Tumor-associated macrophages promote prostate cancer progression via exosome-mediated miR-95 transfer
Han Guan, Rui Peng, and Fang Fang contributed equally to this work.
Abstract
Tumor-associated macrophages (TAMs) are vital constituents in mediating cell-to-cell communication within the tumor microenvironment. However, the molecular mechanisms underlying the interplay between TAMs and tumor cells that guide cell fate are largely undetermined. Extracellular vesicles, also known as exosomes, which are derived from TAMs, are the components exerting regulatory effects. Thus, understanding the underlying mechanism of “onco-vesicles” is of crucial importance for prostate cancer (PCa) therapy. In this study, we analyzed micro RNA sequences in exosomes released by THP-1 and M2 macrophages and found a significant increase in miR-95 levels in TAM-derived exosomes, demonstrating the direct uptake of miR-95 by recipient PCa cells. In vitro and in vivo loss-of-function assays suggested that miR-95 could function as a tumor promoter by directly binding to its downstream target gene, JunB, to promote PCa cell proliferation, invasion, and epithelial–mesenchymal transition. The clinical data analyses further revealed that higher miR-95 expression results in worse clinicopathological features. Collectively, our results demonstrated that TAM-mediated PCa progression is partially attributed to the aberrant expression of miR-95 in TAM-derived exosomes, and the miR-95/JunB axis provides the groundwork for research on TAMs to further develop more-personalized therapeutic approaches for patients with PCa.
1 INTRODUCTION
Prostate cancer (PCa) is the most frequently occurring cancer and the second leading cause of cancer-related deaths among men (Siegel, Miller, & Jemal, 2019). Although secondary androgen deprivation therapy is now the most commonly acknowledged therapeutic measure in PCa, most patients PCa with inevitably develop castration-resistant prostate cancer (CRPC) due to insensitivity to androgen deprivation therapy (Ryan et al., 2013). It is noteworthy that approximately 50–70% of patients with CRPC develop clinically detectable metastasis, the overwhelming cause of death in advanced PCa (Fallowfield, Payne, & Jenkins, 2016). Thus, exploring the molecular pathogenesis underlying tumor-promoting events is urgently needed for developing more promising targeted strategies to benefit patients with PCa.
The tumor microenvironment (TME) has gained increasing attention for its integral role in regulating tumor progression and metastasis (Quail & Joyce, 2013). As the most abundant immune cell population in the TME, macrophages, also known as tumor-associated macrophages (TAMs), have extensively shown their protumoral role in metastatic processes. Macrophages may display different phenotypes in response to various stimuli in local microenvironments. In general, when stimulated by proinflammatory factors, such as lipopolysaccharides, macrophages can differentiate into proinflammatory classical activated (M1) macrophages. However, based on immunosuppressive and metastasis-associated traits, the alternatively activated (M2) macrophages exert an opposite role that facilitates tumor progression (Noy & Pollard, 2014). Therefore, TAMs seem more likely to be classified into M2 macrophages, despite having a mixed phenotype expressing both M1 and M2 markers. Accumulating evidence suggests that TAM-released cytokines or proteins significantly contribute to tumor angiogenesis, invasion, metastasis, and drug resistance. More recent studies have focused on the TAM-released microvesicles, also known as exosomes, to unveil new insights into the potential carcinogenic mechanisms.
Exosomes are lipid bilayer membrane vesicles with diameters ranging from 20 to 200 nm. The cargos encapsulated in the vesicles, including nucleic acids (messenger RNA, micro RNA, and long noncoding RNA), proteins, and other bioactive substances, mediate cellular behaviors under physiological and pathological conditions (Becker et al., 2016). Secreted by cancerous or noncancerous cells, exosomes can be endocytosed by proximal or remote cells to induce a feed-forward signaling loop (Wortzel, Dror, Kenific, & Lyden, 2019). To date, one considerable concern is focused on micro RNA (miRNA) contained in exosomes, through which recipient tumor cells are triggered to accelerate malignant processes.
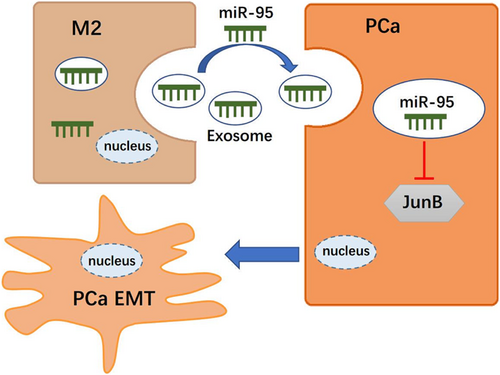
In the current study, we found that TAMs represent a high percentage of cells in PCa tissues and identified the differential miRNA profiles derived from THP-1/M2-TAM exosomes. Further in vitro and in vivo assays demonstrated that miR-95 was responsible for the enhanced ability of PCa cells in proliferation, invasion, and epithelial–mesenchymal transition (EMT). Mechanistically, exosome-shuttled miR-95 downregulates JunB expression, promoting the malignant behaviors of PCa.
2 MATERIALS AND METHODS
2.1 Patients and tissue samples
Two hundred and thirteen tissue samples were obtained from the First Affiliated Hospital of Bengbu Medical College from 2017 to 2019, including 115 PCa tissues from radical prostatectomy and 98 tissues from benign prostatic hyperplasia (BPH) patients who suffered from suprapubic prostatectomy or trans urethral resection prostate. All samples were immediately frozen at −80°C for further study. The samples were collected on the premise of obtaining informed consent from the included patients, and the Ethics Committee of the First Affiliated Hospital of Bengbu Medical College approved the procedures conducted in this study.
