Desferoxamine protects against glucocorticoid-induced osteonecrosis of the femoral head via activating HIF-1α expression
Abstract
Glucocorticoid-induced osteonecrosis of the femoral head (GIOFH) is one of the most common complications of glucocorticoid administration. By chelating Fe2+, desferoxamine (DFO) was reported to be able to activate the HIF-1α/VEGF pathway and promote angiogenesis. In the present study, we examined whether DFO administration could promote angiogenesis and bone repair in GIOFH. GIOFH was induced in rats by methylprednisolone in combination with lipopolysaccharide. Bone repair was assessed by histologic analysis and microcomputed tomography (micro-CT). Vascularization was assessed by Microfil perfusion and micro-CT analysis. Immunohistochemical staining was performed to analyze the expression of HIF-1α, VEGF, and CD31. Our in vivo study revealed that DFO increased HIF-1α/VEGF expression and promoted angiogenesis and osteogenesis in GIOFH. Moreover, our in vitro study revealed that DFO restored dexamethone-induced HIF-1α downregulation and angiogenesis inhibition. Besides, our in vitro study also demonstrated that DFO could protect bone marrow-derived stem cells from dexamethone-induced apoptosis and mitochondrial dysfunction by promoting mitophagy and mitochondrial fission. In summary, our data provided useful information for the development of novel therapeutics for management of GIOFH.
1 INTRODUCTION
Glucocorticoid-induced osteonecrosis of the femoral head (GIOFH) is one of the most serious side effects following corticosteroid treatment (L. Z. Zheng et al., 2018). This usually occurs when corticosteroids are administered in the treatment of autoimmune diseases, such as nephrotic syndrome, rheumatoid arthritis, and systemic lupus erythematosus (A. Wang, Ren, & Wang, 2018). With the development of GIOFH, femoral head will collapse, leads to hip osteoarthritis and dysfunction. Some patients may ultimately require total hip arthroplasty (THA) (Weinstein, 2012). However, as an ultimate treatment strategy, THA is not acceptable for many patients, especially for the young. Therefore investigating new effective measures to treat GIOFH is necessary.
The pathogenic mechanisms of GIOFH have been extensively studied, intravascular thrombus occlusion and extravascular marrow lipid deposition which impairs blood supply are thought to be an important mechanism for GIOFH (Jones, 1993; Weinstein, 2010). In addition, glucocorticoids could directly damage endothelial cells, lead to endothelial cells apoptosis and impair new blood vessels formation, finally decrease the vascular supply to the bone (Zalavras, Shah, Birnbaum, & Frenkel, 2003). The connection between angiogenesis and osteogenesis has been demonstrated by several studies (Li, Fan, Yu, Dang, & Wang, 2015; Liao, Zhang, Yuan, Li, & Bao, 2018). It is reported that GIOFH coexists both bone degenerative and vascular diseases. By disruption or reduction of blood supply to the bone, osteocytes or bone marrow-derived stem cells die and cause bone collapse (Akaike & Matsumoto, 2007). Lack of oxygen, also known as hypoxia is thought to be the main pathophysiological characteristics of osteonecrosis (Hong et al., 2007). Hypoxia inducible factor-1 (HIF-1) is the central master regulator triggered by hypoxia which could promote cell adaptation to the hypoxia environment. HIF-1α plays important roles in bone formation, remodeling, and homeostasis and serves as a survival factor for cells under hypoxia environment (Johnson, Schipani, & Giaccia, 2015).
Nowadays, the role of HIF-1α in osteonecrosis is attracting increasing interest. It was reported that HIF-1α was significantly increased in the epiphyseal cartilage following ischemic injury to the immature femoral head (Kim et al., 2009). Moreover, the HIF-1α downstream factor vascular endothelial growth factor (VEGF) was also increased after ischemia induction and activated the revascularization process (C. Zhang, Li, Cornelia, Swisher, & Kim, 2012). HIF-1α expression was reported elevated significantly after ischemia treatment and kept at high level in the arthromeningitis stage, while the HIF-1α expression fall back to relative low level after 15 days in the pathophysiological process of osteonecrosis and collapse (W. Zhang, Yuan, Pei, & Ma, 2015). HIF-1α is the master transcriptional regulator of cellular response to hypoxia which acts in a wide range of cellular regulation processes including angiogenesis, glycolysis, apoptosis, and erythropoiesis (F. J. Zhang, Luo, & Lei, 2015). Previous studies have demonstrated that HIF-1α activation promotes angiogenesis, cell proliferation, and cell survival (Semenza, 2016). Recent studies indicate that HIF-1α plays an important role in angiogenic–osteogenic coupling (Y. Wang et al., 2007). Transplantation of HIF-1α transgenic bone marrow cells (BMCs) could promote tissue repair of GIOFH in rabbit models (Ding et al., 2013). Activating HIF-1α via PHD inhibitor ethyl 3,4-dihydroxybenzoate could prevent steroid associated osteonecrosis of the femoral head in rabbits by promoting angiogenesis and inhibiting apoptosis of bone cells (Fan, Li, Yu, Dang, & Wang, 2014). These findings implicate that HIF-1α may be a promising target to prevent GIOFH.
