Upregulation of Toll-like receptor 4 through anti-miR-Let-7a enhances blastocyst attachment to endometrial cells in mice
Abstract
Despite encouraging advances in fertility technology, the success rate of an ongoing pregnancy is relatively low and predominantly associated with implantation failure. Inflammatory responses are beneficial in the fetomaternal interface and supposedly accelerate the chances for successful implantation. The current study aims to determine the effect of Toll-like receptor 4 (TLR4) overexpression in mouse blastocysts via Let-7a downregulation using intracytoplasmic sperm injection-sperm-mediated gene transfer on embryo attachment rate. The pLenti-III-GFP-miR-Off-Let-7a vector was transmitted to oocytes derived via in vitro maturation (IVM) and in vivo oocytes by using NaOH-treated spermatozoa. Let-7a and TLR4 expression levels were evaluated by quantitative real-time polymerase chain reaction (qRT-PCR), immunocytochemistry, and western blot analysis in both oocytes and embryos. Blastocyst adhesion on the endometrial cells was monitored by microscopic analysis. qRT-PCR results showed that Let-7a expression decreased in the IVM (GV-MII) oocytes compared to the in vivo oocyte (MII) group (p < .05). TLR4 showed a higher expression in GV-MII oocytes at both the gene and protein levels (p < .05). Following anti-miR-Let-7a transmission, the TLR4 expression level was significantly upregulated in embryos compared with the control groups (p < .05). Attachment and migration of trophoblasts cells towards endometrial cells dramatically increased compared to the control group (p < .05). Based on our results, we concluded that Let-7a might mediate embryo attachment through regulation of TLR4 expression levels.
1 INTRODUCTION
Embryo implantation is a crucial step in the mammalian reproduction process that involves a sequence of steps from apposition, tight attachment, and migration to invasion (Dominguez, Yanez-Mo, Sanchez-Madrid, & Simon, 2005) of the blastocyst into the decidualized endometrial lining (Makrigiannakis, Minas, Kalantaridou, Nikas, & Chrousos, 2006). Despite numerous advances in assisted reproductive technology (ART), the pregnancy rate following this procedure remains relatively low. Implantation failures are believed to be a major cause for this low pregnancy rate (Ata, Yakin, Balaban, & Urman, 2008; Koot, Teklenburg, Salker, Brosens, & Macklon, 2012). Embryo implantation per transfer is approximately 33.2% after in vitro fertilization (IVF) and 31.8% after intracytoplasmic sperm injection (ICSI), which limits the viable pregnancy rate in ART (European et al., 2016). The innate immune system in the fetomaternal interface affects implantation and the pregnancy success rate by promoting tolerance against the allogeneic fetus. In addition, this system is of fundamental importance in maintaining the host defense against the possibility of invading pathogens (Koga & Mor, 2010; Tangerås et al., 2014). Therefore, an appealing strategy to improve embryo implantation would be to target the immune system and stimulate the expression of inflammatory factors.
Toll-like receptors (TLRs) are members of the innate immune system (Kawai & Akira, 2010) that are thought to be implicated in blastocyst implantation and placentation. In addition to immune cells, TLR are also expressed by trophoblastic and decidua cells (Mor, Cardenas, Abrahams, & Guller, 2011; Tangerås et al., 2014). TLR activation leads to rapid, enhanced release of the pro-inflammatory cytokines interleukin-6 (IL-6), IL-8, and tumor necrosis factor-α (TNF-α; Takeuchi & Akira, 2010), which are characteristics of early implantation (Yoshinaga, 2008). The production of cytokines and creation of this inflammatory environment directs the blastocyst to the site of implantation and supports its interaction with the uterine wall (Granot, Gnainsky, & Dekel, 2012; van Mourik, Macklon, & Heijnen, 2009). It also ensures the appropriate reconstruction of the uterine epithelium and removal of cellular debris (Mor et al., 2011). Although cytokines, chemokines, growth factors, and adhesion molecules are involved in the implantation process (Duc-Goiran, Mignot, Bourgeois, & Ferre, 1999; McGowen, Erez, Romero, & Wildman, 2014; van Mourik et al., 2009), the molecular mechanisms behind their regulation should be clearly addressed.
