Dehydroascorbic acid, the oxidized form of vitamin C, improves renal histology and function in old mice
Abstract
Oxidative stress and inflammation are crucial factors that increase with age. In the progression of multiple age-related diseases, antioxidants and bioactive compounds have been recognized as useful antiaging agents. Oxidized or reduced vitamin C exerts different actions on tissues and has different metabolism and uptake. In this study, we analyzed the antiaging effect of vitamin C, both oxidized and reduced forms, in renal aging using laser microdissection, quantitative reverse-transcription polymerase chain reaction, and immunohistochemical analyses. In the kidneys of old SAM mice (10 months of age), a model of accelerated senescence, vitamin C, especially in the oxidized form (dehydroascorbic acid [DHA]) improves renal histology and function. Serum creatinine levels and microalbuminuria also decrease after treatment with a decline in azotemia. In addition, sodium-vitamin C cotransporter isoform 1 levels, which were increased during aging, are normalized. In contrast, the pattern of glucose transporter 1 expression is not affected by aging or vitamin C treatment. We conclude that oxidized and reduced vitamin C are potent antiaging therapies and that DHA reverses the kidney damage observed in senescence-accelerated prone mouse 8 to a greater degree.
1 INTRODUCTION
There is a close relationship between advancing age and increased acute kidney injury and chronic kidney disease. Several recent studies have shown that the aged kidney undergoes various structural and functional changes (O'Sullivan, Hughes, & Ferenbach, 2017; Uzun et al., 2013). Thus, increased serum creatinine levels and microalbuminuria have been detected, together with elevated nitrogenous substances in the blood or azotemia and a yearly decline in the glomerular filtration rate (Lindeman & Goldman, 1986; Succar, Pianta, Davidson, Pickering, & Endre, 2017). Kidneys also have increased oxidative stress markers and inflammation with age (Chung et al., 2009; O'Sullivan et al., 2017).
Different antioxidants have been studied with promising results as antiaging agents. Enzymes or micronutrients, such as vitamins C and D as well as carotenoids, have been studied in animal models and humans (Ames, 2018; Jeremy, Gurusubramanian, & Roy, 2019). Vitamin C exerts a pivotal role in the defense against oxidative stress, reducing DNA oxidation and lipid peroxidation (Levine, Rumsey, Daruwala, Park, & Wang, 1999; Saitoh, Morishita, Mito, Tsujiya, & Miwa, 2013). Vitamin C is found in both reduced and oxidized forms, ascorbic acid (AA) and dehydroascorbic acid (DHA). AA is taken up by high-affinity sodium-dependent transporters, sodium-vitamin C cotransporter isoform 1 (SVCT1), in the kidney (T. Castro et al., 2008; Corpe et al., 2010; Forman et al., 2017; Lee et al., 2006; Nualart et al., 2003; Wang et al., 2000) or SVCT2 in the brain (M. Castro et al., 2001; Dixit et al., 2015; Ferrada, Salazar, & Nualart, 2019; Marcos et al., 2018; Salazar et al., 2014; Salazar et al., 2018; Silva-Alvarez et al., 2017; Tsukaguchi et al., 1999; Ulloa et al., 2019; Warner, Kang, Kennard, & Harrison, 2015), whereas DHA uptake is mediated by facilitative glucose transporters (GLUTs), specifically GLUT1–4, with GLUT1 and 2 expressions in the kidney (Garcia-Krauss et al., 2016; Nualart et al., 2003; Rumsey et al., 1997; Rumsey et al., 2000).
The effect of vitamin C, both reduced and oxidized forms, on renal aging has not been clearly defined. Some studies have reported improvement in endothelial function (Cangemi et al., 2007) after vitamin C treatment, while others showed no effect (Darko, Dornhorst, Kelly, Ritter, & Chowienczyk, 2002).
Vitamin C homeostasis is favored by active reabsorption in renal tubules (M. Martin, Ferrier, & Roch-Ramel, 1983; Toggenburger et al., 1981). Several studies, including those from our research group (T. Castro et al., 2008; Forman et al., 2017; Toggenburger et al., 1981), have shown that SVCT1 is specifically located in the apical membrane along segments S1–S3 of the proximal tubules. Furthermore, we have observed that aging worsens the renal histology in senescence-accelerated prone mouse 8 (SAMP8) mice (Forman et al., 2017).
