Pten deletion in Dmp1-expressing cells does not rescue the osteopenic effects of Wnt/β-catenin suppression
Abstract
Skeletal homeostasis is sensitive to perturbations in Wnt signaling. Beyond its role in the bone, Wnt is a major target for pharmaceutical inhibition in a wide range of diseases, most notably cancers. Numerous clinical trials for Wnt-based candidates are currently underway, and Wnt inhibitors will likely soon be approved for clinical use. Given the bone-suppressive effects accompanying Wnt inhibition, there is a need to expose alternate pathways/molecules that can be targeted to counter the deleterious effects of Wnt inhibition on bone properties. Activation of the Pi3k/Akt pathway via Pten deletion is one possible osteoanabolic pathway to exploit. We investigated whether the osteopenic effects of β-catenin deletion from bone cells could be rescued by Pten deletion in the same cells. Mice carrying floxed alleles for Pten and β-catenin were bred to Dmp1-Cre mice to delete Pten alone, β-catenin alone, or both genes from the late-stage osteoblast/osteocyte population. The mice were assessed for bone mass, density, strength, and formation parameters to evaluate the potential rescue effect of Pten deletion in Wnt-impaired mice. Pten deletion resulted in high bone mass and β-catenin deletion resulted in low bone mass. Compound mutants had bone properties similar to β-catenin mutant mice, or surprisingly in some assays, were further compromised beyond β-catenin mutants. Pten inhibition, or one of its downstream nodes, is unlikely to protect against the bone-wasting effects of Wnt/βcat inhibition. Other avenues for preserving bone mass in the presence of Wnt inhibition should be explored to alleviate the skeletal side effects of Wnt inhibitor-based therapies.
1 INTRODUCTION
The WNT signaling pathway is essential for appropriate bone development, maintenance, and metabolism. Low-density lipoprotein receptor-related protein 5 (LRP5) was the first WNT pathway component linked to skeletal homeostasis in humans, and its importance in bone biology and health was highlighted by work revealing that LRP5 mutations are found in patients with very low (Cheung et al., 2006; Gong et al., 2001) and very high (Little et al., 2002; Van Wesenbeeck et al., 2003) bone mass. Those observations, viewed in the context of LRP5's participation in canonical WNT signaling, implicated the WNT pathway as a high-priority/high-yield cascade for therapeutic targeting to treat skeletal disorders (Johnson & Recker, 2017). For example, targeting of the LRP5 antagonist sclerostin, using a monoclonal antibody against the protein, was recently approved by the Food and Drug Administration (FDA) for the advisory panel for the treatment of osteoporosis in postmenopausal women at increased risk of fracture (Paszty, Turner, & Robinson, 2010).
Tonic activation of the canonical WNT pathway is necessary for skeletal integrity, as highlighted by genetic models of mice with various Wnt-related loss-of-function mutations (e.g. Gpr177, Porcn, Ctnnβ1) in bone cells, which result in severe low bone mass (Glass et al., 2005; Zhong et al., 2012). However, treatments for extra-skeletal diseases such as numerous cancers (Medicine, 2013, 2014a, 2014b, 2014c, 2014d, 2016), cardiac infarction (Moon et al., 2017), and kidney fibrosis (Madan et al., 2016), among others, are being actively addressed by pursuing therapeutic agents that inhibit WNT signaling, which could have severe consequences for bone mass and strength. For example, such is the case for the porcupine inhibitor candidate WNT974, which induces significant regression of breast tumor fragments implanted in mice (J. Liu et al., 2013), but also has severe negative effects on cortical bone mass (Moon et al., 2017). Although there are currently no FDA-approved WNT inhibitors in the market, the sheer number of ongoing clinical trials involving WNT inhibitors suggests that eventual approval for this class of therapy might be realized and implemented as a clinical tool to treat a variety of cancers. It is, therefore, reasonable to be concerned with identifying signaling mechanisms in bone cells that might alleviate or counter the deleterious effects of WNT inhibition on bone mass.