The results of recent studies have shown a possible regulatory role for microRNAs (miRNAs) in the main genes involved in implantation, which would subsequently determine the fate of the fetus (Galliano & Pellicer, 2014). Additionally, embryos undergo changes in miRNA expression profiles during the preimplantation phase (Koot et al., 2012; W. M. Liu et al., 2012; McCallie, Schoolcraft, & Katz-Jaffe, 2010). For example, miR-101a and miR-199a target cyclooxygenase (Chakrabarty et al., 2007) and miR-98 targets the Bcl-xl gene in mouse embryo implantation (Xia, Jin, Cao, Shi, & Ma, 2014). Therefore, misrepresentation of miRNAs can be a cause for implantation failure. Amongst the miRNAs, the Let-7 family exhibits the highest level of expression in mammals and contributes to cell proliferation, replication, and differentiation (Roush & Slack, 2008). Let-7, the first known miRNA in humans, is highly conserved in terms of genomic pattern and function across species such as humans, mice, and Drosophila (Cheong et al., 2014; Roush & Slack, 2008). Liu et al. have observed reduced expression of five members of the Let-7 family (Let-7a, Let-7d, Let-7e, Let-7f, Let-7g) in activated mouse blastocysts for implantation (W. M. Liu et al., 2012). Let-7 family miRNAs post-transcriptionally regulate inherent immune responses (Sathe, Patgaonkar, Bashir, & Reddy, 2014; Schulte, Eulalio, Mollenkopf, Reinhardt, & Vogel, 2011). Androulidaki et al. demonstrated that Let-7e suppressed TLR4 expression in macrophages (Androulidaki et al., 2009). Another research group reported similar findings for Let-7i (Chen, Splinter, O'Hara, & LaRusso, 2007).
In this study, we aimed to explore the impact of Let-7a downregulation on implantation success rate through overexpression of TLR4 in mouse blastocysts derived via ICSI. The quantification of TLR4 and Let-7a in blastocyst-derived in vitro (IVM) and in vivo matured oocytes were assessed by quantitative real-time-polymerase chain reaction (qRT-PCR) after transmission of the pLenti-III-GFP-miR-Off-Let-7a vector using ICSI-sperm-mediated gene transfer (ICSI-SMGT).
2 MATERIALS AND METHODS
Unless otherwise noted, all chemicals used in the present study were purchased from Sigma Aldrich Chemical Company (St. Louis, MO). Female and male B6D2F1 (C57BL/6 ×DBA/2) mice were obtained from the Pasteur Institute (Tehran, Iran). All experimental procedures were performed in accordance with standard guideline procedures approved by the Institutional Animal Care and Ethics of Shahid Beheshti University of Medical Sciences, Tehran, Iran.
2.1 Study design
We designed three experimental groups: (i) test (co-incubation of NaOH-treated spermatozoa with vector), (ii) sham (co-incubation of NaOH-treated spermatozoa with scrambled vector), and iii) control (fresh spermatozoa). In all of the experimental groups, the ICSI procedure was used to inject the spermatozoa into the oocyte-derived IVM (GV-MII) and in vivo oocytes (MII; Figure 1).
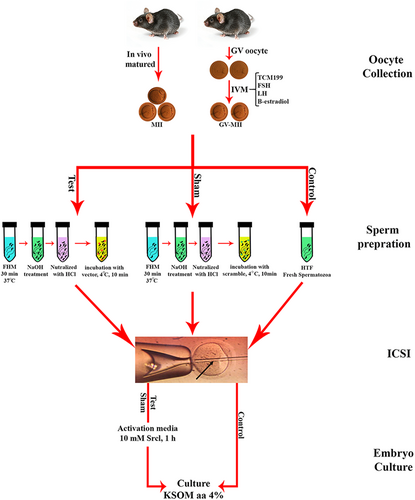
2.2 Oocyte collection
2.2.1 Matured in vivo oocytes
The 6- to 8-week-old female BDF1 mice were superovulated by intraperitoneal injections of 10 IU pregnant mare serum gonadotropin (PMSG). After 48 hr, human chorionic gonadotropin (hCG) was injected to obtain relatively large numbers of oocytes. The mice were killed via cervical dislocation 14 hr after the hCG injection. Subsequently, cumulus-oocyte complex (COCs) were recovered from the oviduct ampulla and immediately immersed in a medium that contained hyaluronidase to disperse the cumulus cells. The denuded oocytes were washed twice in flushing holding medium (FHM) and then transferred into a new drop of potassium simplex optimized medium (KSOM) supplemented with amino acid and 4% bovine serum albumin (BSA).
