packirisamy, 2018), and lipid nanoparticles (Laghezza Masci et al., 2019) are able to dramatically promote the bioavailability of curcumin, thereby enhancing its therapeutic capability.
2 ANTI-TUMOR ACTIVITY OF CURCUMIN
Cancer is still one of the increasing challenges worldwide (Shabaninejad et al., 2019; Tamtaji et al., 2020). The field of cancer therapy has undergone a number of changes. During recent years, phytochemicals have opened a new site for themselves in field of cancer therapy due to their capability of eradication of cancer cells (Fathizadeh, Mirzaei, & Asemi, 2019; Sadeghi et al., 2019; Shafabakhsh, Mirzaei, & Asemi, 2020; Vafadar et al., 2020). Increasing evidence demonstrates that curcumin functions in a multitargeted fashion and its capability of interfering with various molecular pathways has made it a suitable choice for cancer therapy. A search in databases such as PubMed shows that curcumin is able to negatively affect the progression, proliferation, and invasion of cancer cells. It is held that curcumin stimulates apoptotic cell death in tongue cancer cells by targeting oxygen-related signaling pathways including TK1, TDRD3, PDE2A, and ACACB (C. Ma, Zhuang, Su, He, & Li, 2020). ERK/c-Jun axis is involved in the malignant behavior of endometrial carcinoma cells. Administration of curcumin is a promising strategy in suppressing the proliferation and migration of cancer cells via inhibition of the ERK/c-Jun axis (Z. Zhang et al., 2019). The epigenetic effects of curcumin are important in cancer therapy, as curcumin enhances the expression of BRCA1 through inhibition of miR-29b and induction of TET1, leading to the suppression of breast cancer cells (Al-Yousef, Shinwari, Al-Shahrani, Al-Showimi, & Al-Moghrabi, 2020). Notably, curcumin is able to target nutrient supply and metabolism of cancer cells. Curcumin reduces the growth of gastric cancer cells by enhancing ROS production, and subsequent stimulation of YAP and JNK signaling pathways result in suppressing glycolysis (T. Chen et al., 2020). The antitumor activity of curcumin is suggested to be dose-dependent (Khan et al., 2020). Signal transducer and activator of transcription 3 (STAT3) contribute to the malignancy and invasion of cancer cells due to its impact on cell proliferation and differentiation (Qin et al., 2020; D. Sun, Liu, & Wang, 2020). The administration of curcumin downregulates the STAT3 signaling pathway to induce apoptosis in cancer cells (Khan et al., 2020). Interestingly, curcumin is able to modulate miRs, so that curcumin supplementation downregulates the expression of miR-21 to trigger apoptosis, and suppress the proliferation and metastasis of cancer cells (L. Chen, Zhan, Wang, You, & Yao, 2020). Curcumin can be administered with other chemotherapeutic agents. In this way, curcumin can affect various molecular pathways such as STAT3 and ANGPTL4 (San et al., 2020), resulting in sensitivity of cancer cells to gemcitabine chemotherapy. Besides, curcumin enhances the accumulation of chemotherapeutic agents in tumor site by suppressing the activity of P-glycoprotein (P-gp), as a factor involved in efflux of antitumor drugs (Fathy Abd-Ellatef et al., 2020). These studies demonstrate that curcumin supplementation is advantageous in cancer therapy by targeting molecular pathways, transporters, and sensitivity to chemotherapy.
These studies highlight the fact that curcumin has a great potential in treatment of various diseases by targeting molecular pathways and in the next section, we discuss the antitumor activity of curcumin to direct further sections into the special discussion of curcumin and lung cancer therapy.
3 LUNG CANCER: EPIDEMIOLOGY AND TREATMENT
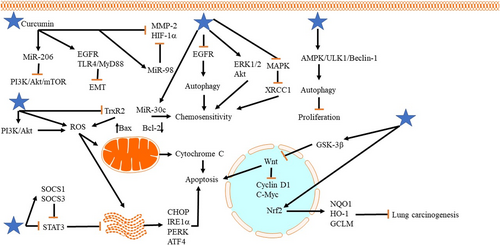
Lung cancer is the leading cause of death in both males and females, and it causes 1.8 million deaths annually (de Alencar, Formiga, & de Lima, 2020). This malignant tumor is the most commonly diagnosed cancer and has poor prognosis; and a 60-month survival rate of patients with Stage I and IV lung cancer is 68% and 0–10%, respectively (Amiri et al., 2020; Duma, Santana-Davila, & Molina, 2019; Torre, Lindsey, & Rebecca, 2016). A variety of factors are involved in the poor prognosis of patients with cancer including diagnosis at an advanced stage, and metastasis into neighboring and distant organs. In the first stages, the patients are free of symptoms, but in advanced stages, various symptoms such as pain, fatigue, dyspnea, and cough emerge that remarkably diminish the quality of life (Cooley, 2000; Ebell, Culp, & Radke, 2016). Notably, treatment is less effective in advanced stages, demanding novel strategies for early diagnosis of lung cancer. This incidence rate of lung cancer is different and depends on regions. Being exposed to cigarette smoke/smoking is considered as the most important factor involved in lung cancer development (Bray et al., 2018). Besides, environmental pollution and epigenetic alterations can lead to lung cancer development (Woodman, Vundu, George, & Wilson, 2020). Histopathologically, lung cancer is divided into two types including small cell lung cancer (SCLC) and non-small-cell lung cancer (NSCLC). NSCLC is the predominant type of lung cancer accounting for about 85% of cases, while SCLC is responsible for 15% of lung cancer cases (Gaspar et al., 2012; Molina, Yang, Cassivi, Schild, & Adjei, 2008; Ramalingam & Belani, 2008; Y. Wang, Zhang, Du, Wu, & Zhang, 2012). There are three major kinds of NSCLC such as adenocarcinoma, squamous cell carcinoma, and large-cell carcinoma. Patients with NSCLC have a poor prognosis, which is due to the resistance of cancer cells to chemotherapy and metastasis. Overall, NSCLC is more dangerous than SCLC. As it was mentioned, cigarette smoking plays a remarkable role in lung cancer development and it has low survival rate (18%) compared with other cancers such as breast cancer (90%), prostate cancer (99%), and colorectal cancer (65%; Alberg, Brock, & Samet, 2005; de Groot, Wu, Carter, & Munden, 2018; L. Gao, Wang et al., 2012; Hecht, 2012). Currently, a variety of techniques are applied in lung cancer diagnosis including computed tomography (CT) scanning, fluorescence bronchoscopy, and polymerase chain reaction (PCR)-based assay of the sputum (Andolfi, Potenza, et al., 2016; Field, Oudkerk, Pedersen, & Duffy, 2013; X. Li, Shen, & Luo, 2017; Pastorino, 2006; Shaw, Akufo-Tetteh, Risk, Field, & Liloglou, 2006; Sutedja, Venmans, Smit, & Postmus, 2001). Chemotherapeutic agents such as paclitaxel (PTX; M. Zhang et al., 2020), docetaxel (DTX; N. Li, Mai, Liu, Gou, & Yang, 2020), cisplatin (CP; H. Zhu, Yang, & Yang, 2020), and gemcitabine (W. Cao et al., 2020) are used in lung cancer therapy. However, the resistance of lung cancer cells has significantly diminished the efficacy of chemotherapeutic agents in the eradication of lung cancer. As a result, molecular biologists and scientists have had a special view into nature, as a source of plant-derived-natural compounds that have high antitumor activity and can significantly suppress the proliferation, viability, and invasion of tumor cells (Banik et al., 2020; Chong et al., 2020; Gupta et al., 2020; Halim et al., 2019; Kashyap et al., 2019; J. H. Lee et al., 2019). In the present review, we demonstrate that how curcumin can be applied in the treatment of lung cancer by emphasizing the molecular pathways to direct further studies into targeting these pathways (Figure 1, Tables 1 and 2).
| Nanocarrier | Size (nm) | Zeta potential (mV) | Entrapment efficiency (%) | Cell line | Major outcomes | References |
|---|---|---|---|---|---|---|
| Lipid-based liposome | 118.4–120.3 | −14.3 | 68–85 | A549 cells | Sustained release of curcumin, enhancing its antitumor activity and targeted delivery | Ibrahim et al. (2018) |
| Polymeric carrier | – | – | 73.6 | A549 cells | Enhancing the level of ROS, induction of MMP loss, and stimulation of apoptotic cell death | Sathuvan et al. (2017) |
| Polymeric micelle | 162 | +25.9 | 21–35 | A549 cells | Enhanced cellular uptake and consequently, higher cytotoxicity against lung cancer cells | Muddineti, Kumari, Ray, Ghosh, and Biswas (2017) |
| Chitosan microsphere | 2.5 ± 0.8 μm | +42.1 | 31.7–54.6 | A549 cells | Increased drug release and induction of apoptosis in cancer cells | Jyoti et al. (2017) |
| Self-assembled micelle | 34.9 | +0.90 | 90.2 | A549 cells | Higher cytotoxicity by induction of cell cycle arrest, apoptosis, and inhibition of migration and invasion via downregulation of MMP-2, MMP-9, VEGF, and Bcl-2 | W.-T. Zhu et al. (2017) |
| Polymeric nanoparticle | 163.8 | −0.31 | 65 | A549 cells | Enhancing ROS production and stimulation of apoptotic cell death | Luo et al. (2017) |
| Lipid nanoparticles | 194.9 | −28.15 | 97.57 | Lewis lung cancer cells | Higher bioavailability of curcumin and induction of apoptosis through PI3K/Akt/FOXO1/Bim axis | S. Li et al. (2016) |
| Micelle | 188 | – | 76.45 | A549 cells | Enhancing the oral bioavailability of curcumin and its cytotoxicity, and inhibition of P-gp expression, leading to reduced proliferation and viability of cancer cells | Patil, Choudhary, Rathore, Roy, and Mahadik (2015) |
| Micelle | 17 | +0.37 | 86.4 | A549 cells | High cellular uptake through caveolae and clathrin mechanism and suppressing the viability and growth of cancer cells | Gu et al. (2016) |
| Nanocrystal | 250 | −40 | – | H460 lung cancer cells | Photoexcited nanocurcumin induces JNK phosphorylation, mitochondrial depolarization, caspase-3 stimulation, and PARP cleavage to induce apoptosis in cancer cells | Paunovic et al. (2016) |
| Solid lipid nanoparticles | 56.2 | −26.2 | 37 | A549 cells | Enhancing the cytotoxicity of curcumin by twofold and stimulation of apoptosis via caspase-3 and -9 activation | S. Jiang et al. (2017) |
- Abbreviations: MMP, mitochondrial membrane potential; ROS, reactive oxygen species; VEGF, vascular endothelial growth factors.