The senescence-accelerated mouse (SAM) is a good model of accelerated aging as it can manifest different senescent phenotypes, including endothelial dysfunction and learning deficits (Gevaert et al., 2017; Takeda, Hosokawa, & Higuchi, 1997). SAMP8, a substrain of the SAM model, has been widely used for the study of age-related diseases (Takeda, 1999); however, few studies have used it for the study of renal aging (Shino, Tsukuda, Omori, & Matsuo, 1987). The relationship between vitamin C with longevity and aging has also been explored with contradictory results (Massie, Aiello, & Doherty, 1984; Selman et al., 2006) in worms, flies, rodents and humans (Traber & Stevens, 2011); however, in SAMP8 mice, scarce information is available (Bayram et al., 2013).
The aim of this study was to investigate the influence of vitamin C treatment (AA or DHA) on renal aging, analyzing age-related histological alterations, renal function and the expression and distribution of the vitamin C transporters, SVCT1 and GLUT1, in renal tissue isolated from SAMP8 mice at different ages.
2 MATERIALS AND METHODS
2.1 Animals
SAMP8 mice from Harlan Laboratories Bicester, UK were used (n = 54). Animals were divided into seven experimental groups: (1) young SAMP8 mice at 2 months of age; (2) adult SAMP8 mice at 6 months of age; (3) old SAMP8 mice at 10 months of age and (4–7) four SAMP8 treatment groups, including adult and old mice treated with AA or DHA (10 mg/kg intraperitoneal, twice a week). DHA was obtained by oxidizing AA with ascorbate oxidase from Cucurbita sp. (Sigma-Aldrich, St. Louis, MO). The animals were housed in controlled light (12-hr light/dark cycle) and temperature (20–24°C) conditions and received a standard diet and water ad libitum. The experiments were performed in accordance with a protocol approved by the Committee for Animal Experimentation of the University of Concepcion.
2.2 Immunohistochemistry and confocal analyses
For microscopy analysis, murine kidneys were fixed by direct immersion in Bouin's solution (F. Nualart, Hein, Rodriguez, & Oksche, 1991) and embedded in paraffin. Sections of 7-μm thickness were cut using a microtome and mounted on poly-l-lysine-coated glass slides. Hematoxylin staining was performed using a solution of hematoxylin and chromotrope 2R both from Merck Millipore (Darmstadt, Germany). For immunohistochemistry, the samples were treated with absolute methanol and 3% hydrogen peroxide to inactivate endogenous peroxidase activity (Poblete, Nualart, del Pozo, Perez, & Figueroa, 1996). Anti-SVCT1 (1:50; D-19 sc-9921; Santa Cruz Biotechnology, Santa Cruz, CA) or anti-GLUT1/anti-GLUT2 (1:50; GT13-A/GT21-A, Alpha Diagnostic International, San Antonio, TX) polyclonal antibodies were used. The sections were incubated overnight at room temperature in a humid chamber. For immunofluorescence, the same antibodies were used together with Phaseolus vulgaris (PHA)-lectin as a marker of the apical brush border of proximal tubules (T. Castro et al., 2008). Subsequently, the samples were incubated for 2 hr at RT with Cy2-, Cy3-, and Cy5-conjugated secondary antibodies (1:200; Jackson ImmunoResearch, West Grove, PA) and analyzed by an LSM-780 LNO confocal microscopy system (Zeiss, Oberkochen, Germany).
2.3 Transmission electron microscopy
Renal samples were immersed in fixative containing 2% paraformaldehyde and 0.5% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4) for 2 hr. Sections of 90- or 150-µm thickness were rinsed in 0.1 M phosphate buffer. Then, the samples were post-fixed in 2% osmium tetroxide in phosphate buffer for 1 hr and stained with 2% uranyl acetate in 70% ethanol for 3 hr at 4°C, dehydrated and incubated with propylene oxide for araldite embedding. Once plasticized, the sections were cured at 60°C for 3 days. Serial semi-thin sections of 1.5 µm were cut on an ultramicrotome (Leica, Wetzlar, Germany) and later stained with 1% toluidine blue. Ultrathin sections (60 nm) were next cut using a diamond knife and the same ultramicrotome and analyzed using an electron microscope (Jeol Jem-1400).