2.2.2 In vitro maturation (IVM) derived oocytes
BDF1 female mice were killed 48 hr after administration of 10 IU PMSG. Their ovaries were excised and placed in a pre-warmed HEPES-tissue cell culture medium (TCM-199) supplemented with 10% fetal bovine serum (FBS; Gibco, Invitrogen, Barcelona, Spain). The follicles were then punctured using a pair of 28G sterile needles under a stereomicroscope. We only utilized immature oocytes that were surrounded by tight unexpanded cumulus cells. The immature oocytes were incubated in TCM-199 media supplemented with 0.2 mM sodium pyruvate, 2 mM l-glutamine, 10 μg/ml follicle stimulating hormone, 10 μg/ml LH, 1 μg/ml β-estradiol, and 10% FBS for 22 hr at 37°C in a humidified atmosphere of 5% CO2. The oocytes were detached from the cumulus cells after IVM by exposure to hyaluronidase and mechanical pipetting. Observation of the first polar body was considered to be the criterion for mature oocytes. For both the in vivo and IVM groups, denuded oocytes were stored in KSOM media within the maximum period of 2 hr until ICSI-SMGT was performed.
2.3 Bioinformatics analyses and plasmid preparation
The miRBase Targets, TargetScan, and PicTar algorithm approaches can predict numerous genes that are targeted by miR-Let-7a. It is well-documented that TLR4 is a target of miR-Let-7. The pLenti-III-GFP-miR-Off-Let-7a vector (anti-miR-Let-7a) was purchased from ABM, Inc. Bacterial colonies were grown in LB-kanamycin broth in a shaking incubator at 37°C for 16 hr. The plasmid was extracted using a Plasmid DNA Purification Kit (NucleoBand PC 2000, Macherey-Nagel, Germany). We confirmed the size of the extracted plasmid by gel electrophoresis and subsequently measured the DNA concentration using a NanoDrop spectrophotometer.
2.4 Collection of spermatozoa and NaOH pretreatment
We removed caudal epididymides from 10- to 12-week-old male B6D2F1 mice. Both epididymal ducts were placed in the dish that contained FHM supplemented with 4 mg/ml (BSA) and minced with sharp scissors. The dense sperm mass was immediately transferred into a microtube that contained 100 μl of FHM and allowed to incubate for 30 min at 37°C. To disrupt the sperm membranes, we mixed the sperm suspension with 10 mM of NaOH and kept this solution at room temperature (RT) for 1 hr. The suspension was then neutralized with the same volume and concentration of HCl. NaOH-pretreated spermatozoa were exposed to the DNA solution at 4°C for 10 min, which resulted in a final DNA concentration of 2 ng/μl in the mixture (Li, Mizutani, Ono, & Wakayama, 2010). The treated spermatozoa were used for ICSI-SMGT.
2.5 Intracytoplasmic sperm injection sperm-mediated gene transfer (ICSI-SMGT)
The spermatozoa were injected into GV-MII and MII oocytes by a PMM-150FU Piezo-actuated micromanipulator (Prime Tech Ltd., Tsuchikura, Japan). We mixed the treated spermatozoa with FHM that contained 10% (w/v) polyvinylpyrrolidone (PVP) and injected them into the oocytes according to a previously described method (Yoshida & Perry, 2007). For NaOH-treated spermatozoa, the injected oocytes were transferred into activation medium that contained 10 mM SrCl2 for 1 hr, after which they were transferred to KSOM medium to resume incubation. To inject fresh spermatozoa for the control group, we separated the head of the sperm from the tail by the application of one or more Piezo pulses, and then injected the head into the oocyte. The surviving oocytes were cultured in KSOM medium and incubated at 37°C and 5% CO2 for 96 hr. Embryo development was ultimately assessed in terms of the percentage of fertilization and blastocyst formation at 24 and 96 hr after fertilization, respectively.