2.2 Cell culture and M2 polarized macrophage induction
All of the PCa cells were purchased from the American type culture collection. The culture medium for the cells was RPMI-1640 (Gibco, Thermo Fisher Scientific) with 10–15% fetal bovine serum and antibiotics (15140122; penicillin–streptomycin; Gibco). Cell culture was conducted in a cell incubator with conditions of 37°C, 5% CO2, and 95% saturated humidity. The human monocyte cell line THP-1 was purchased from Cell Cook (Guangzhou, China) and was cultured in RPMI1640 medium containing 10% fetal bovine serum (Gibco), 100 units/ml penicillin, and 100 µg/ml streptomycin (Gibco). To induce the transformation from THP-1 to M2 macrophages, THP-1 cells were added with 200 ng/ml phorbol myristic acetate (PMA, Spring & Autumn, Nanjing, China) for 24 hr and then cultured with 20 ng/ml IL-4 and 20 ng/ml IL-13 for a further 48 hr, which was based on the established studies (Y. Huang et al., 2019; Tedesco et al., 2018; Tjiu et al., 2009; Xu et al., 2018). The expression of TAM membrane marker CD206 was detected by flow cytometry, and the polarization efficiency was determined by the expression of TAM related cytokines (ARG-1, IL-10) by quantitative real-time polymerase chain reaction (qRT-PCR).
2.3 Isolation and analysis of exosomes
THP-1 cells and M2-TAMs were cultured in RPMI-1640 for 48 hr. Then, the conditioned medium (CM) was centrifuged twice (350g for 10 min and 2,000g for 30 min) to remove cells and cellular debris. We subsequently centrifuged supernatants at 10,000g for 30 min and 100,000g for 70 min. The centrifuged exosomes were resuspended in 50–100 μl phosphate buffered saline (PBS). Ultracentrifugation experiments were conducted with a Beckman Optima L-80XP (Beckman Coulter). Exosomes were observed by electron microscopy. Nanoparticle tracking analysis (Particle Metrix’ ZetaView, Germany) was performed to show that the concentration of exosomes was at about 1.0 × 109 to 1 × 1010 particles/ml. The presence of exosomes was further confirmed using western blot analysis. We labeled isolated exosomes with PKH67, a kind of green lipophilic fluorescent dye, and another kind of red lipophilic fluorescent dye Dil respectively. The PKH67- and Dil-labeled exosomes were incubated with PC3 and DU145 PCa cells for 24 hr. Then, fluorescence microscopy (Olympus FV1200, Japan) was used to view the direct internalization of PKH67- and Dil-labeled exosomes by PC3 and DU145 PCa cells.
2.4 Cell counting kit-8 assay
Cells were collected and equally plated in 96-well plates (1,000 cells/well) 48 hr after transfection. Cells were then incubated at 37°C in a humidified atmosphere with 5% CO2 and cultured for 24, 48, 72, and 96 hr. At each indicated time point, the PCa cells were incubated with 20 µl of cell counting kit 8 (CCK-8) solution (Beyotime Institute of Biotechnology, Shanghai, China) and further incubated at 37°C with 5% CO2 for 4 hr to evaluate cellular proliferative ability. The absorbance value was measured with a spectrophotometer at a wavelength of 495 nm. Each assay was performed in triplicate and repeated three times.
2.5 5-Ethynyl-2′-deoxyuridine assay
A 5-ethynyl-2′-deoxyuridine (EdU) assay was performed to determine cell proliferation ability. Cells were inoculated into a 24-well plate, and EdU medium (Roche, Mannheim, Germany [50 μmol/L]) was added and further incubated at 37°C with 5% CO2 for 2 hr. Fixation with 4% paraformaldehyde, incubation with glycine, Apollo staining, and 4′,6-diamidino-2-phenylindole staining was performed according to the manufacturers’ instructions. A fluorescence microscope was used to observe the representative images.
2.6 TdT-mediated dUTP nick end labeling assay
The transfected cells in each group were inoculated in a dish with a density of 4 × 104/cm2 for 24 hr. Then, the culture medium in the dish was discarded and washed with PBS three times. The cells were next fixed with 4% paraformaldehyde and permeabilized with 0.5% Triton X. DNA fragmentation was administered with TdT-mediated dUTP nick end labeling (TUNEL) according to the manufacturer's instructions (Roche Applied Science, Mannheim, Germany). Fluorescence was detected via an inverted fluorescence microscope (AMG, Bothell, WA), and the apoptosis rate was calculated.
2.7 Colony formation assay
The colony formation assay was performed as described previously (Guan et al., 2019). Briefly, cell proliferation was determined based on colony numbers via the colony formation assay. PC3 and DU145 cells were seeded in six-well plates at a density of 500 cells per well and incubated for 12 days at 37°C in 5% CO2. Next, the cells were washed with PBS three times, fixed with methanol for 15 min, and stained with 300 µl of 0.1% crystal violet for 20 min at room temperature. Colonies containing more than 50 cells were counted using ImageJ 2X software.
2.8 Transwell assays
For invasion assays, PCa cells were transiently transfected with miR-95 inhibitor for 48 hr, after which transfected-PCa cells were diluted to a final concentration of 5.0 × 106/ml. Postdiluted PCa cells in 100 μl serum-free medium were added to the upper chambers of 24-well Transwell units while the bottom chambers were filled with RPMI-1640 containing 10% fetal bovine serum. The top chamber was taken out after placing the chamber in the cell incubator for 24 hr at 37°C. PCa cells invaded through the bottom chambers were fixed with methanol, stained with 0.2% crystal violet, counted, and photographed microscopically (200×).