One such candidate pathway that might exhibit bone-preserving properties, independent of Wnt/β-catenin, is the phosphatase and tensin homolog deleted from chromosome 10 (PTEN)/phosphoinositide 3-kinase (Pi3K)/protein kinase B (Akt) pathway. Akt activates a number of downstream cascades that stimulate bone cell function, including protein translation via mTOR (Jhanwar-Uniyal et al., 2019), glucose transport via Glut4 (Jaldin-Fincati, Pavarotti, Frendo-Cumbo, Bilan, & Klip, 2017), and cell survival/apoptosis via Bim/Bax (Yang et al., 2017). In vivo osteoblast-selective deletion of Pten has potent bone-building effects (X. Liu et al., 2007). Thus it is possible (but unknown) whether inhibition of Pten might counter the osteopenic effects of Wnt pathway inhibition. In this communication, we sought to understand whether the low bone mass phenotype associated with Wnt pathway inhibition—achieved by β-catenin deletion in bone cells—could be rescued if hyperactivation of Pi3k/Akt via Pten deletion (and consequent stimulation of WNT-independent downstream pathways) were achieved in the same cells. To this end, we generated mice with compound homozygous floxed loss-of-function alleles for β-catenin and Pten, recombined with dentin matrix protein-1 (Dmp1)-Cre, and measured the skeletal phenotypes via radiography, histology, mechanical testing, and serum biomarkers. We found that while Pten deletion from bone is osteogenic and β-catenin deletion from bone reduces bone mass, the compound mutants exhibited a phenotype similar to the single β-catenin mutants. Surprisingly, for many measures, Pten deletion exacerbated the low bone mass phenotype induced by β-catenin deletion. Those results suggest that Pi3K/Akt hyperactivation in the skeletons of mice that have other downstream signaling cascades intact except β-catenin, does not improve the low bone mass phenotype induced by β-catenin mutation. Other avenues to preserving bone mass during WNT inhibitor therapy will need to be explored to remedy the concomitant deficit to the bone.
2 MATERIALS AND METHODS
2.1 Mice
The floxed loss-of-function β-catenin mice (βcatf/f) have been described previously (Brault et al., 2001). Briefly, these mice harbor loxP sites in introns 1 and 6 of the Ctnnb1 gene, which results in a null allele upon recombination. The floxed loss-of-function PTEN mice (Ptenf/f) have been described previously (Lesche et al., 2002). Briefly, these mice harbor loxP sites in introns 4 and 5 of the PTEN gene, which results in a null allele upon recombination. 10 kbDmp1-Cre transgenic (TG) mice have been described previously (Lu et al., 2007). These mice harbor a Cre sequence driven by a 10-kb fragment of the Dmp1 promoter, which provides osteocyte and late osteoblast selectivity of expression. All mice were maintained on a C57BL/6 background. Βcatf/f mice were bred to 10 kbDmp1-Cre mice, and the offspring were then bred to Ptenf/f mice over several generations to generate Cre-positive and Cre-negative wild type (βcat+/+;Pten+/+), βcat mutant (βcatf/f;Pten+/+), Pten mutant (βcat+/+;Ptenf/f), and double mutant (βcatf/f;Ptenf/f) mice. Both male and female mice were selected for the experiments. Offspring were same sex housed in cages of 3–5 (independent of genotype; NTG and TG littermates were used) and given standard mouse chow (Harlan Teklad 2018SX; 1% Ca; 0.65% P; vitamin D3 [2.1 IU/g]) and water ad libitum. All animal procedures were performed in accordance with Institutional Animal Care and Use Committee guidelines.
2.1.1 Dual-energy x-ray absorptiometry (DXA)
Whole-body DXA scans were collected on isoflurane-anesthetized mice using a PIXImus II (GE Lunar) densitometer. All mice were scanned at 4 weeks of age, and again at 6 weeks of age, then euthanized at 7 weeks of age. From the whole-body scans, areal bone mineral density (BMD) and bone mineral content were calculated for the entire postcranial skeleton and regionally for the lumbar spine (L3–L5, inclusive) and right hindlimb distal to the acetabulum, using the Lunar ROI tools.