2.6 Observation of GFP expression
The two-cell and blastocyst stage embryos were examined under a fluorescence microscope (Olympus, Tokyo, Japan).
2.7 Immunocytochemistry (ICC)
We used an immunofluorescence technique to assess the presence of TLR4 in the oocytes according to a previously described protocol (Shimada, Hernandez-Gonzalez, Gonzalez-Robanya, & Richards, 2006). Briefly, the zona pellucidae of the oocytes were removed by acidic Tyrode's solution. Next, they were fixed with 4% paraformaldehyde for 20 min and permeabilized with 0.2% Triton X-100 in phosphate-buffered saline (PBS). The fixed oocytes were blocked for 40 min at RT with 3% BSA in PBS and incubated overnight at 4°C with monoclonal anti-TLR4 (Santa Cruz Biotechnology, Santa Cruz, CA; 1:50). The oocytes were finally rinsed three times with PBS and incubated with phycoerythrin-conjugated secondary antibody (Santa Cruz Biotechnology; 1:200) for 1 hr at 37°C. Nuclei were counterstained with 4',6-diamidino-2-phenylindole (DAPI) and visualized by a fluorescent microscope. The fluorescence images were analyzed using the ImageJ software.
2.8 Western blot analysis
Blastocysts derived from each group were pooled and rinsed twice in PBS. We directly added 10 μl Laemmli sample buffer that consisted of 4% sodium dodecyl sulfate (SDS), 20% glycerol, 10% β2-mercaptoethanol, 0.004% bromphenol blue, and 0.125 M Tris–HCl to the blastocysts. Total proteins were denatured in loading buffer for 5 min at 95°C and resolved in SDS–PAGE on a 7% polyacrylamide gel at 70 V. Next, the proteins were transferred to a polyvinylidene fluoride membrane (Bio-Rad Laboratories, Hercules, CA) at 100 V. The membrane was blocked in skim milk for 60 min at RT, then incubated overnight at 4°C with monoclonal anti-TLR4 antibody (Santa Cruz Biotechnology; 1:200). The membrane was finally incubated with anti-mouse HRP-conjugated secondary antibodies (Abcam; 1:2,500) at 37°C for 60 min. After washing in Tris-buffered saline and 0.1% Tween 20, the protein bands were detected by using a Chemiluminescence Detection Kit (Santa Cruz Biotechnology) according to the manufacturer's instructions.
2.9 RNA extraction and complemetary DNA (cDNA) synthesis
Briefly, we added lysis buffer to each microtube that contained the blastocysts and oocytes to lyse the cells. cDNA was synthesized based on a protocol described in our previous study (Hosseini et al., 2017).
2.10 Quantitative real-time PCR (qRT-PCR) analysis
qRT-PCR was performed with a Rotor-Gene Q instrument (Qiagen, Valencia, CA) to assess quantification of TLR4 and Let-7a. All samples were run in duplicate and each experiment was conducted in triplicate. We performed qRT-PCR using a reaction mixture that consisted of a specific primer for each gene, synthesized cDNA, and DNA Master SYBR Green I mix (Roche Applied Sciences). Melting curve analysis was performed for all individual amplification reactions to confirm the occurrence of specific amplification peaks and the absence of primer-dimer formation. β2m and Snord were the internal control genes used for qRT-PCR data normalization. qRT-PCR data were analyzed with the Relative Expression Software Tool (REST, version 2009). Table 1 lists the primer sequences used for qRT-PCR.
| Name | Sequence | Accession no |
|---|---|---|
| miR-Let-7a | F: GGCTGAGGTAGTAGGTTGTATAG | AJ459692.1 |
| R: GAGCAGGGTCCGAGGT | ||
| Tlr4 | F: AGA AAA TGC CAG GAT GAT GC | NM_021297.3 |
| R: TGT CAT CAG GGA CTT TGC TG |
2.11 Mouse embryo attachment assay
2.11.1 Endometrial cell preparation
The uterine tissues were removed from day-8 pseudopregnant mice. The minced endometrial tissues were transferred into a 0.25% collagenase solution (Type IV) and digested for 60 min at 37°C. The enzymatic reaction was stopped by the addition of Dulbecco's modified Eagle's medium (DMEM)/Fl2 (Gibco-BRL) with 10% FBS. The cells were centrifugally washed three times in DMEM/F12 plus 10% FBS for 5 min.