2.4 RNA isolation and reverse-transcription polymerase chain reaction (RT-PCR)
A complete kidney was used for RNA isolation using the instructions provided with the TRIzol Reagent (Invitrogen, Rockville, MD). For RT-PCR, 2 µg of RNA were pretreated with DNase I (Fermentas, ON, Canada) and reversed transcribed into cDNA in a 20 µl reaction volume containing 5X M-MulV reverse transcriptase buffer, 20 U RNAse inhibitor, 1 mM dNTPs, 2.5 µM Oligo(dt)18 primer and 10 U/µl of RevertAid TM H minus M-MuLV reverse transcriptase (Thermo Fisher Scientific, Waltham, MA). Parallel reactions were performed in the absence of reverse transcriptase as a control of purity.
2.5 Laser capture microdissection (LMD)
To measure GLUT1, GLUT2, and SVCT1 mRNA levels in three different kidney areas (cortex, OS/OM, and inner medulla), laser capture microdissection coupled to quantitative RT-PCR (qRT-PCR) was used. The kidneys were removed and fixed in methacarn (60% methanol, 30% chloroform, and 10% acetic acid) for 3 hr. Then, 60-µm-thick longitudinal sections were cut with a Leica VT1000S vibratome (Leica) and mounted on PET frame slides (11505151; Leica). For dissection, an LMD7000 Laser Microdissector (Leica) was employed. For total RNA extraction, an Ambion RNAqueous1-Micro Total RNA Isolation Kit (Ambion, Foster, CA) was used following the manufacturer's instructions followed by cDNA synthesis and qRT-PCR.
2.6 qRT-PCR analysis
qRT-PCR reactions were prepared in a Master cycler Realplex 2 Thermal Cycler (Eppendorf, Hamburg, Germany) with a Brilliant II SYBR Green QPCR master mix kit (Agilent Technologies, Santa Clara, CA) and a final reaction volume of 12.5 µl containing 1 µl of cDNA and 500 nM of specific primers. The following sets of primers were used: SVCT1 forward, CCAAAGCAGCATGAGGTCGTG; SVCT1 reverse, CTCTCCAAGGCCAGGATAGC; GLUT1 forward, AGCAGTGAAGTCCAGGAGGA; GLUT1 reverse, CTGGTCTCAGGCAAGGGAAG; and GLUT2 forward, TTTCTTTGCCCTGACTTCCT; GLUT2 reverse, GGCTAATTTCAGGACTGGTT. To determine the expression of the housekeeping gene, the following sequences were used, GADPH forward, CGTGGTTCACACCCATCACAAAC and reverse, GCAAGTTCAACGGCACAGTCAAG. Relative changes in gene expression were calculated using the method (Livak & Schmittgen, 2001).
2.7 Determination of urea, microalbuminuria, and creatinine levels
Biochemical parameters were measured using commercial kits from the Wiener Lab Group (Rosario–SF, Argentina). Serum and urinary creatinine levels were measured by a kinetic colorimetric assay using the Jaffe method (code 1260360); urinary microalbumin concentrations were estimated by turbidimetry (code 1513266). Urea levels were measured by a urease reaction using the commercial kit number 1810328. All determinations were developed in a Clinical Chemistry Analyzer CB 400i equipment (Wienner Lab, Rosario–SF, Argentina).
2.8 Statistical analysis
Results are expressed as the mean ± SEM. Data were analyzed using analysis of variance followed by Bonferroni posttest. For qRT-PCR analysis, Student's t test with Mann–Whitney correction was used. Data were considered significant when the p value was < .05.