| In vitro | In vivo | Dosage | Duration of experiment | Major outcomes | References |
|---|---|---|---|---|---|
| A549 and H1299 cells | – | Curcumin: 0.78–100 μM | 48 hr | Coadministration of curcumin and palladium induces apoptotic cell death in NSCLC cells via activation of caspase-3 and -7 | Tunc et al. (2017) |
| Palladium: 0.39–50 μM | |||||
| A549 cells | – | 4 μM | 12 and 24 hr | As a derivative of curcumin, MHMD upregulates caspase-3, -8, -9, and -12 and PARP to induce apoptosis in cancer cells. Also, MHMD stimulates actin polymerization to trigger apoptosis through mitochondrial and ER pathways | G.-Z. Zhou, Cao, and Du (2015) |
| NSCLC A549 cells | Lewis lung carcinoma | Curcumin: 10–30 μM | 24 hr | Fenretinide reduced curcumin-mediated GRP78 upregulation to enhance PARP cleavage and stimulate ER stress, leading to reduced invasion and migration of cancer cells | H. Chen et al. (2016) |
| Fenretinide: 2–6 μM | 20 days | ||||
| A549 cell line | – | 100 μM | 24 hr | Decreasing the invasion and proliferation of lung cancer cells through downregulation of GSK-3β and mPGES-1 | Eren and Betul (2016) |
| – | Lewis lung carcinoma | 5, 10, and 20 μM | 24 hr | Curcumin reduces the levels of IL-6 and inhibits the function of myeloid-derived suppressor cells to suppress the growth and proliferation of cancer cells | G.-Y. Liu et al. (2016) |
| A549 cells | – | 1.25 and 2.5 μM | 24 hr | As an analog of curcumin, dimethoxycurcumin enhances ROS production to induce DNA damage and inhibit TrxR2 to sensitize cancer cells to radiotherapy | Jayakumar, Patwardhan, Pal, Sharma, and Sandur (2016) |
| A549 cells | – | 5, 10, 15, 20, 30, 40, and 50 μM | – | Curcumin enhances ROS production to induce apoptosis. Simultaneously, curcumin elevates the levels of ATP to provide adequate energy for apoptosis | Hosseinzadehdehkordi, Adelinik, and Tashakor (2015) |
| A549 and H460 cells | – | 0, 5, 10 and 20 μM | 12 hr | Curcumin elevates the sensitivity of lung cancer cells to chemotherapy and decreases their proliferation via Axl receptor tyrosine kinase downregulation | K. C. Kim, Baek, and Lee (2015) |
| H1299 cells | – | 10 μM | 8, 16, and 24 hr | Induction of necrosis in cancer cells through mitochondrial pathway | F. Li, Chen, Xu, and Zhou (2015) |
| A549 cells | – | 0, 5, 10, and 20 μM | 24 hr | Stimulation of apoptosis and inhibition of cancer growth via upregulation of miR-192–5p and downregulation of PI3K/Akt signaling pathway | Jin, Qiao, Wang, Xu, and Shang (2015) |
| NCI-H460 human lung cancer cells | – | 2 μM | – | Induction of DNA damage, and inhibition of invasion and migration of cancer cells | Chiang et al. (2015) |
| A549 cells | – | 0, 5, 10, 20, 30, 40, 50, and 60 μM | 24 hr | Administration of curcumin significantly diminishes the proliferation and invasion of cancer cells via downregulation of PKCα/NOX-2/ROS/ATF-2/MMP-9 axis | Z. Fan et al. (2015) |
| A549 and H460 cells | – | 1 and 5 μM | 24 hr | Curcumin reduces the DNA methylation of RARβ, as an oncosuppressor factor to inhibit lung cancer malignancy | A. Jiang et al. (2015) |
| Drug-sensitive (A549, SPC-A-1, LTEP-G-2) and drug-resistant (A549/DDP) lung cancer cells | – | 0.6, 1.2, 2.1, and 4.2 μM | 24 hr | A monocarbonyl ligustrazine-curcumin hybrid inhibits Akt, ERK, and P-gp and enhances ROS production to induce apoptosis in lung cancer cells | Ai et al. (2016) |
| A549 and H1299 cells | – | 0, 10, 20, 30, 40, 50, 60, 70, and 80 μmol/L | 24 hr | Curcumin triggers apoptotic cell death in cancer cells by activation of calcium signaling pathway | X. Xu, Chen, Ye, Zhong, and Chen (2015) |
| A549 lung cancer cells | – | 20, 40, and 80 μM | 24 hr | There is a relationship between autophagy and apoptosis, so that curcumin induces apoptosis in cancer cells and inhibition of apoptosis enhances curcumin-mediated autophagy | G. Z. Zhou, Sun, and Zhang (2015) |
| NSCLC 95D cells | – | 0, 10, and 20 μM | 24 hr | Curcumin exerts antiproliferative and anti-migration impacts through inhibition of Wnt, EGR-1, and their crosstalk | Q. Y. Chen, Jiao, et al. (2015) |
| A549 cells | – | 0, 1.25, 2.5, 5, 10, and 20 μg/ml | – | Curcumin sensitizes lung cancer cells to CP chemotherapy via downregulation of FA/BRCA pathway | X. Xu et al. (2015) |
| H460 and A427 cells | – | 0, 5, and 15 μM | 48 hr | Curcumin upregulates the expression of miR-192–5p/215 via p53 induction to inhibit XIAP, resulting in stimulation of apoptosis in lung cancer cells | Ye et al. (2015) |
| Human NSCLC cell lines 95D and A549 cells | – | 0, 15, and 30 μM | 24 hr | Curcumin diminishes the invasion and proliferation of lung cancer cells by inhibition of Wnt/β-catenin signaling pathway via MTA1 induction | Y. Lu, Wei, and Xi (2014) |
| A549 cells | – | 0, 12.5, 25, 50, and 100 μM | 24 hr | Curcumin suppresses the metastasis and invasion of lung cancer cells through adiponectin/NF-κB/MMP inhibition | Tsai et al. (2015) |
| A549 cells | – | 0, 15, 30, 45, and 60 μmol/L | – | Inhibition of lung cancer metastasis through GLUT1/MT1-MMP/MMP-2 downregulation | Liao, Wang, Deng, Ren, and Li (2015) |
| Human lung carcinoma cell line H460 | – | 0.5, 1, 2, 4, and 8 μM | 72 hr | As a curcumin analog, JZ534 stimulates cell cycle arrest and apoptosis via upregulation of caspase-3, Bax, and p53 | J. Wu et al. (2015) |
- Abbreviations: ATP, adenosine triphosphate; ER, endoplasmic reticulum; IL-6, interleukin-6; MMP-2, matrix metalloproteinase-2; NSCLC, non-small-cell lung cancer; PARP, poly (ADP-ribose) polymerase; ROS, reactive oxygen species.
4 CURCUMIN AND LUNG CANCER
4.1 Cellular impacts
4.1.1 Curcumin and migration
Phosphoinositide 3-kinase (PI3K)/Akt signaling pathway can elevate the malignancy and progression of cancer cells by phosphorylation of Akt, whih, in turn, promotes cancer proliferation and induces chemoresistance (Abraham, 2015; Komeili-Movahhed et al., 2015). Newly published articles have confirmed the role of PI3K/Akt in cancer progression. Econazole nitrate can suppress the resistance of cancer cells to chemotherapy via downregulation of PI3K/Akt (Dong et al., 2020). Besides, tripartite motif-containing 26 (TRIM26) inhibits PI3K/Akt to diminish lung cancer growth (Tao et al., 2020). The relationship between curcumin and PI3K/Akt pathway is of importance in lung cancer therapy. In NSCLC cells, curcumin administration (10 and 20 μmol/L for 24 hr) inhibits PI3K/Akt/mTOR axis through upregulation of miR-206 (by 2.5-fold), leading to a decrease in migration and invasion of tumor cells (Y. Song et al., 2020). It is worth mentioning that curcumin can target Akt/mTOR pathway by enhancing the antitumor activity of galbanic acid (GBA). GBA has great antitumor activity and several studies have used GBA as an adjuvant for elevating antitumor activity of chemotherapeutic agents such as DTX (Afsharzadeh, Hashemi, Babaei, Abnous, & Ramezani, 2020; Sajjadi, Karimi, Oskoueian, Iranshahi, & Neamati, 2019). Besides, liposomal nanocarriers are able to enhance the antiproliferative activity of GBA by providing targeted-delivery at tumor site (Nik et al., 2019). However, its potentiality for cancer eradication should be improved. Through downregulation of Akt/mTOR signaling pathway (less than half of normal expression), curcumin (15 mM for 48 hr) elevates the inhibitory effect of GBA on the migration and invasion of NSCLC cells (Z. Zhang et al., 2019), showing that combination therapy with GBA and curcumin can effectively suppress lung cancer metastasis.