2.9 Apoptosis assays
An Annexin V-FITC/propidium iodide (PI) Apoptosis Detection Kit (Beyotime, China) was used to detect the cell apoptosis of the PC3 and DU145 cells. Cells in good condition and in logarithmic growth stage were selected first. The transfected PCa cells were detached using 0.25% trypsin without ethylenediaminetetraacetic acid and washed three times with PBS. Then, PCa cell lines were stained with 5 µl of VFITC and 10 µl PI at room temperature under dark conditions for 15 min. Using a flow cytometer (FACScan; BD Biosciences, Bedford, MA), the number of stained cells was examined and quantified. Data analysis was performed via Cell Quest Pro Software (BD Biosciences, CA).
2.10 Bioinformatics analysis
To search for the putative downstream target genes of miR-95, we utilized three online miRNA target prediction software miRNA target prediction tools including miRDB (http://www.mirdb.org/), TargetScan (http://www.targetscan.org/), mRNA array, and the GSE35988 data set from the Gene Expression Omnibus (GEO) database.
2.11 Oligonucleotide and plasmid transfection
Based on the miRBase database, cy3-miR-95, miR-95 inhibitor, and negative control of miRNA (miR-NC) were obtained from GenePharma (Shanghai, China). Short interfering RNA (siRNA) against JunB (si-JunB) and negative control siRNA with nonspecific sequences (si-NC) were all purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Plasmid lacking the 3′-UTR of JunB and the JunB NC plasmid were transfected using Lipofectamine 3000 (Thermo Fisher) in accordance with the manufacturer's instructions. The indirect coculture to visualize exosomal miR-95 uptake was performed according to Su, Aldawsari, and Amiji (2016). TAMs were transiently transfected with Cy3-tagged miR-95. In total, 1.2 × 106 Cy3-miR-95-transfected M2 macrophages were added to the bottom chamber for 48 hr. Then, 1.0 × 106 of PCa cells were added to the upper chambers. After 24 hr of coculture, cy3 fluorescence in prostate cancer cells was observed under a fluorescence microscope. GW4869 (Sigma, CA), an exosome inhibitor, was used to suppress exosome exocytosis at a concentration of 10 µM. For cell transfection, PC3 and DU145 cells were seeded in six-well plates, and Lipofectamine 3000 (Thermo Fisher Scientific) was utilized to accomplish the transfection process. The transfected cell lines were used in the western blot analysis assays and other biological in vitro and in vivo assays.
2.12 Stable transfection
The reverse complement sequence of miR-95 was synthesized and inserted into the AgeI/EcoR1 site of the GV209 vector (GeneChem, Shanghai, China) to construct a vector that inhibits miR-95 expression. The GFP vector was used for a control. The viruses were used to infect PC3 cells in the presence of polybrene.
2.13 In vivo tumorigenicity assay
Six-week old BALB/C nude mice were purchased from Shanghai SLAC Laboratory Animals. All animal experiments were approved by Institutional Animal Care and Use Committee and the ethics committee of the First Affiliated Hospital of Bengbu Medical College. Experiments were conducted according to the National Institute of Health Guide for the Care and Use of Laboratory Animals. PC3 cells transfected with lentivirus expression plasmid that inhibits miR-95 expression or negative control (scramble) were trypsinized and suspended in PBS. PC3 (4 × 106) cells transfected with miR-95 inhibitor (low-miR-95) or PC3 cells with miR-NC were subcutaneously injected into the flanks of nude mice for a 7-week implantation time. After 7 weeks, the nude mice were killed by cervical dislocation. The longest diameter (a) and shortest diameter (b) of the tumor were measured with a vernier caliper, and tumor volumes were calculated according to the formula: V (mm3) = b (mm2) × a (mm)/2. The excised tissues were partially used for western blot analysis, and the remaining tumor tissues were fixed with 10% formaldehyde and embedded with paraffin to conduct immunohistochemical analysis.
2.14 In vivo metastasis assay and bioluminescence imaging analysis of lung metastasis
PC3 cells stably overexpressing miR-95 vectors (GV209) or control vector constructed by GeneChem (Shanghai, China) were established by infection with lentivirus named LV-miR-95. The viruses were used to infect cells in the presence of Polybrene. After 48 hr, cells were cultured in medium containing puromycin for the selection of stable clones. PC3 (2 × 106) cells transfected with LV-miR-95 or LV-NC for 48 hr were washed and suspended in 0.1 ml PBS and subsequently injected into the lateral tail vein. The injected mice were anesthetized immediately using isoflurance and injected with 150 mg/kg of d-luciferin (15 mg/ml in PBS) intraperitoneally to demonstrate lung location of PCa cells. After a 4-week monitoring time, mice were anesthetized and injected with d-luciferin for 10 min as the first time. The nude mice were imaged with a highly sensitive camera in a light-tight specimen chamber (IVIS200, Xenogen). Living Image software (Xenogen) was conducted to acquire and quantify bioluminescence signals.
