Immune checkpoints in hematologic malignancies: What made the immune cells and clinicians exhausted!
Abstract
Hematologic malignancies comprise a considerable part of cancers with high mortality at any age. Since the introduction of hematopoietic stem cell transplantation (HSCT), the overall survival of patients dramatically increased. The main goal of HSCT is the induction of a graft-versus-leukemia effect to eradicate the residual cancer cells and also reconstitute a healthy immune system for patients. However, relapse is a nettlesome challenge of HSCT. Like many other tumors, hematologic cancer cells induce immune exhaustion leading to immune escape and relapses after HSCT. Besides malignant cells, inhibitory cells such as tumor-associated macrophages and myeloid-derived suppressor cells express various inhibitory receptors capable of inducing exhaustion in immune cells, especially T and natural killer cells. The significance of immune checkpoint blocking in tumor regression in clinical trials led to the 2018 Nobel Prize in Physiology/Medicine. Here, we reviewed the clinical roles of immune checkpoints in hematologic malignancies and post-HSCT relapses.
1 INTRODUCTION
The immune system has a variety of self-limiting mechanisms, called immunological homeostasis, to prevent overreaction and autoimmunity. Following the immune response, inhibitory receptors, known as immune checkpoints, appear on the surface of immune cells and bind to their ligands on other cells (Figure 1). The cytoplasmic tails of these receptors mainly contain inhibitory motifs, such as immunoreceptor tyrosine-based inhibitory motif (ITIM) and immunoreceptor tyrosine-based switch motif, which induce inhibitory signals in the immune cell. In case of chronic inflammation and continuous antigenic stimulation, the upregulation of inhibitory checkpoints caused a collapse and exhaustion in immune responses which promotes the inflammatory conditions (Gholami et al., 2017; Wherry, 2011; Wherry & Kurachi, 2015)
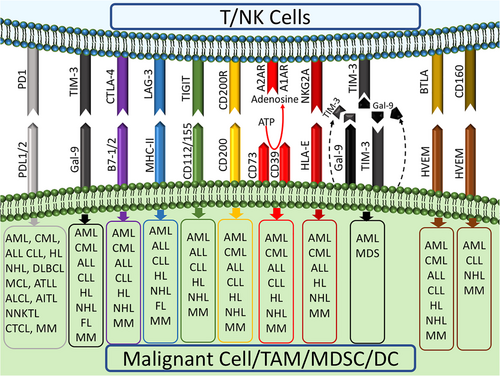
T-cell exhaustion is a state of decline in T-cell proliferation and function (secretion of cytokines and cytotoxicity) defined by the expression of immune checkpoints including programmed cell death protein-1 (PD1), cytotoxic T-lymphocyte-associated protein-4 (CTLA4), T-cell immunoglobulin (Ig) and mucin-domain containing-3 (TIM3), lymphocyte-activation gene-3 (LAG3), T-cell immunoreceptor with Ig and ITIM domains (TIGIT), CD160 (BY55), and V-domain Ig suppressor of T-cell activation (Blackburn et al., 2009; Fourcade et al., 2012; Jin et al., 2010; Joller et al., 2011; Wherry & Kurachi, 2015). This phenomenon was first seen in chronic viral infections such as chronic lymphocytic choriomeningitis virus in mice, hepatitis C virus, and human immunodeficiency virus, then it has been observed that many cancer cells also use this approach to escape from antitumor immune responses (Bakhshaei et al., 2018; Schietinger & Greenberg, 2014; Wherry & Kurachi, 2015; Zajac et al., 1998). Tumor-induced T-cell exhaustion has been studied mainly in solid tumors, but recently was reported in a variety of hematologic malignancies, including chronic myeloid leukemia (CML), acute myeloid leukemia (AML), chronic lymphocytic leukemia (CLL), and lymphomas which are associated with disease progression, resistance to treatment, and relapse after transplantation (Baitsch et al., 2011; Liu et al., 2018; Michonneau et al., 2016; Ozkazanc, Yoyen-Ermis, Tavukcuoglu, Buyukasik, & Esendagli, 2016; Riches et al., 2013; Silva et al., 2017; Wherry, 2011; Yang et al., 2012). One of the most promising ways to treat hematologic malignancies is hematopoietic stem cell transplantation (HSCT), showing considerable success (Ghasemi et al., 2020). However, the disease relapse after transplantation is still a substantial problem in these diseases (Kong et al., 2015). The ultimate goal of HSCT is the induction of graft-versus-leukemia (GVL) to kill cancer cells; hence, a relapse could be indicative of GVL failure. The leading players in GVL are cytotoxic T lymphocytes (CTLs) and natural killer (NK) cells their exhaustion is associated with GVL failure and relapse (Bachireddy et al., 2014). Interestingly, one of the causes of GVL failure or low probability of graft-versus-host disease (GVHD) in some organs rather than the others is the preferential expression of inhibitory ligands in those organs (Michonneau et al., 2016). Formerly, the discrepancy of GVL and GVHD incidence in different organs was attributed to the preferential migration of CTLs to target organs (Sackstein, 2006). Nowadays, the main cause of this distinction is the different expression and involvement of various inhibitory receptors such as PD1 and their ligands in different tissues. This anatomical segregation of inhibitory receptors expression leads to heterogeneity in cell function, differences in the effects of GVL, and provides niches for tumor escape (Michonneau et al., 2016). The use of immune checkpoint blockers will be able to eliminate this anatomical segmentation in immune responses, restore CTLs from exhaustion, and decrease relapse induced by tumor-escaping niches (Michonneau et al., 2016). When leukemic cells are exposed to an inflammatory and antitumor environment, such as interferon-γ (IFN-γ), they rapidly reduce their costimulatory proteins such as CD86 and inducible costimulatory ligand (ICOSL) and increase the inhibitory molecules, especially PD1 ligands (PDL1 and PDL2). On the other hand, continuous stimulation of T cells with costimulatory receptors leads to the expression of inhibitory receptors on the surface of T cells, which decrease the activity of these cells (Ozkazanc et al., 2016; Sackstein, 2006).
More recently, NK cells also play an essential role in the induction of GVL, especially in the early post-transplant stages. NK cells are much safer in allogeneic HSCT and less likely to induce GVHD than T cells (Woan & Miller, 2019). NK cell responses are based on a balance between inhibitory and activatory signals received by their attributed receptors (Carlsten & Järås, 2019). The most prominent inhibitory receptors of NK cells are inhibitory killer-Ig like receptor (iKIR) and NK Group 2 member A (NKG2A), while the well-known activatory receptors are natural cytotoxicity receptors (NCRs), DNAX accessory molecule-1 (DNAM-1), NKG2D, NKp30, and NKp46 (Damele, Ottonello, Mingari, Pietra, & Vitale, 2020). Although NK cells can prevent relapse after HSCT, not all patients are well-responsive to this treatment (Carlsten & Järås, 2019). The importance of NK cell antitumor activity in blood cancers stems from the growing evidence showing that NK cells are often suppressed in leukemia and lymphoma (A. Badros et al., 2017; Damele et al., 2020; Zinzani et al., 2018). Besides the inhibitory receptor of NK cells, expression of inhibitory checkpoints such as PD1 and TIM3 on NK cell surface has been reported in several hematological and nonhematological malignancies (André et al., 2018; Benson et al., 2010; Pesce et al., 2017). The involvement of inhibitory molecules and checkpoints with their ligands on malignant cells results in an exhausted-like phenotype in NK cells with reduced antitumor activity such as degranulation and cytokine secretion (Beldi-Ferchiou et al., 2016; Guo et al., 2016; Ndhlovu et al., 2012; Pesce et al., 2017).
The exhaustion has been reported even in chimeric antigen receptor (CAR)-T-cell therapy and it has been observed that overexpression of inhibitory ligands in the tumor microenvironment, as well as the exhaustion of patients’ T cells, prevent proper CAR-T-cell activity (Cherkassky et al., 2016; Guha et al., 2017; Yao et al., 2017; Ye et al., 2018). Moreover, some CAR-T-cell signals, such as antigen-independent phosphorylation of the CD3 and zeta chains, can induce exhaustion in CAR-T cells (Gomes-Silva et al., 2017; Long et al., 2015). The most important immune checkpoints reported to have roles in T/NK cell exhaustion are shown in Figure 1. We elaborated on the role of T and NK cell exhaustion in disease progression and relapse of hematologic malignancies after transplantation.