2.1.2 Microcomputed tomography (μCT)
After euthanasia at 7 weeks of age, the right femur and 5th lumbar vertebra (L5) were dissected from each mouse, fixed for 2 days in 10% neutral buffered formalin, and then transferred into 70% ethanol for μCT scanning on a high-throughput μCT specimen scanner (μCT-35; Scanco Medical AG). The middle 15% and distal 33% of each femur, and the entire L5 vertebra, were scanned using the following conditions: 50 kV, 120 mA, 151-ms integration time, and 10-μm voxel resolution. Three-dimensional morphometric properties of the cancellous bone in the distal femur and lumbar vertebral body were measured as previously described (Niziolek et al., 2011). Analyses of cortical bone parameters (cortical thickness and area) were collected using a 20-slice stack that was centered around the midshaft slice of the femur, as previously described (Niziolek et al., 2011).
2.1.3 Fluorochrome administration and cortical bone quantitative histomorphometry/histology
Fluorochrome-derived histomorphometric indices of cortical bone formation were measured in the midshaft femur. Mice were injected with calcein (12 mg/kg, i.p.) at 4 weeks of age, demeclocycline (75 mg/kg, s.c.) at 6 weeks of age, and alizarin complexone (20 mg/kg, i.p.) at 6.5 weeks of age. After euthanasia (7 weeks), the left femur was dehydrated in graded ethanols and embedded in methylmethacrylate. Midshaft femur sections were cut in the transverse plane (∼200-μm thick) using a diamond-embedded wafering saw (Buehler, Inc.) and ground to a final thickness of approximately 30 μm. Periosteal bone formation parameters were calculated from the calcein and demeclocycline labels by measuring the extent of the unlabeled perimeter, single-labeled perimeter, double-labeled perimeter, and the area between the double labeling on digitized section images using Image-Pro Plus (Media Cybernetics, Gaithersburg, MD). Derived histomorphometric parameters, including mineralizing surface per unit bone surface (%), mineral apposition rate (μm/day), and bone formation rate per unit bone surface (BFR/BS; μm3/μm2 per year), were calculated using standard equations (Dempster et al., 2013). In addition, to qualitatively evaluate morphological changes induced by the mutations, whole femora were decalcified, embedded in paraffin, sectioned, and stained with hematoxylin and eosin.
2.1.4 Biomechanical measurements of whole bone strength
Whole left femora were collected from carcasses, wrapped in saline-soaked gauze, and stored at −20°C for several weeks before testing. On the day of testing, the samples were brought to room temperature by soaking frozen samples in a room temperature saline bath for 3 hr before mechanical testing. Each femur was positioned posterior side down across the two lower supports (spaced 8 mm apart) of a three-point bending apparatus, mounted in a Bose ElectroForce 3200 electromagnetic test machine, which has a force resolution of 0.001 N. The femora were loaded to failure in monotonic compression using a crosshead speed of 0.2 mm/s, during which force and displacement measurements were collected every 0.01 s. From the force versus displacement curves, the ultimate force, stiffness, and energy to ultimate force were calculated using standard equations (Turner & Burr, 1993).
2.1.5 Serum collection and collagen crosslinks immunoassay
Whole blood samples were collected from female mice via cheek bleeding at 6 weeks of age. Approximately 150 μl of blood was collected into serum separator tubes (BD Microtainer), allowed to clot at room temperature for 40 min, then centrifuged at 10 k×g for 1 min. Serum was removed and stored at −80°C until all samples were collected and assayed together. The serum concentration of C-terminal telopeptide (CTx) was measured by a commercially available ELISA kit (ImmunoDiagnostics, Inc.) according to the manufacturer's instructions. Serum samples were measured in duplicate.
2.1.6 Protein extraction and western blot analysis
Mouse femur, tibia, and fibula were dissected, stripped of soft tissue, flushed to remove bone marrow, pulverized in liquid nitrogen, and solubilized in 4 × sodium dodecyl sulfate polyacrylamide gel electrophoresis sample buffer. About 20 µg of protein was run on a 4–12% polyacrylamide gradient gel (GenScript, Piscataway, NJ) along with prestained molecular weight markers (Bio-Rad) in one of the sample wells. Separated proteins were transferred to nitrocellulose overnight, then stained with Ponceau-S to visualize total protein. Membranes were blocked in Tris-buffered saline with Tween-20 (TBST) then incubated with anti-phospho-AktS473 primary antibody (Cell Signaling Technology, Beverly, MA) overnight. The next day, the blot was washed and incubated with goat anti-rabbit horseradish peroxidase (HRP)-conjugated secondary antibody (Jackson Immunoresearch, West Grove, PA) for 1 hr at R/T then washed in TBST. HRP was reacted with ECL reagent for 5 min (ECL Prime Reagent, Amersham, Little Chalfont, UK). Blots were imaged using an iBrightCL1000 (Invitrogen, Carlsbad, CA).