2.11.2 Coculture of blastocysts and endometria
To determine blastocyst attachment, the zona pellucidae of the blastocysts were removed by acidic Tyrode's solution. The blastocysts were placed on a layer of epithelial and stromal cells in a single well of a four-well plate that contained DMEM/F12 medium with 100 nmol/L of progesterone, 10 nmol/L of estradiol, and 10% FBS for 72 hr at 37°C and 5% CO2. Blastocyst adhesion and migration on the endometrial cells were monitored via microscopic technique.
2.12 Statistical analysis
Statistical analysis was performed on data sets that consisted of at least three independent experiments. We used the unpaired Student's t test and analysis of variance with SPSS software version 22. The confidence level was determined to be p < .05. Data were expressed as mean ± SD.
3 RESULTS
3.1 Tlr4 is expressed in both GV-MII and MII oocytes
We performed qRT-PCR to assess the expression levels of Let-7a and Tlr4 in the oocytes. There was a significant difference in gene expression levels of Let-7a and Tlr4 between MII and GV-MII oocytes (p < .001; Figure 2). The Tlr4 gene had a higher expression in GV-MII oocytes compared to the MII oocytes. In contrast, GV-MII oocytes expressed less Let-7a than the MII group (Figure 2a). We used immunofluorescence staining to assess the protein expression level of TLR4. Figure 2b shows that TLR4 had greater fluorescence in GV-MII oocytes compared to MII oocytes. The quantitative analysis of immunofluorescence images showed an average intensity for TLR4 of 141.4 ± 2.6 in GV-MII oocytes and 68.4 ± 3.08 in MII oocytes (Figure 2c).

3.2 The introduction of anti-miR-Let-7a into the embryo did not adversely affect the rates of fertilization, cleavage, and embryo development
The plasmid was incubated with sperm and transmitted to the matured oocytes derived in vivo and IVM via ICSI-SMGT. We compared the fertilization rate and development of the embryos after plasmid transmission among the groups to examine whether downregulation of Let-7a had an adverse effect on preimplantation development. Table 2 summarizes the percentage of fertilization and the cleavage rate in MII oocytes. The control group had a higher fertilization rate (96.29 ± 4.96) compared to both the test (90.01 ± 1.84) and sham (93.22 ± 1.33) groups. However, there were no significant differences among these groups. We observed an increased cleavage rate from the four-cell to the morula stages in the control group compared with the other groups. This difference was only statistically significant for the eight-cell and morula stages. Similarly, there was a higher rate of blastocyst formation in the control group, but the difference was not significant (p > .05). Figure 3a–d shows the development of embryos derived MII oocytes from the two-cell to the blastocyst stage.
| Group | No. of oocytes | Two-cell stage | Four-cell stage | Eight-cell stage | Morula | Blastocysts |
|---|---|---|---|---|---|---|
| Test | 202 | 90.01 ± 1.84a | 84.80 ± 2.75a | 77.45 ± 3.25a | 73.0 ± 2.49a | 62.62 ± 3.24a |
| Sham | 197 | 93.20 ± 1.33a | 82.37 ± 2.91a | 70.91 ± 4.67a | 62.53 ± 5.89a | 58.12 ± 9.02a |
| Control | 189 | 96.29 ± 4.06a | 95.93 ± 3.39a | 93.87 ± 6.58b* | 89.58 ± 8.29b* | 71.31 ± 9.25a |
- Note: Non-similar letters in each row indicate significant differences. *p < .001.