Epidermal growth factor receptor (EGFR) undergoes abnormal expression in various cancer cells to ensure their migration, invasion, and differentiation (Georgiou, Stewart, Cunningham, Banerji, & Whittaker, 2020; X. Song, Liu, & Yu, 2020). On the contrary, Toll-like receptor 4 (TLR4)/MyD88 plays a significant role in inflammation and enhances the malignancy and proliferation of different cancers (Ding, Peng, Ding, & Peng, 2019; Koliaraki et al., 2019). Both EGFR and TLR4 can act as upstream mediators to stimulate epithelial-to-mesenchymal transition (EMT) in cancer cells, leading to their malignant behavior (Y. Cao, Shen, Zhang, Zhang, & Zhang, 2019; Shao et al., 2019). Notably, curcumin is capable of targeting both the aforementioned molecular pathways in NSCLC therapy. By inhibition of EGFR and TLR4/MyD88, curcumin (5, 10, and 20 μM for 72 hr) inhibits EMT, resulting in reduced migration and invasion of NSCLC cells (Z. Zhang et al., 2019). Curcumin analog also has an inhibitory impact on EGFR that can be used in suppressing the malignancy of NSCLC cells (Shaik, Al-Kreathy, Ajabnoor, Verma, & Banaganapalli, 2019). A recently published article has investigated the effect of curcumin on lung cancer cells. It is said that curcumin administration (0.25–0.5 μM) is a promising strategy in suppressing the invasion and metastasis of cancer cells and curcumin supplementation does not induce chemoresistance in lung cancer cells. Also, curcumin is not associated with the procarcinogenic effect (Smagurauskaite, Mahale, Brown, Thomas, & Howells, 2020). So, curcumin can be considered as a safe agent in lung cancer therapy. Notably, curcumin derivatives have also high potentiality in suppressing the invasion of lung cancer cells (Bland et al., 2019; Fawzy et al., 2019). However, studies have not examined the molecular signaling pathways that these derivative target and further studies should focus on precise molecular pathways.
4.1.2 Curcumin, chemotherapy, and radiotherapy
As was discussed earlier, chemotherapy is a minimally invasive strategy in the treatment of lung cancer. However, the emergence of resistance has limited the potential of chemotherapy. Doxorubicin (DOX) is one of the effective chemotherapeutic compounds that restricts cancer proliferation by inhibiting microtubule disassembly (Hou, Yun, Xue, Jeon, & Kim, 2020). This agent is extensively applied in treatment of lung cancer (E. S. Lee et al., 2020). Curcumin is able to enhance the sensitivity of lung cancer cells to DOX chemotherapy. Octreotide (OCT)-modified curcumin in combination with DOX micelles selectively accumulate in tumor site to inhibit their malignancy (An et al., 2019). Accumulating data exhibits that matrix metalloproteinase-2 (MMP-2) and hypoxia-inducible factor-1α (HIF-1α) are able to enhance the metastasis of cancer cells (Z. Song et al., 2020; Yin, Xia, Sun, & Zhang, 2020). A combination of OCT-modified curcumin and DOX micelles synergistically inhibits MMP-2 and HIF-1α (50% decrease in expression) to suppress vasculogenic mimicry (VM) channels and metastasis, leading to reduced migration of NSCLC cells (An et al., 2019). Using nanoparticles for delivery of curcumin and DOX can elevate their antitumor activity. Multifunctional nanocarriers widely applied in cancer therapy can release a drug in a prolonged-release manner and are sensitive to mild acidic pH of tumor microenvironment (S. Kim et al., 2019; Ni, Li, Yue, Liu & Li, 2020). Codelivery of DOX and curcumin using nanoparticles considerably diminishes tumor growth and volume by releasing drugs into tumor sites and enhancing its cellular uptake (at least 50% reduction in tumor volume; Hong et al., 2019). These studies show that curcumin can effectively elevate the antitumor activity of DOX in lung cancer therapy.
Earlier, we described the role of EGFR in cancer malignancy. Gefitinib is an EGFR-tyrosine kinase inhibitor (TKI) that inhibits EGFR phosphorylation through attachment into ATP cleft of EGFR (Pao & Chmielecki, 2010). On the contrary, EGFR affects downstream targets such as PI3K, Akt, and mammalian target of rapamycin (mTOR) to suppress autophagy in cancer progression (Botti, Djavaheri-Mergny, Pilatte, & Codogno, 2006). Using EGFR-TKIs is associated with the induction of autophagy in NSCLC cells (Gorzalczany et al., 2011; W. Han et al., 2011; Y.-Y. Li, Lam, Mak, Zheng, & Ho, 2013). Autophagy is a lysosomal-dependent mechanism that sequesters organelles and macromolecules to protect against stress and diseases, particularly cancer (Galluzzi & Green, 2019; Hazari, Bravo-San Pedro, Hetz, Galluzzi, & Kroemer, 2020; Jamali et al., 2020). Curcumin targets autophagy in NSCLC cells to sensitize them to gefitinib chemotherapy. Administration of curcumin (5, 10, and 20 μM for 24, 48, 72, and 96 hr) reduces the expression of EGFR by inhibition of Sp1 and its interaction with HADC1 and also suppresses receptor tyrosine kinases, and ERK/MEK and Akt/S6K signaling pathways. As a consequence of EGFR downregulation, autophagy undergoes upregulation in NSCLC cells that subsequently stimulates apoptotic cell death and sensitizes NSCLC cells to gefitinib chemotherapy (P. Chen et al., 2019). Notably, the resistance of cancer cells to chemotherapy is due to the low cellular uptake of chemotherapeutic agents. Cellular uptake is a vital process that regulates the sensitivity and resistance of tumor cells to CP chemotherapy. CRT1 is a cell membrane importer involving in transporting copper and CP (Ishida, Lee, Thiele, & Herskowitz, 2002). There is a reverse relationship between copper and CP so that high levels of copper can inhibit cellular uptake of CP. It is held that copper regulates the resistance of cancer cells to CP in a CRT1-dependent manner and Cu-Sp1-hCRT1 contributes to copper homeostasis regulation (Kuo, Fu, Savaraj, & Chen, 2012; Z. D. Liang, Tsai, Lee, Savaraj, & Kuo, 2012; I.-S. Song et al., 2008). Curcumin is capable of sensitizing cancer cells to CP chemotherapy in a CRT1-dependent manner. Curcumin (5, 10, and 20 μM) acts as a chelator that suppresses copper influx and, in this way, curcumin elevates the attachment of Sp1 into CRT1. Consequently, an increase occurs in CRT1 expression to increase cellular uptake of CP, resulting in a more inhibitory effect on the proliferation and sensitizing of lung cancer cells (T. Zhang et al., 2018). Akt phosphorylation drives tumor resistance to chemotherapeutic agents and ensures their survival and migration (Das, Kundu, Parekh, Bharadwaj, & Mandal, 2019; X. Yu et al., 2019). ERK is another molecular pathway involved in chemoresistance and its inhibition is associated with enhanced sensitivity of cancer cells to chemotherapy (W. Li et al., 2019; X. Li, He, Tian, Weiss, & Martin, 2019). Curcumin (10 ng/ml) dually targets Akt and ERK1/2 pathways to sensitize lung cancer cells to gefitinib chemotherapy. Curcumin supplementation diminishes the expression of ERK1/2 (75% reduction in expression) and Akt (at least 50% reduction in expression) to induce apoptosis in lung cancer cells, leading to their sensitivity to chemotherapy (Xin et al., 2017).
XRCC1 is an oncogenic factor associated with poor prognosis, lymph node metastasis, and differentiation of head and neck cancer cells (Huang, Chien, Liao, Wang, & Wang, 2019). XRCC1 interacts with other factors such as TP53 to induce cancer development and its stabilization stimulates the chemoresistance of cancer cells (G.-C. Liu, Zhou, Su, & Zhang, 2019; S. Singh, Singh, Singh, & Pandey, 2019; Yousafzai et al., 2019). So, inhibition of XRCC1 is a potential strategy in cancer therapy. In CP-resistant lung cancer cells, the expression of XRCC11 undergoes upregulation. The administration of curcumin (40 μM for 1–3 days) downregulates XRCC1 through MAPK inhibition to suppress the chemoresistance of NSCLC cells (Tung et al., 2016). So, MAPK/XRCC1 axis is a potential target in sensitizing NSCLC cells to chemotherapy.