2.1.7 Statistical analysis
Statistical analyses were conducted with JMP (version 4.0; SAS Institute, Inc.). The radiographic, mechanical, histomorphometric, and biochemical endpoints were analyzed using repeated-measures analysis of variance (ANOVA) or one way (within sex and Cre status) ANOVA, with genotype as the main effect. Post hoc comparisons within ANOVAs that achieved overall significance were made using Fisher's protected least significant difference tests. Statistical significance was taken at p < .05. Two-tailed distributions were used for all analyses. Data are presented as means ± SEM.
3 RESULTS
3.1 Mice with loss of both Pten and β-catenin in Dmp1-expressing cells have significantly reduced bone mineral density and µCT properties, similar to or more severe than mice with β-catenin mutations alone
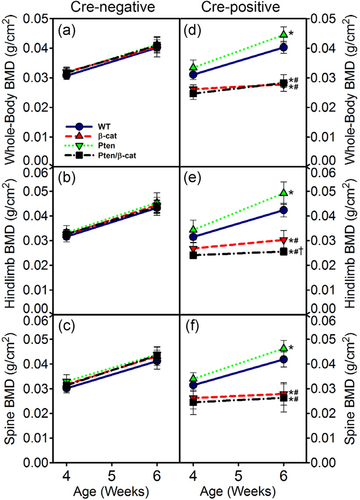
We (Kang, Hong, & Robling, 2016; Kedlaya et al., 2016) and others (Javaheri et al., 2014; Kramer et al., 2010) reported previously that mice with conditional loss-of-function β-catenin alleles induced in Dmp1-expressing cells have low bone mass. As many candidate therapeutics in clinical trials work by suppressing Wnt/β-catenin signaling (Harb, Lin, & Hao, 2019), we explored whether activation of the Akt pathway (via Pten deletion) in the same cells might counteract the bone-wasting effects of β-catenin inhibition. Serial whole-body DXA scans were collected from mice at 4 and 6 weeks of age. Among Cre-positive mice, BMD for the whole body, spine, and hindlimb was significantly increased by the Pten mutation (10–16% increase in male mice and 8–10% increase in female mice at 6 weeks, p < .05), with the exception of the spine ROI in females (Figures 1d–f and s2). BMD was significantly decreased by the β-catenin mutation (29–33% decrease in male mice and 27–35% decrease in female mice at 6 weeks, p < .05). Compound mutants (Pten/βcat double flox) were either statistically indistinguishable from the βcat mutants (e.g., for whole body and spine BMD) or, surprisingly, significantly more osteopenic than the βcat mutants (e.g., hindlimb BMD; Figures 1e and s2). Cre-negative mice were not significantly different from one another in terms of BMD, regardless of the floxed alleles they carried (Figures 1a–c and s2). Bodyweight was not affected by the Pten mutation, but the βcat mutants and compound mutants were significantly lighter than WT controls (Figure S1).
To probe the bone compartment-specific effects of Pten and βcat individual and compound mutations in bone cells, the femur and spine of 7-week-old mice were scanned via µCT to collect distal femur and L5 cancellous properties and midshaft femur cortical properties. Among Cre-positive mice, the trabecular bone volume fraction (BV/TV) and trabecular number (Tb.N) were significantly improved by the Pten mutation in the distal femur among female (27–32% increase, p < .05) but not male mice (Figures 2a–c and s3). Trabecular thickness (Tb.Th) was not affected by the Pten mutation alone in either sex (Figure 2c and S2). The βcat mutant and compound mutant mice were severely osteopenic and nearly completely devoid of cancellous bone in the distal femur (Figure 2f and s3). Trabecular BV/TV was undetectable in both mutants, and Tb.N and Tb.Th were significantly reduced (21–80% reduction, p < .05) in both sexes. In male mice, the compound mutants exhibited significantly lower Tb.Th than was measured for the βcat mutant mice.