3 RESULTS
3.1 The oxidized form of vitamin C improves the renal morphology in old SAMP8 mice
Different areas of the kidney were analyzed using a standard structural nomenclature (Kriz & Bankir, 1988). In, this study, we confirm the structural studies previously carried out in Forman et al. (2017), and we extend the ultra-structural analysis in SAMP8 animals. At 2 months of age, few alterations at the level of the renal tubules were observed (Figure 1a–d). Using toluidine blue staining on semi-thin sections (1 μm), few tubular structures with different types of alterations (small spaces) were observed. However, at the ultrastructural level, most of the renal tissue was found to be in good condition histologically; microvilli, basal membranes, and lateral and basal projections of the plasma membrane of the tubular cells were also without alteration (Figure 1b,c). As previously mentioned, only some cells presented intercellular space dilatation (ICS; Figure 1d). Even so, in these cells, the basement membrane and the basal projections of the tubular cells were without alteration (Figure 1d).
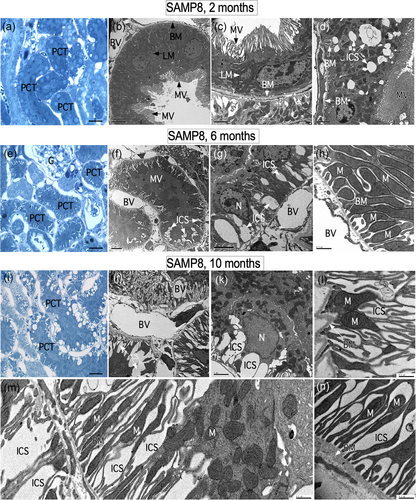
At 6 months of age, a series of structural changes were observed mainly in the proximal convoluted tubules (PCT), segments S1 and S2. Subcellular abnormalities, such as ICS, were also observed in the lateral region of epithelial cells (Figure 1e–g). In sections stained with toluidine blue, alterations were primarily observed in PCT cells that have a narrower lumen due to the abundant microvilli (Figure 1e). Electron microscopy analysis showed epithelial cells with dilated intercellular spaces of variable width at different levels of the tubule (Figure 1f,g). In general, epithelial cells maintain their interaction with the basement membranes, and in addition, there are no alterations in mitochondrial structure (Figure 1g–h). At 10 months of age, the kidney has greatly changed its structure at the tubular level. In semi-thin sections, toluidine blue staining showed that most vacuolar structures are present in the basal region of the PCT epithelial cells (Figure 1i). At an ultrastructural level, it was confirmed that these vacuolar structures are found in the intercellular regions (Figure 1j,k). In addition, we defined that the basal projections of the tubular cells had thinned with a reduced number of mitochondria (Figure 1l–n).
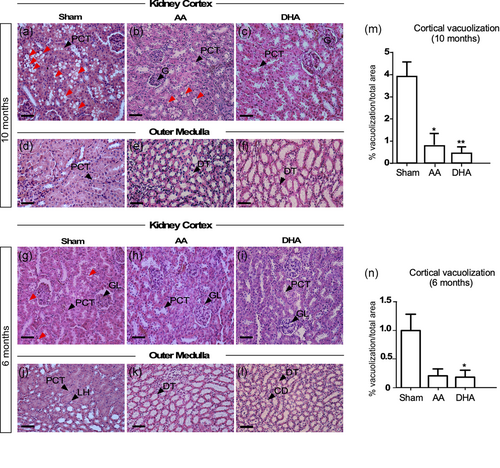
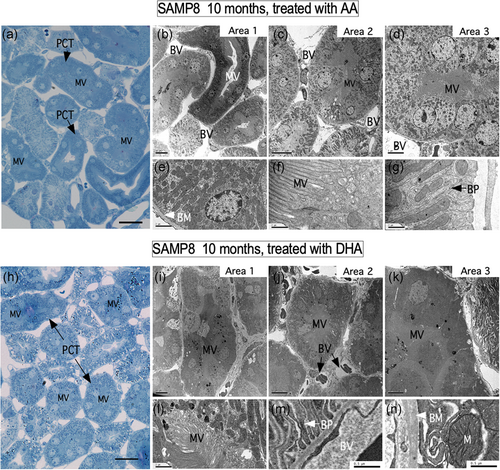
The ultrastructural changes were analyzed in 6- and 10-month-old control animals and in animals treated with AA or DHA. Control animals showed a vacuolar morphology, which was evident at 10 months of age, and to a lesser extent at 6 months of age (Figure 2a,g). At the external medulla, no tissue alterations were found in the periods analyzed (Figure 2d,j). Surprisingly, after vitamin C treatment, the vacuolar structures were minimal in animals treated with AA (Figure 2b,h,m,n) with almost none observed in animals treated with DHA (Figure 2c,i,m,n). In the external medulla, no changes were observed with age or treatments with vitamin C (Figure 2e,f,k,l). Histological features after treatment with vitamin C were also confirmed using ultrastructural analysis (Figure 3). In semi-thin sections after Toluidine blue staining, treatment with AA and DHA inhibited the formation of vacuolar structures in PCT cells (Figure 3a,h). In both treatments, most of the cells that form the PCT presented normal microvilli distribution, and the mitochondria were normally distributed. In addition, the basolateral membranes showed no alterations or loss of basement membrane contact. For each treatment, ultrastructural characteristics were observed in three different zones of the renal cortex (for AA treatment, Figure 3b–d; areas 1–3; higher magnification in e–g and for DHA treatment, Figure 3i–k; areas 1–3; higher magnification in l–n).