HIF-1α is the main regulatory factor induced by hypoxia state. Under normal oxygen condition, the proline residues of HIF-1α are hydroxylated by prolyl-4-hydroxylase domains (PHDs), then the hydroxylated HIF-1α is quickly degraded by Von Hippel–Lindau protein (pVHL). Under hypoxia state or lack of Fe2+, the PHD activity is inhibited, HIF-1α will accumulate and translocate into nucleus to activate numerous HIF target genes (Fernandez-Torres, Zamudio-Cuevas, Martinez-Nava, & Lopez-Reyes, 2017). Besides PHD inhibitor or gene silence, iron chelator could also inhibit HIF-1α degradation by reducing cytolic Fe2+ concentration and inhibiting PHD activity (Park, Bae, & Lee, 2008). In the present study, we choose deferoxamine (DFO) to study the protective effect of iron chelator in GIOFH. DFO has long been used to treat iron overload related diseases such as thalassemia or sickle disease (Jeney, 2017). By promoting HIF-1α and VEGF expression. DFO could accelerate bone regeneration and promote vascularity and has been used to treat bone defect repair and ovariectomy induced osteoporosis (Chen et al., 2016; Guo et al., 2018). Besides promoting angiogenesis and osteogenesis, DFO could also induce mitochondrial autophagy which plays an important role in cell adaptation under hypoxia condition via maintaining intracellular homeostasis (Wu et al., 2010). Mitochondrial dysfunction and oxidative stress are reported responsible for glucocorticoid-induced cell apoptosis and GIOFH development (Tsuchiya et al., 2018). Excess iron could lead to increased formation of reactive oxygen species (ROS) and mitochondrial dysfunction via Fenton reaction and iron deficiency was reported to activate mitochondrial autophagy (Liao et al., 2018). Q. Q. Zheng et al. (2017) reported that DFO could reduce intracellular ROS production and inhibit erythroid apoptosis via HIF-1α activation. However, the effect of DFO in GIOFH and its underlying mechanism has not been well studied.
In the present study, we aim to study whether systemic DFO administration could promote bone formation and revascularization of GIOFH in rat models. Moreover, we wondered whether DFO could promote the survival of mesenchymal stem cells (MSCs) via protecting glucocorticoid-induced mitochondrial function and inhibiting oxidative stress. Our study could provide some insight in both prevention and/or management of GIOFH for clinical applications.
2 MATERIALS AND METHODS
2.1 Cell culture and treatment
Rat bone marrow-derived MSCs (rBMSCs) were isolated from femurs and tibias of 3-weeks-old Sprague-Dawley (SD) rats. Femurs and tibias were dissected, then cells were flushed out using syringe and cultured in Dulbecco's modified Eagle's medium/F12 (DMEM/F12, HyClone) containing 10% fetal bovine serum (FBS, Gibco) and antibiotics (100 U/ml penicillin G sodium, 100 μg/ml streptomycin sulfate; Gibco). After 24 hr, the medium was changed and the unattached cells were washed away by phosphate-buffered saline (PBS). The left attached cells were cultured in growth medium until 80–90% confluence. rBMSCs at Passage 3 were used in the following experiments. Rat endothelial progenitor cells (EPCs) were obtained from iCell Bioscience, Inc. In brief, EPCs were cultured in DMEM/F12 medium containing 10% FBS, 100 µg/ml heparin, and 30 µg/ml endothelial cell growth supplements (BD Biosciences). All procedures were conducted with the approval of the Experimental Animal Ethics Committee of Tongji Medical College.