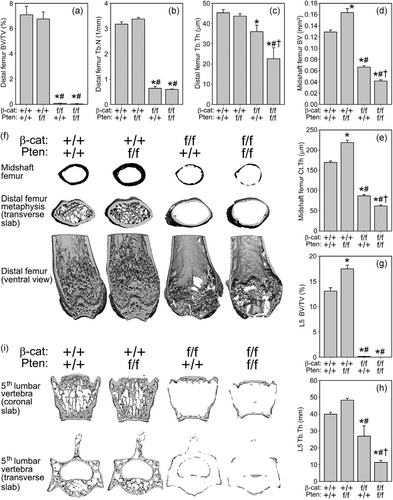
In both sexes, cortical bone properties (bone volume [BV] and cortical thickness [Ct.Th]) were consistently improved by the Pten mutation, impaired by the βcat mutation, and further impaired by the presence of both Pten and βcat mutations (Figures 2d,e and S3). Pten deletion resulted in a 27–32% increase (p < .05) in cortical properties among both sexes, whereas the βcat mutation reduced cortical properties by 45–49% (p < .05). Compound mutants exhibited further significant reductions beyond those measured for βcat mutants in both sexes. µCT properties in Cre-negative mice were not statistically different among genotypes, with the exception of Tb.Th in female mice (Table S1).
Vertebral cancellous properties in Cre-positive mice exhibited trends similar to those reported for the distal femur, where Pten mutants had significantly increased values, βcat mutants had significantly decreased values, and compound mutants were either statistically similar to βcat mutants or had further reductions (e.g. Tb.Th in males).
3.2 Reduced mechanical properties and cortical bone formation parameters in βcat and Pten/βcat mutant mice
The deficit in µCT-derived cortical bone properties among βcat and Pten/βcat mutant mice prompted us to determine whether there were associated deficits in mechanical/structural properties. We tested femora from male Cre-positive mice using 3-point bending (Figure 3a) and found significantly increased ultimate force, stiffness, and energy absorption in Pten mutants compared to WT mice (39–68% increase, p < .05). Those same properties were significantly reduced in βcat mutants (57–90% reduction, p < .05) and double mutants (88–94% reduction, p < .05) compared to WT controls (Figure 3b–d). Double mutants exhibited significantly reduced energy absorption compared to mice with βcat mutation.

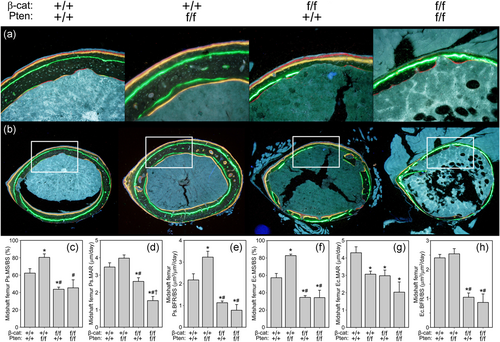
Cortical bone formation parameters were measured in Cre-positive mice using fluorochrome labels injected at 4 and 6 weeks of age (Figure 4a,b). Pten mutants exhibited a significant increase (29–45% increase, p < .05) in mineralizing surface (MS/BS) on both periosteal and endocortical surfaces compared to WT controls, whereas βcat mutants exhibited significantly reduced MS/BS on both surfaces (30–39% decrease, p < .05; Figure 4c,f). Double mutants exhibited similar MS/BS values as βcat mutants but were not different from WT controls on the periosteal surface due to high variability in the measurements. Likewise, bone formation rates on both surfaces were significantly reduced in βcat mutants and double mutants (48–64% reduction, p < .05) compared to WT controls, with no further reduction detected in the double mutants. Qualitatively, femur bone histomorphology revealed the low bone mass phenotype among both morphologies among βcat mutants and double mutants (Figure S4).