3.2 AA and DHA normalizes the level of the SVCT1 but does not alter GLUT1 OR GLUT2 during renal aging
LMD coupled qRT-PCR was used to define the changes in GLUT1, GLUT2, and SVCT1 mRNA expression in three regions of the kidney, cortex, the outer medullary region, and the inner medullary region. In our previous experience, the use of total mRNA tissue extracts masked the real pattern of glucose and vitamin C transporter expression (Forman et al., 2017). Therefore, the LMD technique allows us to overcome this problem. The highest expression of GLUT1 was found in the internal medullary region in both experimental or treatment conditions at all months (Figure 4a). At 6 months, treatment with DHA increased the expression of GLUT1 in the external medullary region, when compared with animals not treated with vitamin C for 6 months or with control animals for 2 months (Figure 4a). At 10 months of age, GLUT1 expression increased in the internal and external medullary regions in control animals as well as those treated with AA or DHA compared with the 2-month-old animals. No differences were observed between the different treatments at 10 months of age. In summary, GLUT1 expression in external and internal medullary regions at 10 months of age does not change even after treatment with vitamin C. In parallel, the highest expression of GLUT2 was found in the cortical region with minor variations with aging or treatment (Figure 4b). A slight decrease in GLUT2 expression was observed at the cortical level at 10 months of age, referring to sham animals of the same age after treatment with DHA (Figure 4b). Also, GLUT2 did not vary in the external medullary region; however, in the internal medullary region, a slight decrease in GLUT2 expression was observed at 10 months of age, which was not altered by vitamin C treatment (Figure 4b).
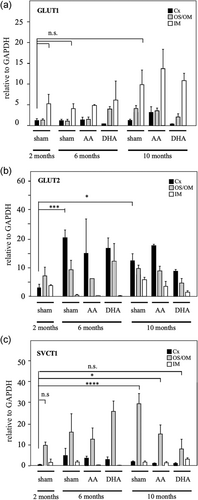
SVCT1 was preferentially expressed in the OS/OM region at 2, 6, and 10 months (Figure 4c). After treatment with AA and DHA, no changes in SVCT1 expression levels were observed at 6 months of age at the OS/OM. However, the increase in SVCT1 expression observed at 10 months of age (sham 2 months vs sham 10 months, OS/OM) was partially or completely normalized after treatment with AA or DHA (Figure 4c). No significant changes in SVCT1 expression were observed in the cortex or internal medullary region under any conditions (Figure 4c).