2.2 Western blot analysis
After reached more than 80% confluence, cells at Passage 3 were cultured with DMEM/F12 medium supplemented with different concentrations of DFO or dexamethasone (Dex) for 24 hr. Then proteins were extracted using radioimmunoprecipitation assay lysis buffer (Boster, China) supplemented with protease and phosphatase inhibitor. Cell homogenates were collected and centrifuged at 12,000 rpm for 20 min at 4℃. Next, the total protein concentration was determined with the bicinchoninic acid method. About 25 μg proteins was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to 0.45-μm polyvinylidine difluoride membranes (Millipore, Billerica, MA). After blocking of the membranes with 5% BSA for 1 hr, membranes were then incubated with the corresponding antibodies at 4°C overnight. The primary antibodies were as follows: anti-HIF-1α antibody (ab2185; Abcam), anti-VEGF antibody (ab46154; Abcam), anti-fission 1 homolog protein (FIS1) antibody (10956-1-AP, Proteintech Group, Inc.); anti-Parkin antibody (14060-1-AP, Proteintech Group, Inc.); anti-dynamic-related protein 1 (DRP1) antibody (#8570, Cell Signaling Technology); anti-mitochondrial fission factor (MFF) antibody (17090-1-AP, Proteintech Group, Inc.); and anti-GAPDH antibody (BM3876, Boster, China). Afterwards, the membranes were washed three times with Tris-buffered saline with Tween-20 and incubated with corresponding secondary antibody (BA1055, Boster, China) at room temperature for 1 hr. The bands were photographed using western ECL substrate kit (Thermo Pierce).
2.3 EPC tube formation assay
Rat EPCs were cultured in different FBS-free condition medium with or without Dex (10 μM) or DFO (100 μM) for 24 hr. Matrigel (BD Biosciences) was thawed at 4℃ and 20 μl Matrigel was applied to each well of 48 well plate for 30 min at 37°C. Thereafter, cell suspension containing a total of 4 × 104 rat EPCs were seeded to each well. After 12 hr, tube formation was monitored by staining EPCs with FITC-conjugated phalloidin (Cytoskeleton) and visualized with fluorescence microscope (Evos fl auto; Life Technologies, Carlsbad, CA). The endothelial tube length was measured with the Image Pro Plus software (Media Cybernetics).
2.4 Evaluation of apoptosis by annexin V-FITC/PI staining
Annexin V-fluorescein isothiocyanate/propidium iodide (FITC/PI) kit (Beyotime Biotechnology, China) was used to evaluate the apoptotic effect of DFO according to manufacturer's instructions. Briefly, EPCs were incubated with Dex (10 μM) with or without DFO (100 μM) for 24 hr. Cells were then harvested and washed with PBS twice. The cells were stained with annexin V-FITC/PI at room temperature for 15 min in the darkness and then analyzed using a FACSCalibur flow cytometer (BD, Franklin Lakes, NJ). Annexin V+/PI− and Annexin V+/PI+ cells were considered as apoptotic cells in the early and late phase respectively.
2.5 Immunofluorescence staining
rBMSCs were seeded in 24-well plate with a density of 1 × 104/ml. After treated with Dex (10 μM) or DFO (100 μM) for 24 hr, 4% paraformaldehyde was used to fix cells for 20 min. Then 0.1% Triton X-100 was used to treat cells for 15 min after washed with PBS for three times. After blocked with 10% FBS for 30 min at room temperature, the cells were then incubated with HIF-1α primary antibody (ab2185; Abcam) at 4℃ overnight. Following rinsed with PBS for three times, the Alexa fluor 555 secondary antibody was added and incubated for 1 hr at room temperature at darkness. Finally, 4',6-diamidino-2-phenylindole was added to stain cells for 5 min, cells were then rinsed with PBS and analyzed with fluorescence microscope (Evos fl auto; Life Technologies, Carlsbad, CA).
2.6 Mitochondrial membrane potential detection
A JC-1 mitochondrial membrane potential (MMP) assay kit was used to examine changes in the MMP. Briefly, rBMSCs were cultured in 24-well plate with Dex (10 μM) or DFO (100 μM) for 24 hr. After washed with DMEM-F12, cells were incubated with JC-1 staining solution (Beyotime Biotechnology, Shanghai, China) for 20 min at 37℃ at darkness. Cells were then washed with JC-1 washing buffer twice and visualized using a fluorescence microscope. Mitochondria with high membrane potential could gather JC-1 in its matrix and labeled in red, JC-1 exists in the form of monomer in the mitochondria with low membrane potential and produce green fluorescence.