Similar to chemotherapy, cancer cells are able to acquire resistance to radiotherapy (Lang et al., 2020). Radiotherapy is still a minimally invasive strategy in cancer therapy (Henson et al., 2020). The resistance of cancer cells has remarkably reduced radiotherapy efficacy and oncologists are not able to enhance irradiation dose due to adverse effects on organs of body (W. Jiang et al., 2020). So, sensitizing tumor cells to radiotherapy is the best strategy. Curcumin can target a number of molecular pathways in radiosensitivity. It is worth mentioning that EGFR signaling pathway plays a considerable role in the induction of radioresistance in tumor cells (Gurtner et al., 2019). The cancer cells demonstrating ectopic expression of EGFR are resistant to radiotherapy (Chu, Niu, Ou, & Hu, 2019). Upstream modulator lncRNA FAM201A triggers radioresistance in tumor cells via the upregulation of EGFR (J.-B. Liu et al., 2019). Administration of curcumin (100 μM for 24 hr) is associated with inhibition of EGFR in A549 cells, which, in turn, significantly diminishes proliferation, invasion, and migration of tumor cells, leading to the sensitivity of cancer cells to radiotherapy. Besides, a regime containing curcumin plus CP is more effective in sensitizing lung cancer cells to radiotherapy compared with curcumin alone (Cai, Sheng, & Liang, 2019). In addition to EGFR, the role of NF-κB signaling pathway in radioresistance of tumor cells has been explored. It is held that Aurora-A stimulates the resistance of hepatocellular carcinoma cells to radiotherapy through NF-κB upregulation (Y. Shen, Chen, & Li, 2019). Besides, aurora-a/NF-κB axis is suggested to be involved in the radioresistance of lung cancer cells (J.-B. Liu et al., 2019). Curcumin effectively inhibits NF-κB signaling pathway to potentiate the antitumor effect of radiation (Sak, 2019). These studies demonstrate that curcumin is a cost-effective adjuvant in radiosensitization.
4.1.3 Curcumin and autophagy
In the previous section, we provided a brief explanation of autophagy. It is worth mentioning that autophagy plays like a double-edged sword in cancer therapy. Several researchers believe that autophagy is not an appropriate option in cancer therapy since it acts as a prosurvival mechanism that elevates the proliferation and viability of cancer cells by inhibition of apoptosis (C. Yang et al., 2020), while other studies highlight the fact that autophagy is a promising strategy in suppressing the malignancy of cancer cells (L. Fan, Li, & Wang, 2020; Tian et al., 2019; Wei, Wu, Liu, & Xie, 2020). Professor Daniel Klionsky from Michigan university believes that the autophagy function relies on cancer type and texture, and further studies are needed to clarify its role in cancer therapy (Klionsky, 2020; Y. Yang & Klionsky, 2020). In suppressing NSCLC cells, a novel analog of curcumin ZYX01 activates autophagy. Through induction of AMPK/ULK1/Beclin-1 axis, curcumin remarkably diminishes the viability and proliferation of NSCLC cells (G. Z. Zhou, Wang, Wang, Wang, & Sun, 2019). Another novel derivative of curcumin known as MOMI-1 shows a modulatory impact on autophagy. MOMI-1 (2.5, 5, 10, 20, and 40 μM for 24, 48, and 72 hr) elevates the levels of Beclin-1 and LC3-I/II, while it decreases p62 expression. This stimulates autophagy, which, in turn, reduces the proliferation of A549 cells and is related to G0/G1 cell cycle arrest (G. Z. Zhou, Shi, Wei, & Sun, 2019).
Gangliosides are sialic acid-bearing glycosphingolipids (GSLs) that regulate various aspects of cell proliferation, differentiation, and molecular signaling (Bektas & Spiegel, 2003; D'Angelo, Capasso, Sticco, & Russo, 2013; Hakomori, 2003; Hwang et al., 2010; Hwang, Lee, Lee, & Suk, 2010; Sonnino & Prinetti, 2010). Gangliosides have demonstrated great potential in stimulation of autophagy (Hwang et al., 2010). Ganglioside GD3 is able to interact with LC3 and LAMP1 to promote the formation of double-membrane autophagosomes in autophagy activation (Matarrese et al., 2014). The same is true for curcumin in lung cancer therapy. Curcumin reduces the malignancy of A549 cells by activation of autophagy by triggering the expression of GD3 synthase (hST8Sia I) gene. On the contrary, AMPK is a sensor of energy and a decreased level of ATP and ATP/AMP ratio is associated with stimulation of autophagy (Krishan, Sahni, Leck, Jansson, & Richardson, 2020; M. Lee et al., 2018; R. D. Ma et al., 2020). Investigation of molecular pathways exhibits that AMPK function as an upstream mediator of hST8Sia I. Curcumin (20, 40, 60 and 80 mM for 12 and 24 hr) upregulates AMPK to induce hST8Sia I-mediated autophagy (M. Lee et al., 2018).
PI3K/Akt pathway is considered as a potent modulator of autophagy. Different studies have investigated the relationship between PI3K/Akt and autophagy in disease therapy (Rong et al., 2020; Shou et al., 2020). Also, mTOR is a negative regulator of autophagy (R. D. Ma et al., 2020). Inhibition of mTOR induces autophagy that is of interest in reducing the viability of lung cancer cells (Xia, Dai, Wang, & Zhu, 2020). Curcumin (40 μM for 48 hr) affects PI3K/Akt/mTOR axis in lung cancer therapy so that curcumin supplementation suppresses PI3K/Akt/mTOR signaling pathway (>90% decrease in expression) in A549 lung cancer cells to upregulate Beclin-1 and LC3-II/I ratio, leading to autophagy and reduced viability and proliferation of cancer cells (F. Liu et al., 2018). The studies show that the induction of autophagy suppresses lung cancer malignancy (J.-J. Yu et al., 2020). Furthermore, stimulation of autophagy is associated with the sensitivity of NSCLC cells to gefitinib chemotherapy (M. Zhang et al., 2020). Various molecular pathways function as upstream mediators of autophagy. Curcumin (0.5, 1, 5, 10, and 20 μM for 24, 48, and 72 hr) administration downregulates mTOR (50% reduction in expression) to induce autophagy, leading to reduced proliferation and viability of NSCLC cells. It is worth mentioning that autophagy induction by curcumin sensitizes NSCLC cells to apoptosis (A. Wang et al., 2017).
4.1.4 Curcumin and delivery
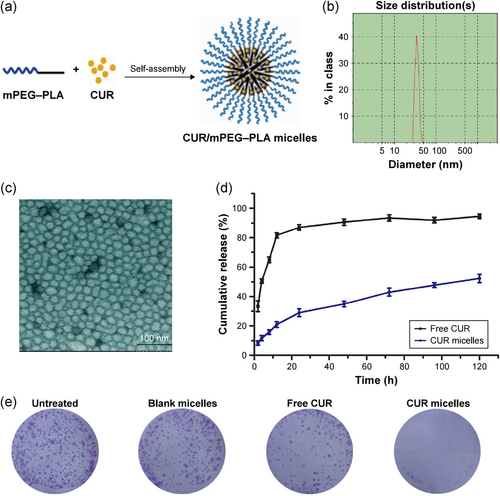
Although curcumin possesses high toxic activity toward cancer cells, it suffers from some limitations such as light sensitivity and poor water solubility (N. Singh, Sachdev, & Gopinath, 2018). Accordingly, to enhance the solubility of hydrophobic drugs and accordingly utilize them in biomedical applications, for example, in drug delivery, different kind of microdevices and nanocarriers are used (Jamaledin et al., 2020; Jyoti et al., 2017; Moeini, Pedram, Makvandi, Malinconico, & d'Ayala, 2020). In light of this, to avail entire benefits of curcumin or at least overlook some of its drawbacks, curcumin-encapsulated systems are being employed (Ibrahim, Tagami, Kishi, & Ozeki, 2018; L. Zhang et al., 2016). This sort of delivery enhances the therapeutic efficacy of curcumin as it ameliorates the bioavailability of curcumin in the local area of the cancer microenvironment. In this direction, different systems including synthetic (e.g., poly(lactic-co-glycolic acid) [PLGA] particles) and natural materials (e.g. polysaccharides such as gum and chitosan) are used to improve the drug ability of cargo (Vakilinezhad, Amini, Dara, & Alipour, 2019; Vijayakurup et al., 2019; Zare et al., 2019; Zare, Makvandi, & Tay, 2019). For instance, micelle self-assembly of polyethylene glycol–polylactide has been used to encapsulate curcumin (Figure 1). The release profile from the nanocarrier showed constant secretion of curcumin. As a result, this prolonged release is in favor for the treatment of NSCLC as the capability of A549 cells to form colonies was clearly changed (W.-T. Zhu et al., 2017).
Benzo [a] pyene (B[a]p) is a carcinogenic agent that is present in fried and grilled food, and also in tobacco smoke (Kazerouni, Sinha, Hsu, Greenberg, & Rothman, 2001). Being exposed to B[a]p is related to the development of cancer by enhancing inflammation, oxidative stress, and stimulation of mitochondrial dysfunction (Omidian, Rafiei, & Bandy, 2017; Wijnhoven et al., 2000). Curcumin-loaded chitosan nanoparticles are suggested to be beneficial in inhibition of B[a]p-mediated lung cancer. Chitosan nanoparticles effectively encapsulate curcumin to enhance its targeted delivery and bioavailability, leading to the amelioration of tumor incidence and more localization in cells by elevating cellular uptake (Vijayakurup et al., 2019). Gold nanoparticles can be applied in plasmonic photothermal therapy (PPTT) triggered by near-infrared light (NIR), leading to hyperthermia and elimination of cancer cells (Bagley, Hill, Rogers, & Bhatia, 2013; Huschka et al., 2012; D. Liu et al., 2016; Sperling, Gil, Zhang, Zanella, & Parak, 2008). However, several cancer cells escape from PPTT and compromise the potential of PPTT in cancer therapy. As a consequence, using novel strategies such as loading anticancer agent enhance their capability in cancer therapy (H. Liu et al., 2011; Wust, Hildebrandt, Sreenivasa, Rau, & Gel, 2002). In agreement with this strategy, multi-stimuli-responsive gold nanorod-curcumin conjugates can be used in cancer therapy. These nanoparticles can penetrate into tumor microenvironment to release curcumin. Under NIR irradiation, an increase occurs in curcumin release with simultaneous photothermal impact of gold nanoparticles, resulting in the effective killing of lung cancer cells and stimulation of apoptotic cell death (Makvandi et al., 2020; Zare et al., 2020; F. Zhu, Tan, Jiang, Yu, & Ren, 2018). For instance, stimuli-responsive curcumin-conjugated gold nanorods have been used for phototherapy and chemotherapy (Figure 2). The in vivo results demonstrated that the existence of both phototherapy and chemotherapy can bring a synergistic effect in mice bearing human lung cancer A549 cells (F. Zhu et al., 2018). Notably, other encapsulating systems, such as β-cyclodextrin, can be used to elevate the entrapment efficiency of gold nanoparticles. As a consequence, the high loading of curcumin on gold nanoparticles significantly enhances their antitumor activity (Hoshikawa et al., 2018).