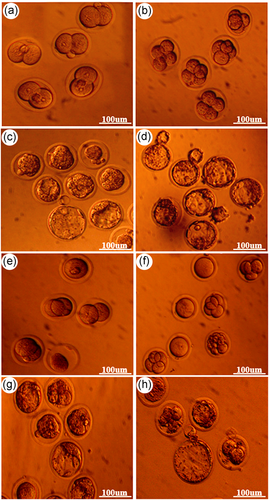
In contrast, in the GV-MII oocytes, the control group had a significantly lower fertilization rate (60.16 ± 4.37) compared to the test (79.96 ± 3.94) and sham (73.62 ± 6.92) groups. We observed no significant differences in cleavage rate and blastocyst formation among the studied groups. The blastocyst formation rates were 34.10 ± 2.67 (test), 31.10 ± 5.54 (sham), and 29.66 ± 3.30 (control; Table 3). Figure 3e–h shows the development of embryos derived from GV-MII oocytes from the two-cell to the blastocyst stage.
| Group | No. of oocytes | Two-cell stage | Four-cell stage | Eight-cell stage | Morula | Blastocysts |
|---|---|---|---|---|---|---|
| Test | 202 | 79.96 ± 3.94a | 60.15 ± 4.59a | 48.97 ± 3.99a | 42.05 ± 4.10a | 34.10 ± 2.67a |
| Sham | 150 | 73.62 ± 6.92a | 60.70 ± 6.91a | 51.90 ± 5.28a | 36.61 ± 5.92a | 31.10 ± 5.54a |
| Control | 115 | 60.16 ± 4.37b* | 49.49 ± 0.87a | 43.17 ± 4.15a | 38.90 ± 3.46a | 29.66 ± 3.20a |
- Note: Non-similar letters in each row indicate significant differences. *p < .05.
3.3 GFP detected in mouse embryos following vector transmission
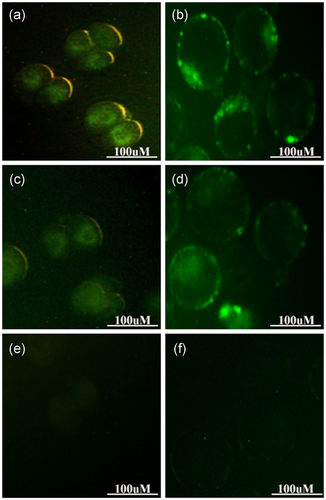
We examined GFP expression, as a reporter gene, at both the two-cell and blastocyst stages to verify the accuracy of the gene that was transferred into the embryos. The fluorescence images showed that most of the embryo-derived MII (Figure 4a,b) and GV-MII (Figure 4c,d) oocytes that had been injected with vector + /NaOH-treated spermatozoa had detectable GFP fluorescence, whereas no GFP was detected in the control group (Figure 4e,f).
3.4 Downregulation of Let-7a increases Tlr4 gene expression levels in the embryos
We quantitatively evaluated the gene expression level of Tlr4 and miR-Let-7a in the embryos. qRT-PCR analysis showed that expression levels of Tlr4 were upregulated by 4.5-fold in the two-cell stage and 7-fold in the blastocyst stage derived MII after transmission of the vector into the oocytes (Figure 5a,b). Likewise, the two-cell embryos and blastocysts derived GV-MII oocytes expressed TLR4 approximately 2.2-fold and 3.5-fold, respectively, more than the sham and control groups (Figure 5c,d). As shown in Figure 5a–d the embryos derived from both GV-MII and MII oocytes expressed similar levels of the Let-7a gene after transmission of anti-miR-Let-7a.
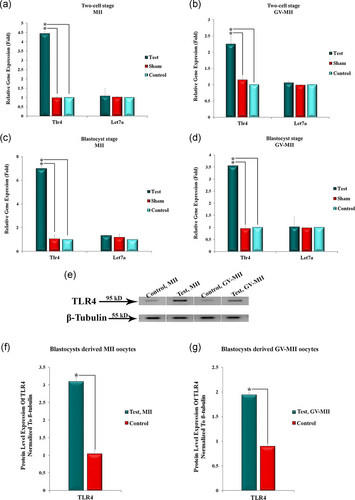
We performed western blot analysis of the TLR4 expression level in the blastocysts. Figure 5e–g shows the expression level of TLR4 at the protein level in embryo-derived GV-MII and MII oocytes after vector transmission. Interestingly, TLR4 expression levels dramatically increased in both groups that received vector compared to the control group (Figure 5e). Normalization of the relevant bands was performed with β-tubulin (Figure 5f,g).