2.7 Mitochondrial specific fluorescence staining
Mito-Tracker Green (C1048; Beyotime; China) was used to detect the shape and number of mitochondria. Mito-Tracker Green is a MMP independent mitochondrial staining reagent. After rBMSCs were treated with Dex (10 μM) or DFO (100 μM) for 24 hr, cells were then washed with FBS-free medium and incubated with diluted Mito-Tracker Green solution for 30 min at 37℃ at darkness. The shapes of the mitochondria were observed under fluorescence microscope (Evos fl auto; Life Technologies, Carlsbad, CA).
2.8 Animal grouping and treatment
The establishment of rat GIOFH was described in our previous studies. Briefly 40 male SD rats (250–300 g, 12 weeks old) were used in this study. All animals were housed at the Animal Care Centre of Tongji Medical College and were given free access to food and water with 12:12 hr light and dark cycles. All experiments were approved by the Institutional Animal Care and Use Committee of Tongji Medical College. Animals were randomly divided into three groups. Rats in the control group received no special treatment. Rats in the methylprednisolone (MP) group received intraperitoneal injection with lipopolysaccharide (LPS, 0.2 mg/kg, Sigma-Aldrich), then rats were intramuscularly injected with MP (Pfizer) 40 mg/kg/d for three continuous days. Rats in the MP + DFO group received intraperitoneal injection with LPS and MP as in the MP group, accompanied by intraperitoneal injection of DFO (250 mg/kg, Novartis, Switzerland) every day after the first injection of MP until animals were killed.
2.9 Microcomputed tomography analysis
After 6 weeks, all animals were killed and the femoral heads were collected and evaluated with microcomputed tomography (μCT, viva CT 40, Scanco 274 Medical, Switzerland) at a resolution of 10.5 μm, 100 kV, 98 μA. Three-dimensional reconstruction and data analysis were accomplished using the built-in software. The following 3D measurement parameters were calculated: bone mineral density (BMD), trabecular number (Tb.N), trabecular separation (Tb.Sp), bone volume per tissue volume (BV/TV), and trabecular thickness (Tb.Th).
2.10 Angiography
Six weeks after the first MP and DFO injection, the rats were anesthetized. After heparinized saline perfusion, Microfil (MV-112, Flow Tech, Inc., Carver, MA) solution was prepared according to manufacturer's protocol. The abdominal vein and aorta were dissected and mixed Microfil solution was injected through the distal abdominal aorta. After a constant white outflow was observed from the abdominal vein, ligate both abdominal vein and aorta. The rats were then put in 4°C overnight. Both femoral heads were separated in the next day and were fixed with 4% paraformaldehyde. After decalcification with 10% EDTA solution, the femoral heads were scanned with micro-CT as described above, and the vessel volume of the femoral head was reconstructed and calculated using the built-in software.
2.11 Histomorphometric analysis
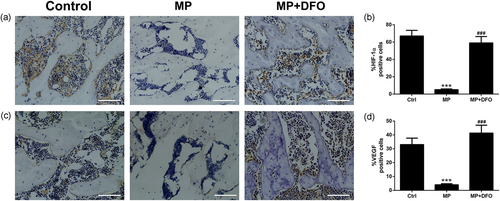
After micro-CT scanning and angiography analysis, the femoral heads were embedded in paraffin wax and sectioned at 5-µm thickness in the coronal plane. Hematoxylin and eosin (H&E) staining was conducted to evaluate the trabecular structure and osteocyte lacunae. For immunohistochemistry assay, the sections were deparaffinized, antigen retrieved, incubated with anti-HIF-1α (ab2185; Abcam), anti-CD31(sc-71873, Santa Cruz Biotechnology), and anti-VEGF(ab46154; Abcam) primary antibodies and then incubated with the corresponding biotinylated goat antirabbit (BA1003, Boster, China) and goat antimouse (BA1001, Boster, China) secondary antibodies. Sections were colored with 3'-diaminobenzidine and counterstained with hematoxylin. Image-Pro Plus software was used to analyze the images of immunohistochemical staining. To quantify CD31-positive endothelial cell, at least 10 random 200 μM × 200 μM random fields were selected and the number of CD31-positive capillaries were counted. To quantify HIF-1α and VEGF positive cells, at least five random fields in the femoral head were selected and the ratio of immune positive cells to total cells were calculated.