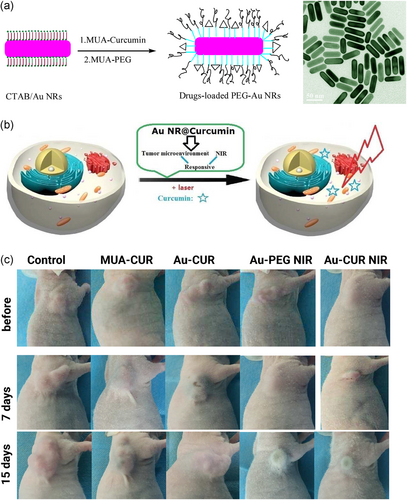
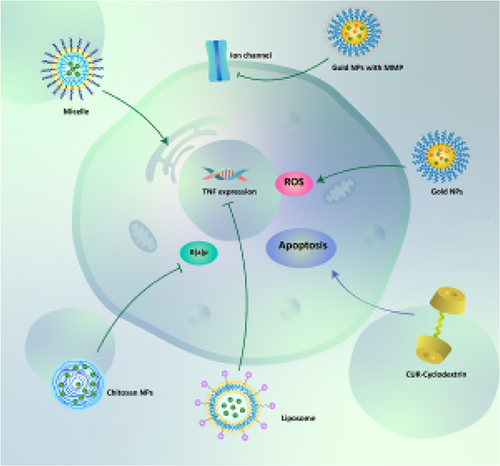
Magnetic nanoparticles (MNPs) have shown great potential in cancer diagnosis and therapy due to their capability in targeting tumor cells (Z. Sun et al., 2016). Conjugation of polyethylene glycol (PEG) into MNPs enhances their circulation time in the blood (Z. Song, Zhu, Liu, Yang, & Feng, 2014). Loading curcumin and chlorotoxin (CTX) on PEG-conjugated gold nanoparticles can inhibit tumor growth (in vivo) and enhance ROS production to induce apoptosis. Also, PEG-modified curcumin- and CTX-loaded gold nanoparticles binds to MMP-2 to suppress ion channels and tumor activity (Y. Yang et al., 2018). Liposomes are other nanocarriers that enhance the bioavailability of hydrophobic drugs by encapsulating them in their bilayer. Liposomes are of interest in cancer therapy as they elevate the cellular uptake of anticancer drugs and enhance their efficacy in the elimination of cancer cells (L. Jiang, Wang, & Chen, 2020; M. Zhang et al., 2020). Notably, liposomes have been used for the treatment of lung disorders such as pneumonia (M. Li, Zhang, Zhu, Wang, & Jin, 2017). Recently, a liposomal curcumin dry (LCD) powder has been developed for the treatment of lung cancer. The LCDs have an average diameter of 5.81 μm and a fine particle fraction of 46.71%, making them appropriate for pulmonary delivery. The LCDs remarkably enhance the cellular uptake of curcumin in A549 lung cancer cells through endocytosis, which, in turn, induce apoptotic cell death (T. Zhang et al., 2018). On the contrary, lung cancer cells stimulate innate immune system to promote the levels of inflammatory factors such as tumor necrosis factor-α (TNF-α; S. Jiang et al., 2017; Patel & Patel, 2017). The LCDs reduce the levels of TNF-α to minimize lung cancer progression (T. Zhang et al., 2018). Surface modification of liposomes can enhance their targeting abilities. RGD peptide elevates the permeability and retention of liposomes in tumor sites (Maeda, Wu, Sawa, Matsumura, & Hori, 2000). So, RGD-modified liposomes are promising candidates in curcumin delivery. RGD-modified PTX- and curcumin-loaded liposomes inhibit cancer cell proliferation and growth in both in vitro and in vivo experiments (K. Jiang, Shen, & Xu, 2018). β-cyclodextrin (CD) is extensively applied in the pharmaceutical industry for delivering drugs due to its solubility, stability, bioavailability, and biocompatibility (Mangolim et al., 2014). CD-curcumin conjugates have been used in prostate cancer therapy so that using CD as a carrier for curcumin delivery significantly enhances its cellular uptake and cytotoxicity (Yallapu, Jaggi, & Chauhan, 2010). CD-curcumin conjugates can also stimulate apoptotic and autophagic cell death in cancer cells to diminish their viability and proliferation (Dhule et al., 2012). In lung cancer cells, curcumin-CD conjugates upregulate the expression of JNK and MAPK to enhance p53 and p21 expression, leading to cell cycle arrest and apoptosis. Also, CD-curcumin conjugates inhibit ERK1/2 expression to downregulate NF-κB signaling pathway, resulting in induction of apoptosis via caspase-3 and Bax upregulation (L. Zhang et al., 2016). This study demonstrates that CD is an efficient carrier for curcumin that significantly elevates its antitumor activity against lung cancer cells. It is worth mentioning that α-cyclodextrin is able to selectively target lung cancer cells by binding into tubulin and microtubules. As a consequence, high levels of curcumin accumulates in tumor site, which, in turn, stimulates apoptosis, inhibits tumor growth, and triggers the expression of p21 and p53 as oncosuppressor factors (Jana et al., 2016). Overall, the studies highlight the fact that using nanoparticles for delivery of curcumin enhances its efficacy in lung cancer therapy by elevating its bioavailability, high cellular uptake, and releasing drug at tumor site (Figure 3; Fahmy, 2019; Khan, Haggag, Lane, McCarron, & Tambuwala, 2018, Ranjan et al., 2016; Roointan, Sharifi-Rad, Badrzadeh, & Sharifi-Rad, 2016; Sadeghzadeh et al., 2017; N. Singh et al., 2018; Su et al., 2019).
4.1.5 Curcumin and apoptosis
High levels of ROS are toxic for cell membrane, protein, and genetic material (Fakhri, Abbaszadeh, Dargahi, & Jorjani, 2018). Induction of oxidative stress via elevating ROS generation is advantageous in cancer therapy (Pelicano, Carney, & Huang, 2004). This is due to the efficacy of ROS in the stimulation of mitochondrial- and endoplasmic reticulum (ER)-mediated apoptosis (Prag, Kula-Alwar et al., 2020; M. Zhang et al., 2020). Notably, curcumin is capable of targeting both mitochondrial and RE in cancer therapy. Utilizing curcumin (12.5, 25, 50, and 100 μM for 24 and 48 hr) can enhance ROS synthesis in cancer cells, which subsequently reduce mitochondrial membrane potential (MMP) to induce mitochondrial dysfunction, which, in turn, activate the intrinsic pathway of apoptosis. On the contrary, curcumin-mediated ROS production negatively affects the homeostasis of ER. As a consequence, ER stress elevates the expression of PERK, IRE1α, BIP, and CHOP that are involved in ER-mediated apoptosis in NSCLC cells (C. Wang et al., 2019). A combination of curcumin and resveratrol inhibits lung carcinogenesis in rats via stimulation of apoptosis in tumor cells (D. Liu, He, Lin, Malhotra, & Yuan, 2019). A monocarbonyl analog of curcumin (Wz35) has the capability of stimulation of apoptotic cell death in NSCLC cells. During ER stress, unfolded protein response (UPR) is activated via the induction of eukaryotic initiation factor 2α (elF2α) phosphorylation (C. Wang et al., 2019, 2020). Then ATF4 undergoes upregulation that subsequently triggers CHOP and ER stress-mediated apoptosis (E.-S. Lee, Yoon, Kim, & Bae, 2007). Wz35 considerably enhances the expressions of elF2α, ATF4, and CHOP in a time- and dose-dependent manner (X. Dai et al., 2018). Besides, Wz35 (1.5, 2.5, and 5 μM for 24 hr) promotes the ROS generation to induce mitochondrial dysfunction and the intrinsic pathway of apoptosis (X. Dai et al., 2018).
Long noncoding RNAs (lncRNAs) have been under attention in cancer therapy and their abnormal expression can be considered as a biomarker for cancer development (Vafadar et al., 2019). The newly published articles confirm the oncogenic role of lncRNA urothelial carcinoma–associated 1 (UCA1). This lncRNA can elevate the progression and proliferation of prostate cancer cells through miR-143 inhibition (Y. Yu, Gao, He, Li, & Ding, 2020). In NSCLC cells, lncRNA UCA1 reduces the expression of miR-143 to induce the resistance of cancer cells to gefitinib chemotherapy (X. Chen et al., 2020). As a consequence, reducing the expression of UCA1 can inhibit cancer progression (Q. Yan, Tian, & Hao, 2020). Curcumin affects the expression of UCA1 in lung cancer therapy. The administration of curcumin (0.2, 0.4, 0.6, 0.8, and 1 μM) reduces the expression of lncRNA UCA1 (twofold reduction in expression) in a dose-dependent manner, leading to the inhibition of lung cancer proliferation and stimulation of apoptotic cell death in cancer cells (W.-H. Wang et al., 2018). On the contrary, lncRNAs act as upstream modulators and are able to affect various molecular pathways. Accumulating data demonstrates that Wnt and mTOR signaling pathways are downstream targets of lncRNA UCA1 and this lncRNA stimulates Wnt and mTOR to enhance cancer malignancy (H. Ma, Su, Feng, Guo, & Su, 2019; Zhong, Wang, He, Xiong, & Cao, 2020). Curcumin downregulates the expression of UCA1 to suppress Wnt and mTOR, leading to apoptosis in A549 cells (W.-H. Wang et al., 2018).