2.15 In situ hybridization and immunohistochemical staining
The double (5′–3′) digoxigenin (DIG)-labeled miR-95 probe and U6 probe was purchased from Boster (Wuhan, China), and ISH was conducted according to the manufacturer's protocol. The ISH results were determined by the combination of positive cell numbers and staining degree. The percentage of positive cells was calculated and the score was given. The expression of JunB and miR-95 in clinical cancer specimens and relative EMT markers in excised tumor tissues was detected by immunohistochemical staining (IHC). The specimen tissues were fixed with 4% paraformaldehyde solution, dehydrated, and embedded in paraffin. After brief proteolytic digestion and peroxidase blocking of the tissue slides, which were cut to a thickness of 4 µm previously, the slides were then incubated overnight at 4°C with E-cadherin, Vimentin, and JunB antibodies (Boster, China). ISH and IHC were scored in a semiquantitative manner. The scoring system was conducted as follows: 0 (<5%), 1 (5–30%), 2 (31–70%), and 3 (≥71%). Also, the staining intensity was classified according to the percentage of positive cells: <10% (+), 11–50% (++), and >50% (+++).
2.16 Luciferase reporter assay
The fragments of the JunB 3′-untranslated region (3′-UTR) containing either putative miR-95 seed sequence were synthesized by GeneChem (Shanghai, China). We subcloned wild type and mutant JunB 3′-UTR into the psiCHECK-2™ vector (Promega) to obtain reporters of psi-CHECK-JunB-WT and psi-CHECK-JunB-MUT. DU145 and PC3 cells were seeded in 24-well plates and cotransfected with either miR-95 mimics or NC and JunB-WT or JunB-MUT plasmid using Lipofectamine 3000 reagent (Invitrogen) at 37°C. The cells were transfected for 48 hr and analyzed by the Dual Luciferase Reporter Assay System (E1910). The luciferase activity was detected by the chemiluminescence method. Measurement was repeated three times to calculate the average value.
2.17 RNA extraction and quantitative real-time polymerase chain reaction
We extracted total RNA from exosomes and PCa cell lines with TRIzol (Invitrogen™, Thermo Fisher Scientific) following the manufacturer's protocol. The concentration and purity of RNA were determined by ultraviolet spectrophotometry. MiR-95 was detected by the TaqMan probe and the mRNA levels of ARG-1 and IL-10 were detected by the Green chimeric fluorescence method. Complementary DNA synthesis was conducted through total RNA and the PrimeScript RT reagent (Takara, Kusatsu, Japan). PCR results were analyzed by OpticonMonitor3 software (BioRad, Irvine, CA) and mRNA expression were measured by the method.
2.18 Statistical analysis
SPSS version 22.0 (SPSS, Inc., Armonk, NY) was used to perform statistical analyses. Student's t test and two-way analysis of variance analysis were used to compare the significance of two groups. The RT-qPCR and western blot data were analyzed by Wilcoxon signed-rank tests. The Fisher's exact test was used for any 2 × 2 tables. Spearman correlations were calculated for the expression levels between miR-95 and JunB. All experiments above were repeated three times, and differences among groups in in vitro or in vivo studies were assessed by two-tailed Student's t-test. Data are presented as means and standard deviation. A p < .05 was considered statistically significant.
3 RESULTS
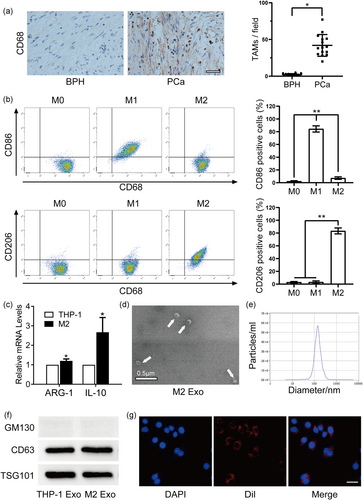
3.1 TAMs enriched in PCa tissues and the characteristics of exosomes derived from TAMs
To measure the infiltration of TAMs in PCa tissues, we performed IHC to detect the expression of the TAM marker CD68 in PCa tissues and BPH tissues. An extensively higher infiltration of TAMs was detected in PCa tissues than in normal tissues, as verified by a higher number of CD68-positive cells (Figure 1a). Thus, we hypothesized that TAMs contribute to PCa progression. To reach the M2-TAMs, M2 polarized macrophages were induced according to the aforementioned methods. Then, the flow cytometry analysis revealed a higher percentage of CD206+ cells (Figure 1b). As shown by qRT-PCR, M2-TAM-related cytokines, including ARG-1 and IL-10, were prominently elevated following continuous stimulation by PMA and IL-4 plus IL-13 (Figure 1c). To explore the role of TAM-secreted exosomes, which may mediate the exchange of small RNAs between TAMs and PCa cells, we extracted exosomes from CM by gradient centrifugation. Transmission electron microscopy revealed a representative double-layer membrane structure of exosomes, with about 100 nm in diameter (Figure 1d,e). Western blot analysis also showed the enrichment of CD63 and TSG101, whereas the absence of GM130 in the exosomal fraction suggested a diminutive amount of contamination and a higher level of purity of the isolated exosomes, which paved the way for further study (Figure 1f). To visualize the direct uptake of exosomes by recipient PCa cells, we incubated PCa cells with prepurified M2 exosomes labeled with DiI, a lipophilic fluorescent dye. Then, fluorescence microscopy was used to view the direct internalization of DiI-labeled exosomes by PCa cells (Figure 1g). To further verify the results, M2 exosomes were also labeled with PKH67, another kind of lipophilic fluorescent dye, were incubated with PC3 and DU145 respectively. Then, green fluorescence was viewed on the membrane of PC3 and DU145 cell lines, demonstrating a direct internalization of PKH67-labeled exosomes by PCa cells (Figure S1B).