2 MYELOID LEUKEMIA
2.1 Acute myeloid leukemia
Relapse of AML after allogeneic-HSCT results in the death of patients within 4 months after relapse and only 5% has a 5-year survival (Frassoni et al., 1988; Mielcarek et al., 2007). Immature CD34+ leukemia precursors (not CD34+ hematopoietic stem cells) express high levels of inhibitory ligands, including PDL1, which bind to specific alloreactive T lymphocytes and inhibit their activity (Norde et al., 2011). PD1+ and TIM3+ exhausted T cells present in the peripheral blood of relapsing patients with AML have defective production of inflammatory cytokines, which is associated with relapse after transplantation (Kong et al., 2015). To evaluate the effect of the tumor microenvironment on T-cell exhaustion, Noviello et al. investigated the expression of inhibitory receptors in bone marrow-infiltrated tumor-specific T cells in 32 patients with AML who had a relapse within 1 year after allogeneic HSCT or were in complete remission (CR). They reported that the expression of inhibitory receptors on the surface of bone marrow-infiltrated T cells in relapsed patients was significantly higher than those in patients with CR, and these exhausted T cells had leukemia-specific T-cell receptors but failed to respond functionally to leukemic cells (Noviello et al., 2019). The strength of this study, in addition to studying the underlying microenvironment of AML, was considering a 1-year period after transplantation for selection of relapsing and remitting patients. This time period seems necessary regarding the fact that inflammatory conditions resulting from transplantation could drive T-cell phenotype and function to exhaustion which is not related to relapse. The study also showed that early detection of extremely exhausted memory stem T cells (PD1+, Eomes+, and T-bet−) is able to predict relapse (Noviello et al., 2019).
Although inhibitory receptors are expressed and acted superficially, evidence has been found that secretory forms of these inhibitory molecules are also present. AML cells and leukemic stem cells are able to express TIM3 on their surface as well as secreting TIM3 (secretory TIM3) in a complex form with its ligand, Galectin-9 (Gal9), which are capable of suppressing the cytotoxicity of NK and T cells. Gal9 secreted by myeloid leukemia cells can activate nuclear factor-κB and B-catenin signaling pathways in an autocrine manner, leading to the growth and self-renewal of TIM3+ leukemic blasts and conversion of preleukemic conditions to leukemia (Kikushige et al., 2015). Thus, the TIM3/Gal9 pathway not only regulates the antitumor immune response but also causes the survival of tumor cells and disease progression. Accordingly, Gal9 can be used as a marker of immune-escaping and the TIM3/Gal9 pathway could be an effective therapeutic target in patients with AML (Kursunel & Esendagli, 2017).
Most studies of T-cell exhaustion have been performed in CTLs. Lately, the exhaustion of helper T cells (THs) has also been addressed (Antoine et al., 2012; Goding et al., 2013). A subset of exhausted TH cells expressing PD1, TIM3, and LAG3 that are defective in cytokine secretion has been found in the bone marrow aspirates of patients with AML and myelodysplastic syndrome (Ozkazanc et al., 2016). Interestingly, CD8+ T cells function is facilitated by TH cells, and the presence of functional TH cells prevents CD8+ T cells from being exhausted (Church, Jensen, Antony, Restifo, & Fox, 2014; Hunziker, Klenerman, Zinkernagel, & Ehl, 2002). Therefore, TH cell exhaustion also has negative effects on CD8+ T-cell function. Expectedly, the increase in the number of exhausted TH cells is associated with relapse after allogeneic transplantation in AML. In the coculture of TH cells with leukemic cells, TH cell responses are stimulated initially. After a while, due to the high intensity and continuation of stimulatory signals, TH cells become exhausted by overexpression of inhibitory receptors and reduced tendency to proliferation and production of interleukin-2, tumor necrosis factor (TNF)-α, and IFN-γ (Ozkazanc et al., 2016). Thus, the costimulatory molecules expressed in myeloproliferative diseases are able to induce exhaustion, which is known as the mechanism of immune dysregulation in cancer. That is why some studies have linked high expression of costimulatory molecules such as CD86 and ICOSL in myeloproliferative diseases with poor clinical prognosis (Graf et al., 2005; Tamura et al., 2005). The alterations in the effects of leukemia cells from immune-stimulating to immunosuppressive effects are called adaptive resistance, which is mainly associated with increased expression of PDL1 (Ribas, 2015).