2.12 Statistical analysis
All numerical data were presented as mean ± standard deviation. Differences in numerical data between two groups were determined with Student's t test. One-way analysis of variance was utilized to determine differences among groups more than two followed by Bonferroni post hoc test to specifically compare difference between two groups. SPSS 13.0 was used for the general statistical analysis. Values of p < .05 were considered statistically significant.
3 RESULTS
3.1 DFO improved histological and micro-CT outcome of GIOFH
H&E staining was performed 6 weeks after MP and DFO treatment. The incidence of osteonecrosis was 0% (0/10) in the control group, 93% (14/15) in the MP group, and 20% (3/15) in the MP + DFO group. As shown in Figure 1, MP group exhibited obvious osteonecrosis compared with the control group and the DFO treatment group, which was characterized by empty lacunae. The necrotic area was mainly located in subchondral region of the femoral head and the cancellous bone adjacent to the femoral neck. The bone trabecular was thinner and numerous empty lacunae were observed in the MP group. The bone marrow structure was destroyed, containing necrotic bone marrow stromal cells and aggregated debris. The empty lacunae was significantly increased in the MP group and it was reversed after DFO cotreatment.
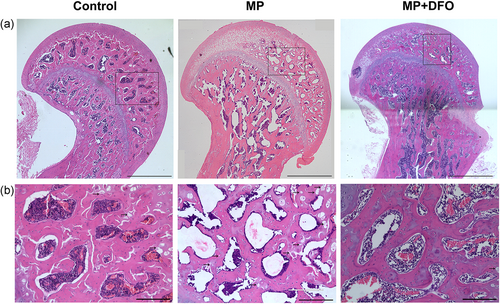
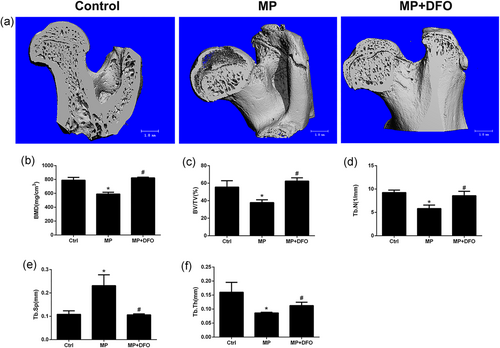
Micro-CT showed an obvious subchondral bone collapse in the MP group compared with the control group and the MP + DFO group. There was significant lower BMD, BV/TV, Tb.N, Tb.Th, and higher Tb.Sp, indicating the deleterious effects of MP on bones at the proximal subchondral bone trabecular of the rat femoral head. Treatment with MP + DFO partially reversed the deleterious effect of MP on bones with increased BMD, BV/TV, Tb.N and Tb.Th and decreased Tb.Sp compared to the MP group (Figure 2).
3.2 DFO improved angiogenesis of femoral head and inhibited Dex-induced EPC apoptosis
In this study, microangiography of femoral head was performed using Microfil perfusion. The blood vessels of femoral head were perfused with Microfil solution and examined by micro-CT. The reconstructed 3D images of femoral head showed a significantly decreased blood vessels in the MP group (Figure 3a). However, blood vessels in the MP + DFO group were significantly denser, although they were fewer compared with the control group (Figure 3a). Immunohistochemical staining of CD31 was also performed to detect angiogenesis in the rat femoral head. As shown in Figure 3b, there was a significantly decreased CD31-positive capillaries in the MP group, indicating the inhibitory effects of MP on capillary formation. Treatment of DFO significantly promoted CD31-positive capillary formation in the subchondral bone trabecular of rat femoral head (Figure 3c,d).