The PI3K/Akt molecular pathway is a potential target in cancer therapy via antitumor drugs. There are controversial data about the relationship between PI3K/Akt and apoptosis. Some of the antitumor drugs induce apoptosis via inhibition of PI3K/Akt (X. Gao et al., 2020), whereas others stimulate PI3K/Akt to trigger apoptotic cell death in cancer cells (Yoon et al., 2020). However, the studies show that there is a close relationship between apoptosis and PI3K/Akt signaling pathway (Reddy, Kumavath, Tan, Ampasala, & Kumar, 2020). A novel derivative of curcumin known as T59 (2.5, 5, and 1 μM for 24 hr) enhances the levels of ROS, caspase-3, and Bax through activation of PI3K/Akt signaling pathway to induce apoptosis in lung cancer cells (Z. Zhao, Yang, Liu, & Li, 2018). Notably, newly published articles demonstrate that novel derivatives of curcumin mainly act by elevating ROS synthesis, upregulating caspases and Bax, and inducing intrinsic pathway apoptosis through mitochondrial dysfunction (G.-Z. Zhou, Li, Sun, & Sun, 2018). However, another study provides controversial results. It is held that a combination of curcumin and paris saponin II (PSII) stimulates apoptosis in lung cancer cells via inhibiting the PI3K/Akt signaling pathway (Man et al., 2018). So, more studies are needed to clarify the effect of curcumin on PI3K/Akt in lung cancer therapy.
Thioredoxin reductase (TrxR2) is a mitochondrial factor that elevates the proliferation of cancer cells and its inhibition is of interest in cancer therapy (Du et al., 2019). In NSCLC cells, TrxR2 downregulation diminishes the growth and metabolism of cancer cells and stimulates apoptosis (Bu et al., 2017). Mitocurcumin is a derivative of curcumin that selectively affects mitochondria. By enhancing ROS production and induction of DNA damage, mitocurcumin (0.5, 1, 2.5, and 5 μM for 24 hr) increases Bax/Bcl-2 ratio to release cytochrome C from mitochondria and stimulate apoptosis. Mitocurcumin inhibits TrxR2 to enhance ROS accumulation in mitochondria, resulting in more apoptosis in cancer cells (Jayakumar et al., 2017).
A combination of curcumin and quercetin negatively affects the phosphorylation of p53 in lung cancer cells. By modulation of p53 at the posttranslational level, curcumin and quercetin effectively induce apoptosis through caspase-3 and -9 activation, leading to reduced proliferation and viability of cancer cells (P. Zhang & Zhang, 2017). A novel derivative of curcumin known as MHMD affects genes involved in apoptosis including caspase-7, p53, TGFA, and prkar1b to induce apoptosis in lung cancer cells (G. Zhou, Sun, Zhou, & Wang, 2017). Another analog of curcumin, 2,2/-fluorine monocarbonyl curcumin (25 μM for 20–140 hr) elevates ROS production in lung cancer cells to induce MMP loss, resulting in apoptotic cell death through the mitochondrial pathway (G.-Y. Liu, Zhai, Chen, Zhang, & Yang, 2016). A novel derivative of curcumin known as compound 10 (2.7, 4.1, and 5.4 µM) has a more inhibitory effect on P-gp expression compared with curcumin. Subsequently, the accumulation of compound 10 enhances tumor cells to stimulate apoptosis and G2 cell cycle arrest (Lopes-Rodrigues et al., 2017).
4.1.6 Curcumin and extracellular matrix (ECM) receptors
The ECM receptors are transmembrane proteins that modulate the cell-cell attachment or cell attachment into extracellular molecules (Multhaupt, Leitinger, Gullberg, & Couchman, 2016). The ECM-receptor interaction has an oncogenic role in elevating the migration, proliferation, and differentiation of cancer cells (Berrier & Yamada, 2007; Hynes, 2009; Wozniak, Modzelewska, Kwong, & Keely, 2004). Tumor cells adhere to ECM via collagen and integrin to stimulate oncogenic signaling pathways such as PI3K/Akt, MAPK, ERK, and Rho/ROCK that induce chemoresistance and cancer malignancy (Carragher & Frame, 2004; Golubovskaya, 2013; J. Zhao & Guan, 2009, 2011). Furthermore, collagen and laminin can enhance the malignant behavior of cancer cells through EMT activation (F. Liu et al., 2018). Administration of curcumin (2–32 μg/ml for 48 hr) targets EMT receptors such as collagen (COL1A1, COL4A1, and COL5A1), integrin (ITGA2B and ITGA3), and laminin (LAMA5). By reducing the expression of the aforementioned genes, curcumin stimulates apoptotic cell death in A549 cells and restricts their migration and metastasis (X. Li et al., 2017).
4.1.7 Curcumin and angiogenesis
Angiogenesis is the formation of new vessels from pre-existing ones (Hashemi Goradel et al., 2018; Ramadan, Zaher, Altaie, Talaat, & Elmoselhi, 2020). Proangiogenic molecules such as vascular endothelial growth factors (VEGFs) and antiangiogenic molecules such as thrombospandins and angiostatin interact with each other to regulate angiogenesis (Ferrara, 2019; Folkman, 2006; Mashreghi et al., 2018; Muppala et al., 2017; Ren, Yee, Lawler, & Khosravi-Far, 2006). Angiogenesis is a vital mechanism for physiological processes including wound healing and embryogenesis. However, stimulation of angiogenesis can improve oxygen and nutrient strategies, leading to enhanced proliferation and growth of tumor cells. Inhibition of angiogenesis is a potential strategy in lung cancer therapy. Recently published articles have explored the role of angiogenesis in lung cancer therapy. The lncRNA F630028O10Rik reduces the expression of VEGF A through miR-223-3p downregulation, leading to the inhibition of angiogenesis and malignancy of lung cancer cells (Y. Qin et al., 2020). Curcumin follows the same route in lung cancer therapy. Pectin-PVP-based-curcumin particles (PECTIN-PVP-CUR) are able to accumulate in lung due to their low size (2.74 μm). The PECTIN-PVP-CUR inhibits the angiogenesis of lung cancer cells to minimize their migration and localize these cancer cells (Gaikwad et al., 2017). The drawback of the mentioned study is the lack of investigation of molecular pathways. Further studies should demonstrate PECTIN-PVP-CUR effect on molecular pathways that inhibit angiogenesis in lung cancer cells. The hepatocyte growth factor (HGF) is an upstream activator of the c-Met signaling pathway that is associated with the poor prognosis of patients with cancer (D. Gao, Vahdat, Wong, Chang, & Mittal, 2012; Gentile, Trusolino, & Comoglio, 2008). HGF-c-Met axis can stimulate EMT to elevate the malignant behavior of lung cancer cells (Z.-T. Shen et al., 2019). Furthermore, HGC can enhance angiogenesis and stimulate tumor microenvironment remolding to elevate the progression of cancer cells (D. Gao et al., 2012; Gentile et al., 2008). Application of curcumin (10, 20, and 30 μmol/L for 12 hr) prevents EMT and angiogenesis via downregulation of the HGF/c-Met pathway and its downstream target PI3K/Akt/mTOR axis, resulting in inhibition of angiogenesis and EMT of lung cancer cells (D. Jiao et al., 2016).
Investigation of antitumor activity on rats and mice is a step into paving the road for using of curcumin in clinical trials. In line with this strategy, Li et al. administered curcumin (100 mg/kg) through intraperitoneal route. The results demonstrated that curcumin is a potent antitumor agent in reducing tumor growth and weight in mice bearing lung cancer A549 cells. In this way, curcumin effectively downregulates the expression of VEGF, HIF-1 and NF-κB (X. Li et al., 2018). Further studies should be allotted into using curcumin-loaded nanoparticles to enhance its antitumor and biocompatibility in vivo experiments.
4.1.8 Curcumin and T cells
The escape of cancer cells from immune surveillance is associated with their tolerance and enhanced proliferation (Sommariva et at., 2020). The CD4+ CD25+ forkhead box protein-3 (FOXP3) regulatory T cells (Treg) contribute to immune tolerance of cancer cells by production of TGF-β and other cytokines to ensure the growth of cancer cells and their escape from cancer-specific cytotoxic CD8+ T cells (B.-Q. Wang et al., 2011). This remarkably enhances cancer growth and various studies have examined the anti-Treg strategies in tumor therapy (W. Li et al., 2019). Curcumin has demonstrated great potential in targeting Treg cells in various diseases (Shafabakhsh et al., 2019). In patients with lung cancer, administration of curcumin (1.5 g/capsule for 2 weeks) enhances the conversion of Treg cells into Th1 cells via downregulation of FOXP3 and upregulation of interferon-γ (IFN-γ). As a consequence, an upregulation occurs in the levels of Th1 cells, while the levels of Treg cells decrease, resulting in immune responses against lung cancer cells (Figure 4; Zou et al., 2018).