Exhaustion occurs following the executive immune response, and the markers of exhausted T cells are similar to those of effector T cells in the final phase of their activity (Legat, Speiser, Pircher, Zehn, & Fuertes Marraco, 2013). In the functional phase of effector T cells, the expression of CTLA4 and PD1 is earlier than that of TIM3 and LAG3, so the two molecules TIM3 and LAG3 are probably better markers for exhaustion (Crespo, Sun, Welling, Tian, & Zou, 2013; Legat et al., 2013; Speiser et al., 2014). However, the expression of inhibitory receptors alone does not define exhaustion and cell function must be taken into account. Accordingly, some studies have shown that the expression of PD1 and even CD244 molecules does not impair T-cell function in relapsing patients with AML, indicating their differentiation into functional memory subsets rather than exhaustion (Noviello et al., 2019; Schnorfeil et al., 2015). TIGIT is another immune checkpoint that has ITIM in its signaling domain and induces inhibitory signals in T and NK cells (Boles et al., 2009; Stanietsky et al., 2009; Stengel et al., 2012; Yu et al., 2009). As for CTLA4 and CD28, TIGIT shares its ligands, CD155 and CD112, with the CD226 costimulatory receptor (A. Shibuya et al., 1996; K. Shibuya et al., 2003). TIGIT expression increases on CD8+ T-cell surface of patients with AML and is related to drug resistance and relapse after transplantation as well as disease outcome (Kong et al., 2016). These TIGIT+ CD8+ T cells exhibit an exhausted phenotype with expression of other inhibitory receptors, functional defects in cytokine production, and high susceptibility to apoptosis. Decreased expression of TIGIT is associated with improvement in T-cell function and provides therapeutic potential in relapsing AML (Kong et al., 2016).
Clinical studies on allogeneic HSCT have shown the critical role of NK cells in the prevention of relapse (Miller et al., 2005; Ruggeri et al., 2002). In AML, higher cytotoxicity of NK cells as well as more expression of activatory receptors, NKp30 and NKp46, are associated with more prolonged remission phase of patients (Alcasid, Ma, Gotlib, Arber, & Ohgami, 2017; Chretien, Devillier et al., 2017; Chretien, Fauriat et al., 2017; Khaznadar et al., 2015). Miller et al. (2005) showed that IL2-activated NK cells in haploidentical transplantation led to CR in more than a quarter of patients. One of the reasons for the insufficient and temporary success of NK cell therapy in preventing relapse is the exhaustion of NK cells. Continuous exposure of NK cells to ligands expressed by AML blasts reduces activatory receptors such as NKG2D, DNAM-1, and NCRs, while increases the expression of PD1, TIM3, TIGIT, and CD200 on NK cells, causing exhaustion and consequently disease progression (Carlsten et al., 2010; Carlsten & Järås, 2019; Coles et al., 2011; Damele et al., 2020; Fauriat et al., 2007). Interestingly, TIGIT is proposed to be a better marker than PD1 and CTLA4 for exhausted NK cells, which requires further investigations in hematologic malignancies (Voutsadakis, 2003). However, recent evidence suggests that blocking PD1/PDL1 interaction is necessary to activate NK cells, indicating the importance of combining checkpoint blockers with HSCT and NK cell therapy in myeloid malignancies (Hsu et al., 2018).