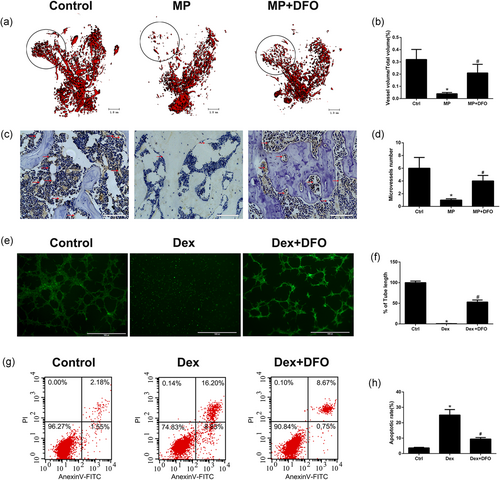
To further detect the effects of Dex and DFO on tube formation of EPCs in vitro, the matrigel tube assay was conducted. As shown in Figure 3e,f, dex treatment showed a strong antiangiogenesis capacity with no tube formation and a smaller tube length. However, more tube formation and longer tube length were observed in the DFO + Dex treatment group. Considering Dex at high concentration could promote cell apoptosis, cell apoptosis was tested by the Annexin V-FITC/PI staining. Our results showed that Dex at 10 μM significantly promoted EPCs apoptosis and Dex-induced EPCs apoptosis was significantly inhibited by DFO treatment (Figure 3g,h). These results indicated that DFO could protect against Dex-induced apoptosis and angiogenesis inhibition.
3.3 The regulatory effect of Dex and DFO on HIF-1α and VEGF protein expression in vitro and in vivo
HIF-1α and its downstream target gene VEGF in rBMSCs play important roles in osteogenesis and angiogenesis coupling in bone tissue (Wan et al., 2008). As shown in Figure 4a, western blot analysis revealed that Dex inhibited HIF-1α and VEGF protein expression in a dose-dependent manner. Dex at 1 μM and 10 μM significantly downregulated HIF-1α and VEGF protein expression. Our results also showed that DFO promoted HIF-1α expression in a dose-dependent manner (Figure 4b). DFO at 100 μM showed the most significant effect in promoting HIF-1α protein expression and rescued the HIF-1α and VEGF protein expression which was inhibited by Dex (Figure 4c). Similar results were observed by immunofluorescence assay (Figure 4d).
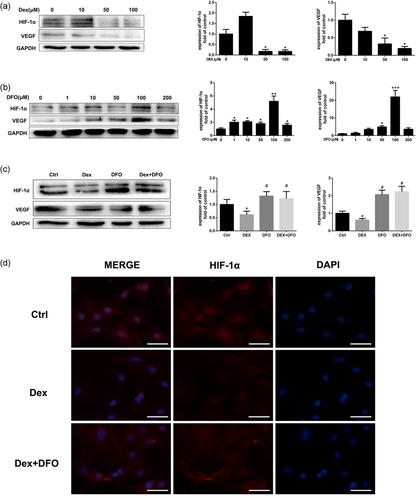
The HIF-1α/VEGF pathway plays important roles in bone homeostasis and regeneration in osteonecrosis of the femoral head. To further explore the effect of glucocorticoid and DFO on HIF-1α and VEGF protein expression in vivo, immunohistochemical staining of HIF-1α and VEGF was conducted. As shown in Figure 5, MP treatment significantly downregulated HIF-1α and VEGF protein expression, while DFO administration abrogated the inhibitory effect induced by MP treatment.
3.4 DFO improved mitophagy in rBMSCs exposed to Dex in vitro
Apoptosis of osteoblasts and mesenchymal stem cells (BMSCs) triggered by prolonged or excessive use of glucocorticoids has been identified as a dominant contributor to the development of osteoporosis and osteonecrosis. Recent studies demonstrated that autophagy principally acts as a cytoprotective form via maintaining the intracellular homeostasis and plays important roles in regulating mesenchymal stem cells adaptation to hypoxia condition in GIOFH (Liao et al., 2018). To explore whether DFO could induce mitochondrial autophagy (mitophagy) in rBMSCs, western blot assay was conducted to evaluate the expression of parkin protein expression. As shown in Figure 6a,b, DFO significantly promoted parkin expression in a dose-dependent manner. The Dex-induced mitochondrial dysfunction and the subsequent ROS overproduction is crucial in contributing BMSCs apoptosis. As shown in Figure 6c, the mitochondria of healthy BMSCs in the control group exhibited long and wire-like shape, while after treatment of Dex, the number of mitochondria decreased. The Dex and DFO cotreatment promoted the number mitochondria compared with the Dex group, however, the left mitochondria showed shortened and granulated shape, indicating that DFO might promote BMSCs mitochondrial autophagy and rescue the mitochondrial function.