4.1.9 Curcumin and cancer stem cells (CSCs)
CSCs are subsets of cancer cells possessing self-renewal and differentiation capabilities (Dalerba, Diehn, Weissman, & Clarke, 2020). The CSCs were first isolated from leukemia and then, they were shown to be in other cancers, particularly lung cancer (Hsu et al., 2020). Concerning the role of CSCs in the progression and recurrence of cancer, targeting CSCs is of importance in cancer therapy. Curcumin has great capability in an effective eradication of lung cancer so that curcumin (0, 5, 10, 20, and 40 μM for 7 days) suppresses Wnt/β-catenin signaling pathway to inhibit CSCs markers, colony formation, and reduce the number of CD133-positive cells, leading to the apoptotic cell death and inhibition of proliferation of CSCs and eradication of lung cancer (W.-T. Zhu et al., 2017). Targeting the self-renewal capability of CSCs inhibits lung cancer progression. Administration of curcumin stimulates DNA damage in CSCs and suppresses DNA repair mechanisms to inhibit lung cancer malignancy and proliferation (Mirza, Vasaiya, Vora, Jain, & Rawal, 2017). It is worth mentioning that curcumin is capable of enhancing the inhibitory effect of chemotherapeutic agents on CSCs. The administration of curcumin (10, 20, 30, and 40 μM for 48 hr) induces cell cycle arrest in CSCs through cyclin D1 downregulation. Besides, curcumin upregulates the expression of p21 to stimulate caspase-9 and Apaf-1, leading to apoptotic cell death in CSCs and decreased invasion and metastasis of NSCLC cells (Baharuddin et al., 2016). In the previous sections, we discussed the role of the STAT3 signaling pathway in cancer cells and the effects of curcumin therapy on STAT3. Notably, STAT3 is able to target CSCs and elevates their radioresistance (Park et al., 2019). The lncARSR stimulates the chemoresistance of CSCs (C. Yang et al., 2019). So, STAT3 activation is associated with poor prognosis by preserving the tumorigenesis effect of CSCs (X.-F. Xu et al., 2019). Curcumin is able to suppress the proliferation and colony formation of CSCs to inhibit lung cancer growth. The investigation of molecular pathways exhibits that curcumin (20 μm for 24 hr) downregulates JAK2/STAT3 to exert its inhibitory effect on CSCs in lung cancer therapy (J. Wu et al., 2015).
4.2 Signaling pathways
4.2.1 Curcumin and Wnt/β-catenin signaling pathway
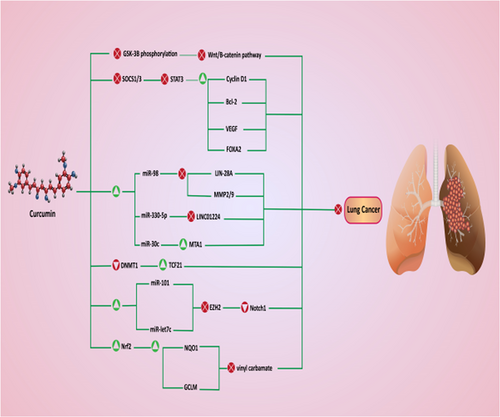
Wnt/β-catenin signaling pathway is able to regulate various biological processes such as cell growth, cell differentiation, cell apoptosis, and cell migration (Chiarini, Paganelli, Martelli, & Evangelisti, 2020; Patel, Alam, Pant, & Chattopadhyay, 2019). Briefly, there are transmembrane receptors known as Frizzled (Fzd) and Wnt ligands bind into them. In the absence of Wnt ligands, a destruction complex containing adenomatous polyposis coli (APC), an axis scaffold protein, glycogen synthase kinase-3β (GSK-3β), and casein kinase 1 (CK1) directly get involved in proteosomal degradation of β-catenin to inhibit its nuclear translocation and activate downstream targets such as c-Myc, cyclin D1, CCND1. By attachment of Wnt ligand into Fzd, the destruction complex activity is inhibited, which, in turn, triggers nuclear translocation of β-catenin (Patel et al., 2019; Stamos & Weis, 2013). Increasing evidence exhibits that Wnt/β-catenin contributes to cancer progression and its inhibition is of importance in cancer therapy (Fendler et al., 2020; van Schie & van Amerongen, 2020). Curcumin supplementation (2.5–80 μm for 24 hr) inhibits GSK-3β phosphorylation at Serine9 (Ser9) to maintain its activity. As a result, β-catenin undergoes proteosomal degradation and its nuclear translocation is inhibited (2.5-fold reduction in its expression). Downstream targets such as c-Myc and cyclin D1 undergo suppression that consequently inhibits the growth of NSCLC cells (W.-H. Wang et al., 2018).
4.2.2 Curcumin and STAT3 signaling pathway
Briefly, the STAT3 signaling pathway possesses a pivotal role in the regulation of biological processes and its abnormal expression occurs in various cancers (Dutta et al., 2020; H. Yu, Pardoll, & Jove, 2009). Both upregulation and downregulation of STAT3 can mediate cancer progression and proliferation (Z. Liang et al., 2020; Y. Qin et al., 2020). So, restoring its normal expression is of importance in cancer therapy. The STAT3 acts as an upstream modulator that affects various targets. FOXA2 is suggested to be a downstream target of STAT3 signaling pathway (Hao et al., 2014). The FOXA2 is an oncosuppressor factor and its downregulation enhances the migration of tumor cells (Bow et al., 2020). Besides, FOXA2 and PGC-1β cooperation is involved in suppressing the malignancy and proliferation of cancer cells (J. Cao et al., 2019). Curcumin targets the STAT3/FOXA2 axis in lung cancer therapy. Administration of curcumin (10 and 40 mM for 24 hr) reduces the proliferation of lung cancer cells by FOXA2 overexpression (up to 5-fold increase in expression) through STAT3 inhibition (Tang et al., 2018). Noteworthy, there are endogenous inhibitors that negatively affect STAT3 expression to suppress its activity. Suppressor of cytokine signaling 1 and 3 (SOCS1 and SOCS3) are STAT3 inhibitors and their downregulation elevates the proliferation of cancer cells (N. Li et al., 2020; K. J. Zhang, Tan, & Guo, 2020). Curcumin is capable of enhancing the expression of SOCS3 and SOCS1 to inhibit STAT3 and lung cancer growth (Tang et al., 2018). It is held that curcumin (1, 2, and 4 hr for 1–12 hr) downregulates the expression of the STAT3 signaling pathway (>50% decrease in expression) in a dose-dependent manner to inhibit colony formation and cell growth, and induce cell cycle arrest (Feng et al., 2017). Several downstream targets of STAT3 including VEGF, Bcl-2, and cyclin D1 mediate the stimulatory impact of STAT3 on invasion and proliferation of cancer cells (X. Chen et al., 2019; L. Chen, Lin, Xian, & Zheng, 2019; A. Singh et al., 2019). Curcumin has demonstrated a great potential in inhibition of STAT3 and its downstream targets in both in vitro and in vivo experiments. By inhibition of STAT3, curcumin (100 mg/kg for 60 days) significantly decreases the expressions of cyclin D1, Bcl-2, and VEGF to inhibit cancer cell proliferation, formation, and migration (X. Xu & Zhu, 2017). In fact, by targeting STAT3 signaling pathway, curcumin impairs a dynamic signaling network in which tumor cells require.
4.2.3 Curcumin and miRs
MiRs are endogenous, short noncoding RNAs that were believed to be “junk factors” in the genome (Nahand et al., 2020; O'Neill & Dwyer, 2020). Nowadays, it is hypothesized that miRs have evolutionary and regulatory roles. They form dynamic and complex networks with other molecular pathways to regulate cellular metabolism and growth as well as other important biological mechanisms (X. Jiao et al., 2020; X. Lu et al., 2020; Nahand et al., 2019). Consequently, abnormal expression of miRs is associated with cancer development and progression (Z. Liang, Feng, & Shim, 2020). On the contrary, curcumin is able to affect miRs in disease therapy (Z. Han, Zhang, Zhang, & Zhao, 2020). In lung cancer cells, curcumin targets miRs. The miR-98 is an oncosuppressor miR that reduces cancer malignancy through inhibition of EMT (M. Zhu, Zhang, Chen, Chen, & Zheng, 2019). Besides, miR-98 can sensitize cancer cells to CP chemotherapy (Guo et al., 2019). In NSCLC cells, miR-98 suppresses the viability and growth of cancer cells (F. Jiang et al., 2019). Administration of curcumin (25–100 μM for 6–48 hr) upregulates the expression of miR-98 (up to 2.5-fold increase) to inhibit LIN-28A, MMP-2, and MMP-9, leading to a decrease in cancer migration and proliferation (W.-L. Liu et al., 2017). The miR-330-5p is another oncosuppressor miR that is induced by lncRNA LINC01224 in hepatocellular carcinoma to suppress their proliferation (Gong et al., 2020). Stimulation of miR-330-5p also reduces the migration and invasion of cervical cancer cells (S. Chen & Wang, 2019). In lung cancer cells, curcumin (10 μM for 14 hr) exerts the most stimulatory impact on miR-330-5p. Enhanced expression of miR-330-5p has a reverse relationship with metastasis of lung cancer cells and diminishes their invasion (Zhan et al., 2017). Noteworthy, miRs can sensitize cancer cells to chemotherapy (Lafin et al., 2020). MiR-30c is an oncosuppressor miR that inhibits the chemoresistance of cancer cells by targeting IL-1 and TWF1 (Bockhorn et al., 2013). Besides, miR-30c downregulates the expression of metastasis-associated gene-1 (MTA1) to inhibit cancer migration and invasion (H. Zhou et al., 2012). The resistance to chemotherapy is a common phenomenon in NSCLC cells. Administration of curcumin (10–50 μM for 48 hr) diminishes the expression of MTA1 (75% decrease in expression) via miR-30c upregulation to enhance the sensitivity of NSCLC cells to PTX chemotherapy (Y. Lu et al., 2017).