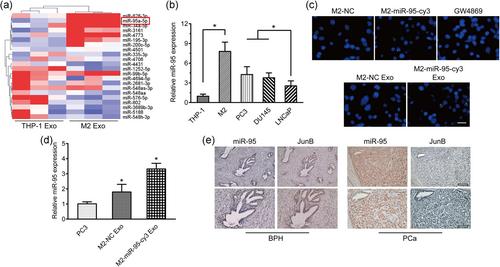
3.2 Direct transfer of TAM-derived exosomal miR-95 to PCa cells
The horizontal transfer of miRNAs released by exosomes has been fully demonstrated to participate in cancer evolution. Thus, we selected and profiled exosomal miRNAs between monocytes (c) and M2 macrophages (Figure 2a) and we found that 63 miRNAs were upregulated and 32 miRNAs were downregulated with 2 fold-change, among which miR-95 in upregulated gene lists was selected for further exploration (Figure 2a). qRT-PCR was also conducted to confirm that in contrast to THP-1 with lower miR-95 expression, TAMs and PCa cell lines presented a higher level of miR-95, which was consistent with the sequencing results (Figure 2b). The expression of miR-95 in TAMs- and THP-1-derived exosomes also showed a statistic difference (Figure S1C). To visualize the direct shuttling of miR-95 from TAM exosomes to recipient PCa cells, we cocultured TAMs transiently transfected with Cy3-tagged miR-95 with PC3 and DU145 cells to observe green fluorescence of marked miR-95 in PCa cells. As anticipated, we found that Cy3 fluorescence was observed in >90% of the PC3 and DU145 cells. We subsequently treated TAMs with GW4869, an exosome inhibitor, and the green fluorescence of Cy3 was abolished in the PC3 and DU145 cells. Likewise, green fluorescent signals were also detected in PC3 and DU145 cells incubated with the exosomes isolated from miR-95-transfected TAMs (Figure 2c; Figure S1A). We also observed an increase in miR-95 expression in the recipient PC3 cells after treatment with exosomes that were derived from Cy3-labeled miR-95-transfected TAMs (Figure 2d). To confirm that miR-95 increment in PC3 cells was ascribed to exosomes incubation rather than the transformation of pri-miR-95 within PC3 cells, we transiently transfected Cy3-tagged miR-95 or miR-NC into PC3 cells. We next detected the expression of miR-95, or pri-miR-95, in the recipient PC3 cells treated with exosomes from Cy3-labeled miR-95-transfected M2 cells, miR-NC-transfected M2 cells, and miR-95-transfected M2 plus GW4869, respectively. The results demonstrated that miR-95 production was significantly elevated in PC3 cells cocultured with exosomes derived from TAMs that transiently transfected with miR-95, whereas pri-miR-95 production was almost unchanged under exogenous TAMs exosome interference (Figure S1D, E). Meanwhile, to demonstrate the protective effect of exosomes on miR-95. We treated TAMs exosomes/miR-95 with RNase in the presence or absence of the vesicle-disrupting detergent Triton X-100. qRT-PCR indicated that RNase treatment did not dramatically alter miR-95 in cocultured PC3 cells whereas the combination of RNase and Triton X-100 dramatically reduced the detection of miR-95 abundance in PC3 cells cocultured with exosomes/miR-95. These results suggest that miR-95 is protected from degradation by encapsulation within TAMs-derived exosomes (Figure S1F). To validate the microarray results, 10 pairs of BPH tissues and PCa tissues with a miR-95 probe were subjected to the in situ hybridization (ISH) staining with a miR-95 probe. Consistently, significantly higher miR-95 expression was detected in the PCa specimens than that in the BPH specimens (Figure 2e). We next assessed the correlation between miR-95 expression and the clinicopathological features of PCa patients (it was deemed as high expression as the expression of miR-95 level was more than 2/3 of the highest value in primary PCa patients). As shown in Table 1, the production of miR-95 was positively associated with Gleason score and metastasis
| miR-95 low | miR-95 high | |||
|---|---|---|---|---|
| N | (n = 90) | (n = 110) | p -Value | |
| Mean ages (years) | 115 | 65 ± 7.02 | 68 ± 10.57 | <.05 |
| PSA (ng/ml) | ||||
| <10 | 18 | 7(38.9) | 11(61.1) | .742 |
| ≥10 | 97 | 45(46.4) | 52(53.6) | |
| Gleason score | ||||
| <8 | 78 | 38(48.7) | 40(51.3) | .045 |
| ≥8 | 37 | 10(27.0) | 27(73.0) | |
| Clinical stage | ||||
| <T2A | 67 | 40(59.7) | 27(40.3) | .133 |
| ≥T2A | 48 | 21(43.8) | 27(56.3) | |
| Pathological stage | ||||
| T2A-T2C | 71 | 37(52.1) | 34(47.9) | .791 |
| T3A-T4 | 44 | 21(47.7) | 23(52.3) | |
| Metastasis | ||||
| Negative | 94 | 59(62.8) | 35(37.2) | .026 |
| Positive | 21 | 7(33.3) | 14(66.7) |
- Abbreviation: PSA, prostate-specific antigen.