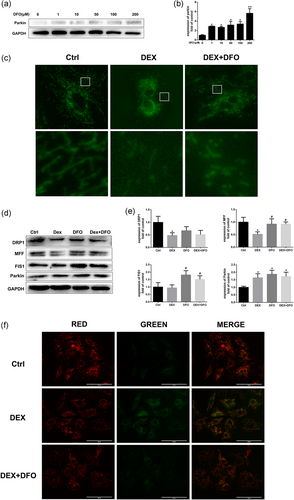
To further investigate the protective effect of DFO in mitochondrial function, the mitochondrial fission proteins and MMP were evaluated. The mitochondrial fission is reported to be related to mitophagy and could regulate the morphology and function of mitochondria (Tsuchiya et al., 2018). As shown in Figure 6d,e, the mitochondrial fission-related protein levels of DRP1, MFF, and FIS1 decreased after the Dex treatment and were reversed by the DFO treatment. Similar results were observed in the MMP assay. The MMP was reduced after Dex induction and was restored by DFO treatment (Figure 6f). Interestingly, Dex promoted mitophagy-related protein parkin expression and DFO treatment further promoted parkin protein expression. Our results indicated that DFO might protect Dex-induced MMP reduction and mitochondrial dysfunction via promoting mitochondrial fission and autophagy process.
4 DISCUSSION
In human body, bone tissue is highly vascularized and constantly suffer bone resorption and formation process. Once the blood supply is disrupted, the bone marrows die and eventually results in bone collapse (Weinstein, 2010). Due to the special anatomy of femoral head, osteonecrosis of the femoral head is common with 8.12 million among Chinese people aged >15 years old. Among patients who were diagnosed with osteonecrosis of the femoral head, 26.35% of males and 55.75% of females reported that they had received glucocorticoids treatment (Zhao et al., 2015). The pathogenic mechanism of GIOFH might attribute to the intravascular thrombus formation and oxidative stress, which results in impairment of blood supply and increased osteoblasts as well as MSCs apoptosis (G. Zhang et al., 2009). Failure of blood supply would further decrease oxygen supply in bone tissue and promote MSCs apoptosis.
HIF-1α is the main transcriptional regulator-induced by hypoxia and plays important roles in cell survival and energy metabolism under hypoxia condition. HIF-1α plays important roles in angiogenesis–osteogenesis coupling during bone remodeling and bone regeneration (Shao et al., 2015; Y. Wang et al., 2007). Recently the role of HIF-1α in osteonecrosis has attracted increasing attention. However the relationship between HIF-1α and osteonecrosis of the femoral head still remains elusive. Hong et al. (2007) reported that HIF-1α might play an important role in the pathogenesis and risk factor for GIOFH in Korean population. However, studies made by Chachami et al. (2013) indicated that HIF-1α gene was not important for the risk of developing osteonecrosis. Perhaps this is because the expression of HIF-1α changes with the development of osteonecrosis of the femoral head. It is reported that HIF-1α expression increased greatly after ischemic operation and kept at relative high level in the arthromeningitis stage and declined in the stages of osteonecrosis and reconstruction (W. Zhang et al., 2015). HIF-1α is the central transcriptional regulator for sensing local oxygen concentration and activates a variety of downstream genes that influence angiogenesis and cellular metabolism (Chen et al., 2016).
By chelating Fe2+ required for the PHD activity, iron chelator could activate the HIF-1α/VEGF pathway, thereby promoting angiogenesis. Besides its angiogenic capacity, DFO could also promote osteoblasts and mesenchymal stem cells osteogenic differentiation via wnt/β-catenin pathway (Baschant et al., 2016). Previous studies have demonstrated the capacity of DFO in treating osteoporosis and bone defect (Grewal, Keller, Weinhold, & Dahners, 2014; Jia et al., 2016), however, the effect of DFO in GIOFH is rarely investigated. In the present study, in vivo and in vitro studies were conducted to investigate the effect of DFO on angiogenesis and bone repair in GIOFH. Our in vivo studies revealed that MP treatment obviously increased the osteonecrosis rate in rats as evaluated by H&E staining and micro-CT analysis. The empty lacunae in H%E staining and subchondral bone collapse in micro-CT images demonstrated that MP treatment successfully caused osteonecrosis, while DFO administration exhibited obvious protective effect in GIOFH.