Previously, we have established that SVCT1 is expressed as a gradient in renal proximal tubules with the highest levels detected in the OS/OM in mouse and human cells (T. Castro et al., 2008). Aging increased SVCT1 levels in the OS/OM, segment S3, and in PCT cells (Forman et al., 2017). In the present work, we confirmed the aforementioned results (Figure 5a–d). Interestingly, AA and DHA treatments were able to reduce SCVT1 apical staining (brush border membranes) in both PCT and OS/OM segments (Figure 5e–l,x,y) at 10 months. Comparative studies analyzing the expression of GLUT1 (Figure 5m,n) in the inner medulla showed no changes with AA or DHA treatments (Figure 5o–r,z). When performing confocal microscopy analysis at the OS/OM, we observed that SVCT1 concentrates in the microvilli of PCT-segment S3 (PCT-S3) (Figure 5q,q1), colocalizing with PHA lectin (Figure 5r,r1,s,s1, white label). We also noted that GLUT1 is preferably expressed in the collecting duct (CD) where it does not colocalize with SVCT1 or PHA lectin (Figure 5q,r,r1,s,s1). The subcellular distribution of SVCT1 or GLUT1 was not modified with AA or DHA treatments (Figure 5t–w).
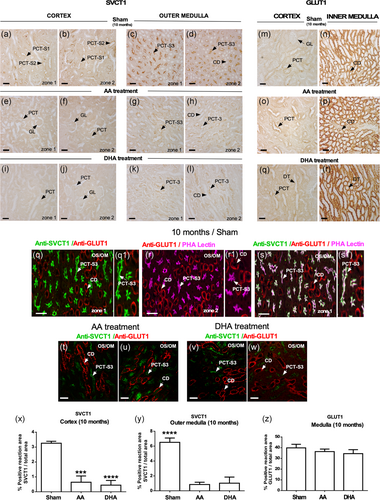
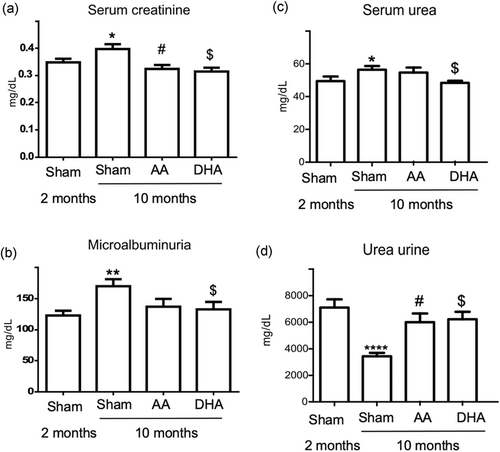
3.3 DHA normalizes microalbuminuria and serum creatinine levels in old SAMP8 mice
Alterations in the structural tissue integrity have been associated with functional disorders. Thus, we detected that histological age-related kidney alterations were correlated with modifications of the classical renal biochemical parameters related to renal function. Significantly higher serum creatinine levels were observed with advancing age (Figure 6a; p < .05). In addition, the presence of microalbuminuria was also detected in old SAMP8 mice (p < .005), suggesting poor renal function (Figure 6b). Both AA and DHA reduced the creatinine levels, showing values similar to those observed in young animals although DHA had a greater effect (Figure 6a, DHA; p < .005) as compared to AA (Figure 6a, AA; p = 0.01). In addition, the presence of microalbuminuria was normalized after DHA treatment, suggesting an improvement in renal function in old mice (Figure 6b, DHA; p < .05). During renal aging, signs of azotemia were also observed as shown in the increased retention of serum urea (Figure 6c; p < .05) and reduced urinary excretion (Figure 5d; p < .0001) in old SAMP8 mice. An improvement in the degree of azotemia after vitamin C treatment was established. DHA significantly reduced the urea serum levels (Figure 6c; p < .05) and increased the urea fraction in urine (Figure 5d; p = .0007). Only AA was able to increase renal urea excretion (Figure 6d; p < .002).