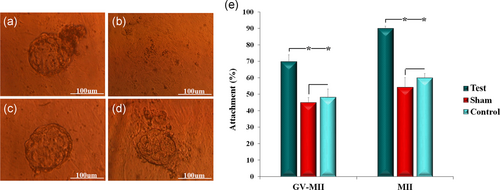
3.5 Increased TLR4 expression in blastocysts resulted in a higher attachment rate
We cultured the blastocysts on mouse endometrial cells in vitro to determine the effect of overexpressed TLR4 on embryo attachment. Blastocyst attachment and migration in these endometrial cells were examined at days 1 and 3 (Figure 6a). We observed an increase in attachment of blastocysts that received the vector onto the endometrial cell by approximately 20% and 30% compared to sham and control in GV-MII and MII oocyte groups, respectively (p < .05; Figure 6b).
4 DISCUSSION
Implantation is a complicated process that requires synchronized interaction between the hatched blastocysts and receptive endometrium. The Let-7 family is a regulator of gene expression during oogenesis and initial embryonic development (Tang et al., 2007). Let-7 expression is naturally reduced during preimplantation in the embryo (Viswanathan et al., 2009) and it cannot be identified in mouse and human embryonic stem cells (Melton, Judson, & Blelloch, 2010). Accordingly, overexpression of Let-7a in the blastocyst inhibits embryo implantation in mice (Liu et al., 2012). Hence, the current study was designed to determine the impact of TLR4, as one of the gene targets of Let-7a, in the process of embryo attachment.
This is the first evaluation of the expression level of TLR4 in mouse oocytes. Various studies have reported the expression of TLRs, members of innate immunity, by nonimmune cells in the female reproductive system. Follicles and granulosa cells of chicken ovaries express TLR2, 4, 5, 7 and TLR4, 5, respectively (Subedi, Isobe, Nishibori, & Yoshimura, 2007). The results of other studies have shown the presence of TLR4 in pig ovaries (B. Alvarez et al., 2006), TLR5 in human ovaries (Chaudhary et al., 1998), and TLR2, 4, 8, 9 in mouse granulosa cells (Shimada et al., 2006). Sasaki et al. reported TLR9 expression in mouse oocytes and trophoblast cells (Sasaki et al., 2018). Our qRT-PCR and ICC results confirmed that mouse oocytes expressed TLR4 at both the gene and protein levels. We observed that a higher level of TLR4 was expressed by GV-MII compared to MII oocytes, which might be attributed to IVM. Various factors in IVM, such as the type of culture medium and the number of free radicals, affect oocyte quality. IVM supposedly alters the transcription of genes involved in oocyte signaling pathways (Fair, Carter, Park, Evans, & Lonergan, 2007). Adona et al. have compared the expression levels of 1488 genes in matured oocytes derived from in vivo and IVM. They observed that 51 genes were downregulated, while 56 genes were upregulated in IVM-derived oocytes compared to the in vivo group (Adona et al., 2016). As recorded in TargetScan, TLR4 is a target gene of Let-7a. Upregulation of Let-7a leads to suppression of TLR4 and vice versa. According to our qRT-PCR results, there was significantly reduced expression of Let-7a in GV-MII oocytes compared to MII oocytes. As expected, a decrease in the amount of Let-7a expression caused upregulation of the TLR4 gene in the GV-MII oocytes.