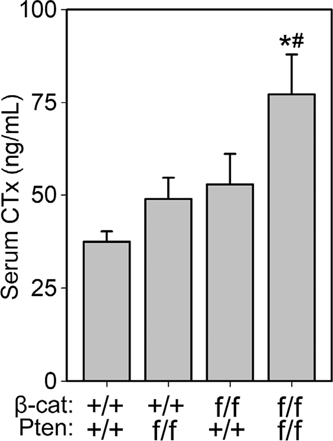
Serum collected from 6-week-old female mice revealed a significant increase in carboxy-terminal collagen crosslinks among Pten/βcat double mutants, compared to both WT and Pten mutants (Figure 5). Surprisingly, CTx was not significantly elevated in the βcat mutants despite very low bone mass. CTx levels were not affected by the Pten mutation at the 6-week time point.
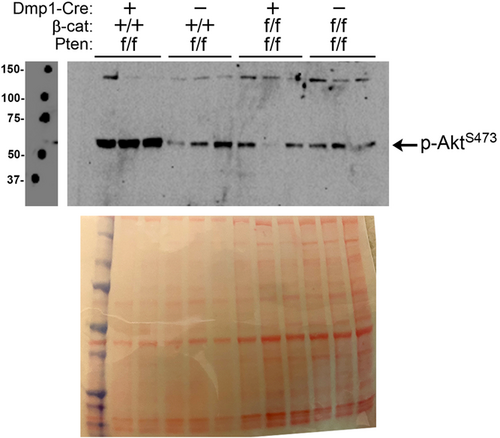
3.3 Pten deletion activates Akt signaling in βcat-WT but not βcat mutant mice
The failure of Pten deletion to rescue the bone-wasting effects of βcat deletion in the same cell population prompted us to investigate whether Pten deletion activated Akt in the absence of βcat. To this end, we collected fresh protein lysates from cortical bone samples of 6-week-old mice and probed for activated Akt. As expected, western blot analysis for phosphorylated Akt (at serine 473) revealed that Akt was heavily activated in bone from Cre-positive Ptenf/f mice, compared to Cre-negative Ptenf/f littermates (Figure 6). However, the same Pten comparison (i.e., Cre vs. no Cre) on a βcatf/f background indicated no increase in Akt activation by Pten deletion in the absence of βcat. Those observations might explain the lack of skeletal improvement in Pten mutant mice when βcat was co-deleted. βcat deletion alone (on a Pten-WT background) had no effect on Akt activation (Figure S5).
4 DISCUSSION
Our main goal in this study was to determine whether Pten inhibition can overcome the bone-compromising effects of Wnt pathway inhibition, which we enacted experimentally by deleting β-catenin in the same cells in which Pten was deleted. We reported previously that β-catenin deletion from Dmp1-expressing cells causes a low bone mass phenotype (Kang et al., 2016; Kedlaya et al., 2016), a result that was confirmed in the present report. Conditional deletion of Pten from bone cells earlier in the osteogenic lineage (e.g., Bglap-Cre-mediated) causes a very high bone mass phenotype (X. Liu et al., 2007) and improves fracture healing (Burgers et al., 2013; Collins et al., 2015). The main target of Pten–Pi3k–participates in a wide range of cellular processes, including proliferation, differentiation, cell motility, and survival (Yamada & Araki, 2001). Some of these Pten/Pi3k-mediated mechanisms do not involve Wnt signaling and so our aim was to explore whether co-activation of Pi3k could restore or improve the deleterious bone phenotype in βcat mutant mice, perhaps by accelerating Pi3k/Akt-driven bone formation despite the reduction induced by β-catenin deficiency. Our results suggest that the severe osteopenic phenotype induced by β-catenin deletion is not improved by the co-deletion of Pten. Surprisingly, for many endpoints, the β-catenin skeletal phenotype was significantly compromised further by the absence of Pten in the same cells.