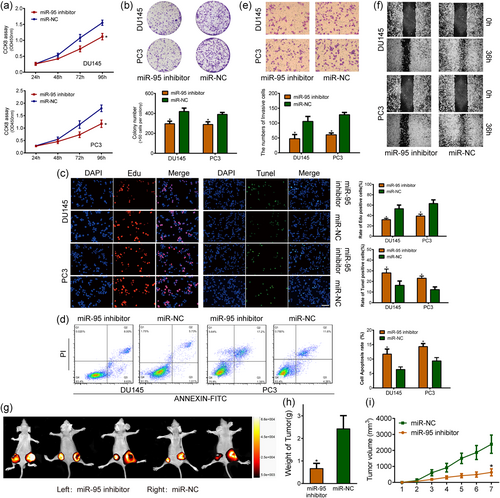
3.3 miR-95 promotes PCa cell proliferation and invasion and inhibits apoptosis in vitro and in vivo
Growing evidence has proved that TAMs exosomes are capable of facilitating the progression of malignancies, such as colon cancer (Lan et al., 2019) and breast cancer (Yang et al., 2011). In this regard, we preliminarily hypothesized that TAMs-isolated exosomes have the likelihood of promoting PCa progression, which was evidenced by in vitro CCK-8 and invasion assays, with the result showing that the capacity in proliferation and invasion of PCa cells were markedly increased when incubated with TAMs-isolated exosomes (Figure S4A, B). Based on the preliminary work, we, therefore, choose the “hot-button”, microRNA, in exosomes for further exploration. To determine the effects of miR-95 on PCa biological behaviors, we treated PC3 and DU145 cells with miR-95 inhibitor or miR-95 mimics. The silencing of miR-95 suppressed PCa cell growth compared with that of miR-NC-transfected cells, as shown by the CCK-8 assay, colony formation assay, and EdU assay (Figure 3a-c). Flow cytometry analysis also showed that the miR-95 inhibitor markedly increased the number of apoptotic cells (Figure 3d). To evaluate the invasion ability of miR-95, we performed invasion and scratch assays and found that the depletion of miR-95 was unable to increase the motility of PCa cells (Figure 3e,f). In contrast, overexpression of miR-95 promoted cell proliferation ability in comparison to miR-NC and showed a lower percentage of apoptosis than the control group (Figure S2A-F). Then, an in vivo subcutaneous xenograft tumor model was established to confirm whether miR-95 contributed to tumorigenesis and EMT phenotypes. We stably transfected a lentivirus expression plasmid that encodes a miR-95 inhibitor, or miR-95 mimics, into PC3 cells. Next, we subcutaneously coinjected PC3/miR-95 inhibitor and PC3/miR-NC, or PC3/miR-95 mimics and PC3/miR-NC into nude mice. After a 7-week monitoring period, significantly larger tumor sizes and weights were observed in the mice bearing PC3/miR-NC tumors than in the control animals (Figure 3e,f). Similarly, the upregulation of miR-95 significantly facilitated tumor growth as verified by comparatively smaller tumor sizes and weights (Figure S3A). The results indicated that the protumorigenic capacity may be partially ascribed to aberrant miR-95 expression. We then measured the effect of miR-95 on tumor metastasis in vivo by injecting PC3 cells transfected with miR-95 inhibitor or NC into the tail veins of nude mice. Bioluminescence imaging demonstrated that silencing miR-95 suppressed metastasis of PC3 cells to the lungs, potently suggesting a metastasis-promoting potential of miR-95 (Figure S3B).
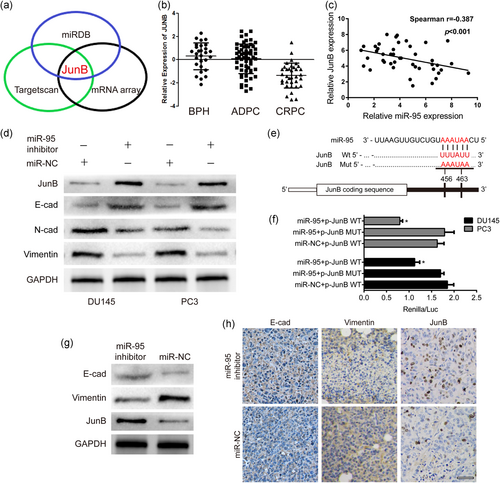
3.4 JunB is a direct target of miR-95, and the inhibition of miR-95 facilitates EMT in PCa
To elucidate the mechanism by which miR-95 promotes cell proliferation and invasion, we used miRNA target prediction tools (miRDB, TargetScan, and mRNA array) and the GSE35988 data set from the GEO database. We found that JunB was a potential target gene, and the abundance of JunB was higher in patients with BPH (Figure 4a,b). We next conducted qRT-PCR of 44 PCa cancer specimens and found that JunB expression levels were inversely correlated with miR-95 in the 44 PCa tissues (r = −.387), with a p< .001 (Figure 4c). Similarly, IHC results showed that greater JunB staining was accompanied by weaker miR-95 expression in PCa samples (Figure 2e). Moreover, western blot analysis revealed that silencing miR-95 enhanced the enrichment of JunB (Figure 4d). To identify whether miR-95 could affect EMT, we evaluated the recognized EMT markers, including E-cadherin and Vimentin. Consistent with the results of the Transwell assay, the depletion of miR-95 resulted in an increased expression of E-cadherin and a decreased expression of the mesenchymal markers N-cadherin and Vimentin (Figure 4d). The luciferase reporter assay further confirmed our hypothesis that JunB is a direct target gene of miR-95, with the results showing that miR-95 could significantly reduce WT-JunB (harboring the wild-type miR-95 binding site in the JunB 3′-UTR downstream of the firefly luciferase gene) luciferase activity in PCa cells, whereas the suppressive effect was completely abolished in MUT-JunB (containing a mutated miR-95 binding site in the JunB 3′-UTR) group (Figure 4e,f). Additionally, we compared the expression of E-cadherin, Vimentin, and JunB in the xenograft tissues of PC3 cell lines by western blot analysis and IHC staining analyses. Xenograft tumors without miR-95 induced an increase in E-cadherin but a decrease in Vimentin and JunB at the protein level, which was consistent with the in vitro results (Figure 4g,h).