Dysfunction of blood supply caused by glucocorticoid is believed the main cause of osteonecrosis of the femoral head (Akaike & Matsumoto, 2007). Consistent with previous studies, our MP-treated rat GIOFH models exhibited obvious destruction of blood vessels, however, we found that DFO had significant protective effects in the blood supply of the femoral heads. Our in vitro studies further demonstrated that Dex could directly injure endothelial cells. We found that Dex at 10 μM significantly inhibited angiogenesis and promoted apoptosis in endothelial cells which was consistent with previous studies made by Y. Zhang, Yin, Ding, Zhang, and Gao (2016). DFO treatment could partly abolish the deleterious effect of Dex and promote angiogenesis of endothelial progenitor cells.
Several studies have demonstrated that HIF-1α coupled osteogenesis and angiogenesis in bone tissues (Wan et al., 2008). HIF-1α could activate VEGF gene expression which is important in angiogenesis by regulating endothelial cell migration, proliferation, and vascularization. Activation of HIF-1α in BMSCs could not only promote angiogenesis of EPCs via VEGF secretion, but also regulate osteoclastogenesis and bone resorption via direct regulation of osteoprotegerin (Shao et al., 2015). Previous in vivo study has demonstrated the protective effect of HIF-1α transgenic BMCs in glucocorticoid-induced osteonecrosis of femoral head (Ding et al., 2013). In the present study, our in vitro study showed that Dex obviously inhibited the protein expression of HIF-1α and VEGF in BMSCs, while DFO with a concentration of 100 μM significantly abrogated this effect of Dex in BMSCs. Our in vivo experiments were consistent with previous studies that MP treatment decreased the HIF-1α and VEGF expression (W. Zhang et al., 2015), however, DFO administration partly reversed the HIF-1α inhibition induced by MP, indicating DFO might exhibit protective effect in GIOFH via HIF-1α.
The mesenchymal stem cells apoptosis and the subsequent reduction of bone formation is the direct cause of osteonecrosis (Deng et al., 2018). Destruction of blood vessels results in reduced blood supply in bone and promotes cell apoptosis. Meanwhile, HIF-1α was found activated to counteract the hypoxia environment. Besides promoting angiogenesis via VEGF secretion, HIF-1α could also promote cell adaptation to hypoxia and regulate intracellular energy homeostasis via autophagy. Mitochondrial fission is cellular process that remove damaged mitochondria and restore mitochondrial function and morphology. DRP1 mediates mitochondrial fission. During mitochondrial fission, Drp1 is recruited and FIS1 and MFF are activated. The damaged mitochondria are subsequently degraded by parkin dependent mitophagy (Pilsl & Winklhofer, 2012). The mitochondrial dysfunction plays important roles in the progression of GIOFH (Tsuchiya et al., 2018). It is reported that Dex could impair the mitochondrial fission and fusion process, results in the respiratory damage, and ROS overproduction (Deng et al., 2018; Liu et al., 2018). In the present study, we found that Dex treatment suppressed the mitochondrial fission protein level and decreased the number of healthy mitochondria in BMSCs. DFO significantly enhanced the mitochondrial fission level and restored the number of mitochondria. Moreover we found that DFO could promote mitophagy-related protein parkin expression which is a key process in the mitochondrial quality control.
In the present study, we demonstrated the protective effect of DFO in glucocorticoid-induced osteonecrosis of femoral head. Our in vivo and in vitro experiments demonstrated that DFO might exhibit its protective roles via promoting HIF-1α activation. Activated HIF-1α could not only promote angiogenesis via VEGF secretion, but also regulate mitochondrial function via enhancement of mitochondrial fission and autophagy. Our results demonstrated that DFO is a promising anti-GIOFH drug worth further clinical trials.
ACKNOWLEDGMENT
This study was supported by grants from the National Natural Science Foundation of China (No. 81702155 and 81572094).
CONFLICT OF INTERESTS
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
AUTHOR CONTRIBUTIONS
J. X. Z., L. T., and J. Z. S. designed the research study. J. X. Z., Y. X. X., Z.W. M., W. G. D., and L. X. Y. performed the research. D. T., J. X. Z., and L. T. analyzed the data. J. X. Z., D. T., and J. Z. S. wrote the paper.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