4 DISCUSSION
In the present study, we analyzed the antiaging potential of AA and DHA in renal aging. Our research group along with other groups have previously analyzed age-related renal alterations in old SAMP8 mice (Forman et al., 2017; Shino et al., 1987), including increased urinary albumin-to-creatinine ratio and glomerulosclerosis (Zeng, Wang, Zhang, & Du, 2016). Interestingly, no increase in serum creatinine was observed in these old mice compared with age-matched senescence-resistant mice (Zeng et al., 2016). Chronic kidney disease induction increased urinary oxidative and pro-inflammatory markers, such as interleukin-18, without increasing serum creatinine levels, suggesting that extensive structural damage within the kidney must be present before serum creatinine increases (Succar et al., 2017). Of note, damage was also observed in our present results. Furthermore, the excretion rate of albumin is considered the most used biomarker of renal injury (Tesch, 2010). Thus, the expansion of intracellular spaces in proximal tubular epithelial cells along with the perivascular lymphocytic infiltrate and glomerular congestion detected previously by our group in the kidney cortex of old SAMP8 mice (Forman et al., 2017) likely promoted increased creatinine levels, azotemia and microalbuminuria in old mice as markers of kidney cellular injury, which was also observed in the present results. Also, old SAMP10 mice had significant increases in serum creatinine and urea levels together with a lower level of superoxide dismutase in the kidney (Hu et al., 2013).
In the present results, DHA had enhanced protective effects on aged kidneys as compared to AA. DHA improves renal function by normalizing microalbuminuria, reversing azotemia and decreasing serum creatinine levels, all effects that have been previously related to supplementation with vitamin C. In mice, vitamin C decreases renal artery reactive oxygen species, serum urea, creatinine and renal artery resistance (Zhu, Zhang, Zhang, & Zhang, 2016). Evidence suggests that DHA is the preferred vitamin C in the kidney, probably related to urinary tubular acidity given that serum obtained from the renal veins contained AA largely in the oxidized form, DHA. This finding is consistent with the hypothesis that the kidney facilitates the transport of AA by oxidizing the vitamin to an un-ionized form that readily penetrates cellular barriers (G. R. Martin, 1961). Notably, in the present study, only DHA in old SAMP8 mice was able to reduce microalbuminuria. AA only partially reverses the azotemia and decreased serum creatinine levels, but to a lesser degree than DHA. The effects of vitamin C (AA or DHA) are probably related to a decrease in pro-oxidative status observed during aging. A bioactive compound derived from the rhizome of rhubarb (RHL) reduced the upregulation of creatinine and urea in SAMP10 mice (Hu et al., 2013). RHL is able to diminish MDA levels and increases the levels of superoxide dismutase and glutathione peroxidase in kidney tissues. Thus, the antioxidant properties of vitamin C (AA and DHA) could be exerting a similar effect.
We also have to consider that DHA is preferentially transported across plasma membranes by GLUT1 (Arrigoni & De Tullio, 2002). In the present study, immunohistochemistry and qRT-PCR analyses showed that GLUT1 is present along the tubular network, unlike SVCT1 which is restricted to the proximal tubule. Therefore, it is possible that old kidneys uptake more DHA than AA. As DHA is more lipid-soluble than AA, DHA would be expected to cross cellular barriers more readily (G. R. Martin, 1961).
In studies using human fibroblast cultures, there is increased AA uptake in senescent cells compared to young cells (Saitoh et al., 2013). Thus, the aforementioned results are related to the beneficial effects induced by AA treatment in the renal cells, such as improvements of renal histology, SVCT1 normalization (cortex and OS/OM), improved serum creatinine, and decreased urinary urea levels, which were more attenuated with DHA treatments. Previously, we have proposed that increased SVCT1 expression and epithelial polarization in the kidney cortex favors AA absorption in S2 and S3 segments to compensate for the age-related decline of vitamin C production. Furthermore, previous studies have shown that high concentrations of AA reduce SVCT1 expression and vitamin C uptake (T. Castro et al., 2008).
We conclude that AA and DHA are promising antiaging agents, improving renal function and modulating the age-related expression of SVCT1 in aged kidneys. DHA displayed better protective effects in the kidney from old SAMP8 mice as compared to AA.
ACKNOWLEDGMENTS
The authors thank Dr. Marjet Heitzer for suggestions on the manuscript. This study was supported by Grants from Fondecyt Iniciación 11160949 (K.F), CONICYT REDI170373 (P.T), Fondecyt regular 1181243 (F.N), CONICYT PIA ECM-12 (F.N).
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
K. F., F. M., M. C., F. N. developed the original idea, performed experiments, analyzed the literature and results, drafted the manuscript. M. F., R. B., P. T., K. S. analyzed the literature and results, made a critical reading of the document and directly contributed to the discussion.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon request.