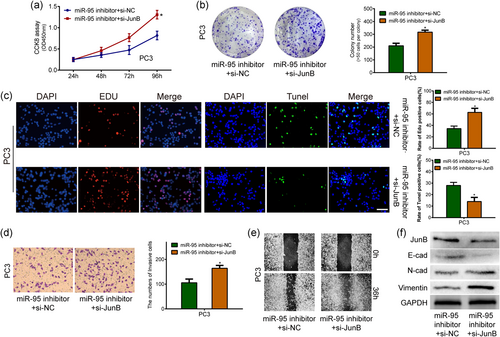
3.5 JunB can reverse miR-95-mediated biological effects in PCa cells
The above findings raised a question about whether JunB was responsible for the miR-95-mediated oncogenic effects. To confirm our hypothesis, PC3 cells with anti-miR-95 were further cotransfected with siJunB. Evaluation by CCK-8, colony formation, EdU, and TUNEL assays showed that the knockdown of JunB partially attenuated the miR-95-induced suppression of PCa cell proliferation (Figure 5a-c). Similarly, the invasion and scratch assays showed the antagonistic effects of siJunB against the miR-95 inhibitor (Figure 5d,e). Western blot assays also demonstrated that the knockdown of JunB significantly antagonized the enrichment of JunB and E-cadherin and alleviated the downregulation of Vimentin and N-cadherin caused by the suppression of miR-95 in PC3 cells (Figure 5f). Therefore, our data strongly suggested that JunB was functionally involved in miR-95-mediated biological behaviors in PCa cells.
4 DISCUSSION
Using experimental and clinical studies, researchers have been exploring the effects of TAMs on tumor cell growth and cancer metastasis within the TME. TAM-derived chemokines and cytokines, such as CCL-5, CCL-17, and TGF-β, significantly influence various aspects of cancer progression, including immune escape, tumor dissemination, distant engraftment, and treatment resistance (Pathria, Louis, & Varner, 2019). Furthermore, studies have strongly demonstrated that the infiltrating macrophage population is positively correlated with the biological behaviors of cancer cells and closely related to poor patient prognosis (Feng et al., 2019). In this study, we found that the density of TAMs was higher in PCa tissues than in BPH patient tissues, indicating a contributing role of TAMs to PCa.
Although most studies have focused on cytokines, molecules, or other proteins secreted by macrophages, greater emphasis is needed on miRNAs considering their regulatory effects on cancer cell progression. miRNAs are noncoding, single-stranded RNA molecules that bind to the 3′-UTR of target genes to posttranscriptionally regulate mRNA expression. The bidirectional infiltration of miRNAs between stromal and cancerous cells is believed to be a key factor in cancer progression. However, previous studies have performed miRNA profiling limited to merely cells or tissues, while other microvesicles, especially exosomes, were neglected, which may also mediate the cell-to-cell interaction via shuttling functional miRNAs. For example, exosome-mediated shuttling of apolipoprotein E (ApoE) is involved in the PI3K-Akt signaling pathway activation, which promotes the migration of gastric cancer cells (Wan et al., 2018). Additionally, TGF-β secreted by bladder cancer cells induces the phenotypic transformation of fibroblasts into cancer-associated fibroblasts (Ringuette et al., 2018). For TAMs, Lan et al. (2019) found that miR-21 and miR-155 contained in TAM-derived exosomes promote colon cancer cell migration and invasion by targeting BRG1 expression. Another study reported that Treg/Th17 cell imbalance in epithelial ovarian cancer is mainly ascribed to the aberrant miRNA levels originating from TAM exosomes (Zhou et al., 2018). Therefore, we speculated that miRNAs released by TAM exosomes influence the malignant behaviors of PCa cells. To validate our hypothesis, we conducted a microarray analysis to screen out miRNAs in exosomes from THP-1 and M2 macrophages.
Based on the miRNA sequence results, miR-95 was conspicuous in the upregulated miRNAs. Of note, miR-95 is involved in tumorigenic processes in human colorectal and pancreatic carcinomas and shows a positive correlation with EMT in glioblastoma (Zhang et al., 2018). More important, miR-95 is significantly elevated in patient with PCa specimens and is positively associated with the risk of biochemical-free relapse survival, which makes it worthwhile for further research (X. Huang et al., 2013; Stuopelyte, Daniunaite, Jankevicius, & Jarmalaite, 2016). The subsequent qRT-PCR results also echoed the findings from the aforementioned microarray analysis. Next, to validate that exosomal miR-95 could be directly absorbed by the recipient PCa cells, we utilized confocal microscopy and detected the red fluorescent signals of Cy3-tagged miR-95 in PCa cells, while no signals were detected when the exosome inhibitors were added.