We chose to induce recombination of β-catenin and Pten floxed alleles in the osteocyte, using the Dmp1-Cre transgene. Like many of the approved skeletal therapeutics currently in the market target osteocyte products (e.g., burosumab, romosuzumab, denosumab), the osteocyte is a crucial regulatory cell in skeletal homeostasis. Deletion of Pten earlier in the osteoblast differentiation pathway produces a much more robust effect on bone mass. For example, recombination of floxed Pten alleles using Bglap-Cre increased BMD by 33% at 6 weeks of age, which is more than double the effect size we report in the current communication at the same age (see Figure 1). Moreover, the Bglap-Cre mice exhibited a 43% increase in Ct.Th compared to controls at 12 weeks, whereas the Dmp1-Cre mice we use had a more mild cortical phenotype, reaching a ~30% increase at 6 weeks. We did not observe the sclerotic phenotype in long bones from Dmp1-Cre/Pten flox mice as was reported for the Bglap-Cre/Pten mice; the Dmp1-Cre femora had normal size and shape, with slightly increased bone mass. However, we terminated our experiments at 7 weeks of age and thus cannot compare µCT values or images at identical ages to mice in the Bglap-Cre study. Thus, it is possible that the Dmp1-Cre/Pten mice would have developed a much more robust, perhaps even sclerotic phenotype, had they been permitted to age longer. To this point, our preliminary experiments were designed to collect measurements until 12 weeks, but both βcat and Pten/βcat mice did not live much beyond 8–9 weeks of age (data not shown) and therefore all mice were terminated at the 7-week time point.
The mechanism of action for increased bone mass in mice with Pten-deficient osteocytes is not clear. A wealth of signal transduction literature on Pten indicates that a number of mechanisms could be responsible; the most widely studied and obvious downstream pathway is the Pi3k/Akt axis. However, other pathways are modulated directly by Pten. Pten directly regulates the phosphorylation status of focal adhesion kinase (Fak), a protein important for in vitro mechanical signaling in bone cells.(Castillo et al., 2012) It also functions as a scaffolding protein for the androgen receptor (Lin, Hu, Lee, & Chang, 2004) and the melanocortin-1 receptor (MC1R) (Cao et al., 2013); as a binding partner for transcription factors in the nucleus (e.g., p53, Smad3) (Freeman et al., 2003; Hjelmeland et al., 2005), and as a regulator of Ca2+ release from the ER (Bononi et al., 2013). Recently, a translational variant of Pten (Pten-L) was found to be secreted into the extracellular space, where it can enter into neighboring cells and dephosphorylate PIP3 into PIP2 (i.e., inhibit Pi3k; Hopkins et al., 2013).
The failure of Pten deletion to induce any measurable improvement in bone properties among mice with β-catenin deficiency in the same cell population suggests that either Pten→Pi3k→Akt→Gsk3β→βcat is the dominant mechanism in bone cells that regulates bone mass, or that the Pten and βcat cascades operate independently to control bone mass but the βcat effect overwhelms bone homeostasis despite signals for improved properties originating from Pten deletion. Our immunoblots indicate that the Akt pathway is not activated by Pten deletion when βcat is absent, suggesting that there might be significant crosstalk between the Wnt and Akt pathways (Georgopoulos, Kirkwood, & Southgate, 2014) in osteocytes. It is puzzling that for many endpoints, Pten deletion further deteriorated an already compromised skeleton induced by βcat deletion. We did not detect an obvious suppression of Akt signaling in double mutants, but it is possible that other pathways might have been affected by the compound mutation and suppression of both Wnt and Akt pathways.
In summary, deletion of Pten from the osteocyte/late-stage osteoblast population results in increased bone mass, density, strength, and formation parameters, whereas deletion of βcat has the opposite effects. Co-deletion of Pten and β-catenin in the same cell population has no beneficial effect on the low bone mass phenotype generated by βcat deletion, and for many indices, Pten deletion further impaired the poor bone properties associated with β-cat deletion. A broad range of emerging pharmaceutical therapies are being designed to inhibit Wnt/βcat signaling for a variety of disorders and can have a significant, unintended impact on skeletal fragility. For example, small molecule inhibitors of the o-acyl transferase porcupine have severe consequences on bone mass, but those bone-wasting effects can be countered by bisphosphonate therapy (Madan et al., 2018). Agents that work on the Pten enzyme or its downstream effectors are unlikely to counter the bone-wasting effects of pharmacologic Wnt inhibition.
ACKNOWLEDGMENT
This study was supported by NIH grants AR065971 (to WAB), AR070624 (to WAB), AR069029 (to FMP and AGR), AR053237 (to HK and AGR); and by VA grants BX001478 and BX003783 (to AGR).
Open Research
DATA AVAILABILITY STATEMENT
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.