We evaluated the developmental rate of the embryos after transmission of anti-miR-Let-7a to address the effect of Let-7a downregulation on preimplantation development. The results indicated that downregulation of Let-7a had no adverse effects on preimplantation development. Our results showed that the test, sham, and control groups had similar percentages of fertilization and blastocyst formation. Given the fact that Let-7a targets numerous genes involved in the cell cycle (www.mirz.unibas.ch.), downregulation of miR-Let-7a would perturb the developmental process of the embryo. Moreover, W. M. Liu et al. (2012) reported that Let-7a expression continuously decreased during embryonic development (oocyte to blastocyst stages) in humans and mice. Therefore, this finding suggested that other miRNAs that played a role in the cell cycle would mediate the embryo development process during the preimplantation phase. The GV-MII oocytes had a significantly lower fertilization rate in the control group compared to the test and sham groups. The elevated fertilization rate in the sham and test groups might be related to transfer of the eggs to the activation medium after the microinjection. On the other hand, an increase in fertilization rates in mature eggs in the laboratory by microinjection compared to IVF has been reported (Hwang, Lin, & Tsai, 2000; J. Liu, Rybouchkin, Van der Elst, & Dhont, 2002). This finding was not consistent with the result obtained in our study. The discrepancy in fertilization rate among the studies could be explained by the differences in oocyte maturity caused by different culture media, culture duration, the composition of the culture media, addition of growth factors, serum and hormones, and species differences (C. Alvarez, Garcia-Garrido, Taronger, & Gonzalez de Merlo, 2013; Chian & Tan, 2002; Roberts, Franks, & Hardy, 2002).
We sought to address the relationship between TLR4 and Let-7a in terms of embryo attachment. The use of anti-miR is a widespread approach in bioresearch to eliminate the function of miRNA. In this method, anti-miR with mRNA competes with the target for binding to miRNA, which results in inhibited miRNA activity and increased expression of its target genes. Therefore, qRT-PCR analysis showed that after vector transmission, the Tlr4 gene had significantly increased expression, whereas there was no considerable change observed in the Let-7a expression level in the embryos. Similarly, Western blot data showed an increase in TLR4 expression at the protein level in the blastocysts after the vector transmission. After transferring the blastocysts to endometrial cells, the binding rate and the migration of trophoblasts in the recipient group were significantly higher than the control group. Interestingly, the adhesion rate increased by more than 30% and 20% in the embryos obtained from the MII and the GV-MII oocytes. At the time of implantation, along with cellular changes (decidualization; Dunn, Kelly, & Critchley, 2003), a high level of pro-inflammatory cytokines (IL-6, IL-8, and TNF-α) are expressed both in the endometrium and blastocyst (Paria, Reese, Das, & Dey, 2002). The production of cytokines and creation of an inflammatory environment is required for directing the blastocyst to the site of the attachment and its interaction with the uterus (Granot et al., 2012; van Mourik et al., 2009), and to ensure appropriate reconstruction of the uterine epithelium and removal of cellular droplets (Koga & Mor, 2010). At the time of implantation, along with cellular changes (decidualization; Dunn et al., 2003), a high level of pro-inflammatory cytokines (IL-6, IL-8, and TNF-α) are expressed both in the endometrium and blastocyst (Paria et al., 2002). The activation of TLRs as an essential part of the intrinsic defense system results in rapid release of inflammatory cytokines and chemokines such as IL-6, IL-8, IFN-γ, and TNF-α (Tangerås et al., 2014). Studies have reported that there are 10 types of TLRs in primary first-trimester trophoblasts and the placenta (Abrahams et al., 2004; Mulla et al., 2013; Tangerås et al., 2014). Tangerås et al. (2014) reported that activation of TLR 1, 4, and 5 in the early trophoblasts increased the release of IL-6 and IL-8 from these trophoblasts. Koga and Mor (2010) observed that TLR4 activation in first-trimester trophoblasts produced a mild inflammatory response characterized by a brief increase in cytokines. TLR expression in trophoblasts (Chatterjee et al., 2012; Pineda, Verdin-Teran, Camacho, & Moreno-Fierros, 2011) shows that this receptor may play an important role in the formation of an inflammatory response during pregnancy. Therefore, the increased expression of TLR4 in our study could possibly have increased pro-inflammatory cytokine secretion levels in trophoblast cells, which would result in an enhanced blastocyst attachment rate.
Therefore, our results showed a higher attachment rate in the blastocysts that contained off-miR-Let-7a, which was attributed to the upregulation of the Let-7a-targeted genes involved in the implantation process. We conclude that Let-7a could mediate embryo attachment through regulation of TLR4 expression.
ACKNOWLEDGMENT
We would also like to thank the staff at the Cellular and Molecular Biology Research Center for their assistance with this study.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
Open Research
DATA AVAILABILITY STATEMENT
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.