Then, we incubated PCa cells with the exosomes of TAMs transfected with the miR-95 inhibitor. After performing loss-of-function assays, we discovered that the depletion of miR-95 markedly decreased the proliferation and invasion abilities of PCa cells and suppressed apoptosis in vitro. Additionally, the in vivo xenograft tumor model exhibited the ability of miR-95 in tumorigenicity and in maintaining mesenchymal characteristics, suggesting that the exosomes derived from TAMs primed PCa cell proliferation and EMT transition by shuttling miR-95 into PCa cells. Therefore, we surmised that miR-95 behaved as an oncogenic factor in the progression of PCa. To further determine the potential target that may be responsible for the protumorigenic effects of miR-95, we performed bioinformatics analysis and predicted JunB as a downstream target gene of miR-95, as verified by a luciferase reporter assay and western blot analysis.
JunB is a member of the activator protein-1 (AP-1) transcription factor family composed of Fos (c-Fos, FosB, Fra-1, and Fra-2) and Jun (c-Jun, JunB, and JunD). These proteins constitute the AP-1 complex with different combinations of heterodimers and homodimers and participate in various processes, including cell proliferation, invasion, and metastasis (Eferl & Wagner, 2003). The AP-1 family facilitates the EMT process in multiple malignancies, which is strongly exemplified by the fact that c-Jun in synergy with FosL1 promotes the invasive ability of triple-negative breast cancer cells (Qiao et al., 2015). Likewise, JunB is believed to be oncogenic. Fan et al. (2017) demonstrated a critical role of JunB in multiple myeloma cell proliferation, survival, and drug resistance within the bone marrow environment. Recently, JunB, as an EMT-promoting factor, has been suggested to participate in uveal melanoma (UM) aggressiveness. It is also required for cell invasion and metastasis in head and neck squamous cell carcinoma (HNSCC; Gong et al., 2018; Hyakusoku et al., 2016). Nevertheless, JunB as a pro-oncogenic factor has been increasingly challenged. In PCa, Konishi et al. delineated that JunB is inversely correlated with the pathologic grade in PCa and cooperates with p16/pRb to induce cell senescence (Konishi et al., 2008). Another finding strengthened the suppressing role of JunB, which illustrates the lower expression of JunB in high-grade PCa and demonstrates that the combined knockdown of PTEN and JunB in prostate epithelial cells leads to invasive transformation by downregulating the cell cycle inhibitors p16Ink4a and p21CIP1(Thomsen et al., 2015). Consistently, we found that miR-95 exerted its promoting role of PCa via inhibiting JunB expression. Further rescue assays were conducted, and the knockdown of JunB by siJunB transfection moderately attenuated the promotion of cell proliferation and invasion and the epithelial characteristics induced by the downregulation of miR-95, suggesting that JunB participates in the process of miR-95-mediated EMT activation. EMT is a “hallmark” required for increased cell migration and invasiveness, and the AP-1 family has been increasingly embodied in contributing to the EMT process in malignancies. In this regard, AP-1 acts synergistically with other oncogenes, such as ZEB2 and KDM4A, to induce tumor invasion in breast cancer and HNSCC (Hyakusoku et al., 2016). In contrast, very little is known of the contribution of JunB to EMT. In our study, we corroborated that JunB is inversely related to EMT in PCa. However, further studies are needed to elucidate the details of the mechanisms underlying the JunB-mediated suppression of tumor invasion in PCa (Figure 6).
There are still limitations in our current study that need to be taken into account. Although we confirmed a prominent difference in miR-95 expression in THP-1/M2-exosomes, we did not analyze cellular miRNA profiles. However, the in vivo lung metastasis experiments could also be utilized to determine whether miR-95 could influence PCa cell metastasis since EMT is a key step in tumor metastasis. Additionally, our data do not clearly illuminate the EMT pathway, which may also function through other distinct target genes and multiple downstream pathways. The identification and verification of additional targets, as well as their effects, are therefore imperative to provide a rationale for therapies.
In summary, our study provides the first evidence demonstrating that TAMs can transfer functional miR-95 via exosomes to PCa cells and that miR-95 promotes PCa cell proliferation, invasion, and EMT pathway activation by modulating JunB. Therefore, the miR-95/JunB axis may be a potential therapeutic target for preventing PCa progression and metastasis.
ACKNOWLEDGMENTS
The authors thank Wiley Editing Services (www.wileyauthors.com/eeo/preparation) for its linguistic assistance during the preparation of this manuscript. This study was supported by grants from National Science Foundation of China [81801573], Science Foundation of Anhui Province [KJ2018A0214, KJ2019A0355], Science Foundation of Bengbu Medical College [BYKY2019014ZD], The First Affiliated Hospital of Bengbu Medical College Science Fund for Outstanding Young Scholars[2019BYYFYYQ09], First Affiliated Hospital of Bengbu Medical College Science Foundation [BYYFYKJ201805], Municipal Social Development Science and Technology Demonstration Project [N20192002], Jiangsu Province Science and Education Program [CXTDA2017047], and Jiangsu Provincial Key R&D Program [BE2018629].
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Designing of the study and critical revisions: H. G., R. P., and N. H. F.; laboratory measurements: F. F., L. K. M., and B. X.; data collection and analysis: H. G., S. Y., C. Y. D., and B. X.; manuscript drafting: H. G., R. R., and F. F.; conceptualization and data curation: H. G., R. P., and F. F.; formal analysis: H. G., R. P., and N. H. F.; funding acquisition: N. H. F., B. X., and M. C.; Investigation: Z. J. C., S. Y., and C. Y. D.; methodology: R. P., S. Y., and C. Y. W.; software: F. F., H. L. W., and B. X.; resources: F. F. and N. H. F.; supervision: B. X. and F. F.; validation: Z. J.C., and B. X.; writing–original draft: H. G. and R. P.; writing–review and editing: H. G., N. H. F., B. X., and M. C.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