4.2.4 Curcumin and TCF21
Several studies have demonstrated that curcumin has the capability of epigenetic targeting of genes by affecting DNA methylation (Alexander et al., 2015; Taverna et al., 2015). To influence the methylation of cancer-related genes, curcumin decreases the catalytic activity of DNMT1, as a methyltransferase (A. Jiang et al., 2015; Z. Liu et al., 2009). The transcription factor 21 (TCF21) is an oncosuppressor protein that can be modulated by methylation (Y. Dai et al., 2016). The recent literatures have shed some light on the role of TCF21 in cancer therapy. Enhancing the expression of TCF21 is associated with decreased viability of gastric cancer cells and their sensitivity to chemotherapy (Z. Yang et al., 2019). TCF-21 upregulation induces apoptosis in breast cancer cells and inhibits their migration via angiogenesis downregulation (W. Li et al., 2019). The invasion of bladder cancer cells undergoes upregulation by inhibition of TCF21 via miR-3648 (W. Sun et al., 2019). Curcumin positively affects TCF21 in lung cancer therapy. Curcumin (5 and 10 μM for 48 hr) elevates the expression of TCF21 (up to fourfold increase) via DNMT1 downregulation (75% decrease in expression) to suppress the proliferation, migration, and colony formation of lung cancer cells (G.-Q. Wu et al., 2016).
4.2.5 Curcumin and enhancer of zeste homolog 2 (EZH2)
The EZH2 is an oncogenic factor that its stability elevates the progression and proliferation of lung cancer cells (Zheng, Chu, Lin, He, & Wang, 2020). Inhibition of EZH2 suppresses angiogenesis and migration of endometrial cancer cells (Roh et al., 2020). The upstream modulators such as lncRNA PVT1 and linc01088 are able to upregulate the EZH2 to ensure the malignancy and invasion of NSCLC cells (J.-Q. Liu, Feng, Zeng, & Zhong, 2020; Qiu, Li, Sun, & Yang, 2020). Notch1 is another oncogenic factor that stimulates EMT, metastasis, and chemoresistance in cancer cells (Peng et al., 2020; Zeng et al., 2020). On the contrary, EZH2 acts as an upstream modulator of Notch1 in cancer cells (S. Wang et al., 2020). In lung cancer cells, curcumin (1–15 μmol/L) upregulates the expression of miR-101 and miR-let7c to inhibit EZH2 (25–50% reduction in expression). Due to the presence of a reciprocal loop between EZH2 and Notch1, inhibition of EZH2 reduces Notch1 expression, resulting in suppressing of growth and metastasis of lung cancer cells (G.-Q. Wu et al., 2016).
4.2.6 Curcumin and Nrf2 signaling pathway
The increased level of oxidant molecules leads to damages in DNA and cell membrane (Cordani et al., 2020). Enhancing ROS generation is an efficient strategy in killing cancer cells by stimulation of mitochondrial- and ER stress-mediated apoptosis (J. R. Liang & Yang, 2020; X. L. Sun, Zhang, Zhai, Zhang, & Ma, 2020). However, increased levels of oxidant molecules have carcinogenesis impact on normal cells (Lesiow, Komarnicka, Kyziol, Bienko, & Pietrzyk, 2019). So, reinforcing antioxidant defense system can inhibit carcinogenesis effect of oxidant agents. The Nrf2 signaling pathway enhances the antioxidant defense system to ameliorate ROS production (Kasai, Shimizu, Tatara, Mimura, & Itoh, 2020). During normal condition, Kelch-like ECH-associated protein 1 (Keap1) and cullin-3 E3 ligase cooperate to induce proteosomal degradation of Nrf2 through ubiquitination (La Rosa, Bertini, & Piemonte, 2020). This inhibits nuclear translocation of Nrf2 and stimulation of antioxidant factors (Itoh, Ye, Matsumiya, Tanji, & Ozaki, 2015). However, increased levels of ROS trigger structural alterations in Keap1 and cullin-3 E3 ligase, so that they are no longer capable of ubiquitination of Nrf2 and its proteosomal degradation (Yamamoto, Kensler, & Motohashi, 2018). Subsequently, Nrf2 accumulates in cytoplasm and then, translocates into nucleus, where it induces downstream targets such as superoxide dismutase (SOD), heme oxygenease-1 (HO-1), and NADPH quinone dehydrogenase 1 (NQO1), resulting in improvement in antioxidant defense system and attenuation of oxidative stress (Smolkova et al., 2020). A novel derivative of curcumin known as Bis[2-hydroxybenzyldiene] acetone (BHBA) has demonstrated great potential in targeting the Nrf2 signaling pathway. In lung cells exposed to vinyl carbamate, an increase occurs in the level of oxidative stress through elevating ROS production. Administration of BHBA (1.3–80 μM for 16 hr) effectively suppresses vinyl carbamate-induced lung cancer by stimulation of the Nrf2 signaling pathway and upregulation of downstream targets such as NQO1 and GCLM (T. Shen et al., 2015). However, Nrf2 induction is a potential strategy in suppressing procarcinogenic impact of vinyl carbamate in lung cells. The elimination of lung cancer cells relies on elevating ROS levels through Nrf2 inhibition. More studies are needed to clarify the aforementioned discussions (Figure 5).
5 CONCLUSION AND REMARKS
Minimal toxicity and great therapeutic impacts have led to the extensive application of plant-derived natural compounds in cancer therapy (Banik et al., 2020; X. Chen et al., 2019; J. H. Lee et al., 2019). The naturally occurring compounds target various molecular and cellular aspects of cancer cells (Kashyap et al., 2019; J. H. Lee et al., 2019). In the present review, we demonstrated the potential of curcumin as a plant-derived-natural compound in cancer therapy. Curcumin has great pharmacological activities and is able to affect different molecular pathways in cancer therapy. We comprehensively discussed the role of curcumin in lung cancer therapy.
The metastasis and invasion of lung cancer cells into neighboring and distant tissues have resulted in high deaths. Curcumin inhibits PI3K/Akt/mTOR axis to suppress the migration of lung cancer cells and it can also enhance the antitumor effects of drugs such as GBA through inhibition of Akt/mTOR. Besides, curcumin downregulates EGFR and TLR4/MyD88 to suppress EMT, resulting in reduced migration and invasion of cancer cells. Curcumin and its derivatives sensitize cancer cells to chemotherapy and radiotherapy via inhibition of MMP-2, HIF-1α, EGFR, XRCC1, MAPK, and NF-κB. Curcumin and its derivatives are able to stimulate apoptotic and autophagic cell death in lung cancer cells to diminish their viability and proliferation.
Despite having excellent antitumor activity, poor solubility of curcumin has limited its efficacy in the elimination and eradication of lung cancer cells. Consequently, several nanocarriers such as polymeric nanoparticles, liposomes, lipid-based nanoparticles, carbon-based materials (e.g., quantum dots and graphene-based nanosheets) and inorganic nanostructures, that is, gold nanorods, have been developed to elevate its antitumor activity against lung cancer cells by enhancing its cellular uptake. These platforms enhance the therapeutic efficacy by ameliorating the bioavailability of curcumin in the tumor microenvironment. The nanocarriers overcome the abovementioned hurdles by improving the stability of curcumin before being deactivated in the body as well as using the sustained release of this biotherapeutic to the cancer cells (Ahmadi Nasab, Hassani Kumleh, Beygzadeh, Teimourian, & Kazemzad, 2018; Kotcherlakota et al., 2016).
It is worth mentioning that curcumin and its derivatives are capable of stimulating epigenetic modification in lung cancer cells to diminish their viability, proliferation, and migration. The lncRNA UCA1, miR-98, -230-5p, and 30c are among factors affected by curcumin. Curcumin suppresses immune tolerance of lung cancer cells and decreases their migration capability via inhibition of angiogenesis. STAT3 and Wnt/β-catenin signaling pathways, as oncogenic factors undergo downregulation by curcumin to restrict the malignancy of lung cancer cells. Our review demonstrates that curcumin is a potential antitumor agent in suppressing the migration, invasion, and proliferation of lung cancer cells. Besides, curcumin minimizes the relapse and recurrence of lung cancer cells by suppressing the self-renewal and proliferation of CSCs. However, more studies are needed to clarify the role of curcumin in lung cancer therapy.
Finally, in addition to low bioavailability of curcumin, its side effects should be considered. The studies demonstrate that curcumin is well-tolerated. However, to enhance the therapeutic effects of curcumin, higher doses of curcumin may be prescribed. It has been demonstrated that digestive problems, headache, nausea, and skin rash are the most prevalent side effects of curcumin, when the high doses of curcumin are administered. All of these statements direct us toward using chemical formulations and nanostrategies (mentioned earlier in text) for elevating the bioavailability of curcumin to minimize its adverse effects and simultaneously, maximize its therapeutic and biological effects.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Study conception and design: S. S. and M.A. Acquisition of data: M.N. and T. F. Drafting of the manuscript: S. S., M. A., and M. N. Critical revision: S. S., P.M., and A. Z. contributed in revising and drawing figures for this article.