2.2 Chronic myeloid leukemia
CML is generally treated by tyrosine kinase inhibitors (TKIs), and cases in blast phase (blast crisis) are treated with allogeneic HSCT (Apperley, 2015). Evidence of allogeneic transplantation in CML revealed that functional CTLs and NK cells are able to lyse CML blasts (Pattengale, Sundstrom, Yu, & Levine, 1983). CML patients with a higher percentage of functional NK cells have more appropriate molecular responses to TKIs and a longer remission time when TKI is discontinued (Ilander et al., 2017). However, the success of allogeneic HSCT was temporary, largely due to the dysfunction of NK/T cells in these patients (Mattias Carlsten & Järås, 2019). Therefore, T/NK cell exhaustion is associated with the relapse of the disease in CML (Bachireddy et al., 2014). During the progression of CML, NK cells decrease in number and exhibit severe defects, including reduced expression of activating receptors (DNAM-1, NKp46, and NKp30), increased NKG2A inhibitory receptor, suppressed cytotoxicity and proliferation, as well as low secretion of IFN-γ and TNF-α all of which lead to poor clinical outcomes (Fauriat et al., 2007; Khaznadar et al., 2015; Stringaris et al., 2014).
Various mechanisms including cell–cell interactions, secretory factors, and other regulatory elements in the tumor microenvironment are involved in this process (Carlsten & Järås, 2019). In addition to cell surface inhibitory receptors, leukemic blasts are able to suppress T and NK cells activity by secreting interleukin-10 (IL-10), transforming growth factor-β, reactive oxygen species, and indoleamine 2,3-dioxygenase (Damele et al., 2020). In line with the role of NKG2A in regulating NK cell responses, Ruggeri et al. (2016) reported that targeting NKG2A with blocking antibodies resulted in a strong antileukemic response by NK cells. Studies have also shown that blocking iKIRs and NKG2A together enhances the lethal responses of NK cells to leukemic blasts (Godal et al., 2010).
One of the useful therapeutic strategies for the induction of GVL, especially in patients with CML, is the donor lymphocyte infusion (DLI) in which the CD4+ T cells are infused to help bone marrow-resident CD8+ T cells. For this reason, the amount of bone marrow CD8+ T cells during DLI and their functionality is of great importance in predicting antileukemia responses so that even 1% infiltration of CD8+ T cell at DLI is sufficient to elicit an appropriate response to the tumor (Collins et al., 1997). The response to DLI in relapsing patients with CML whose T cells are exhausted is dysfunctional, and the reversal of exhaustion by blocking the PD1/PDL1 pathway is associated with improved DLI-induced antileukemia responses (Bachireddy et al., 2014). Taken together, targeting immune checkpoints could be served as a complementary treatment for HSCT and DLI in patients with CML.
3 LYMPHOID LEUKEMIA
3.1 Acute lymphoblastic leukemia
Acute lymphoblastic leukemia (ALL) is a common malignancy in children. B-ALL originated from B precursors associated with high recurrence of disease after allogeneic transplantation and high mortality (Poon et al., 2013). Only a few patients have long-term survival after cutting the chemotherapeutic and immunosuppressive drugs, with approximately 20% of the 2-year survival (Poon et al., 2013; Yan et al., 2016). It has been shown that CD4+ and CD8+ T cells in patients with B-ALL who relapsed after transplantation have exhausted phenotypes with high expression of PD1 and TIM3 (Liu et al., 2018). Also, the simultaneous appearance of these two inhibitory receptors on the surface of bone marrow-derived effector T cells is greater than their expression on the peripheral T cells of relapsing patients with B-ALL (Liu et al., 2018). This finding that the severity of exhaustion in the tumor microenvironment is more than other sites is seen in other cancers including AML, multiple myeloma (MM), hepatocellular carcinoma, and colorectal cancer (Chew et al., 2017; Kong et al., 2015; Xu et al., 2015; Zelle-Rieser et al., 2016). Similar to AML, B-ALL has been shown to induce PD1 expression specifically on the surface of effector memory T cells (Liu et al., 2018; Schnorfeil et al., 2015). Simultaneous expression of PD1 and TIM3 in the antigen-experienced T cells such as effector and effector memory cells is associated with B-ALL relapse after transplantation (Liu et al., 2018). In addition, these exhausted cells also showed a decreased capacity of proliferation, cytokine, and granzyme B production and defects in cytotoxicity in both CD4+ and CD8+ subgroups (Liu et al., 2018). Reversion of exhausted T cells in patients with B-ALL was associated with improved response to treatment, and patients who reached the CR stage showed lower levels of PD1+ TIM3+ cells (Feucht et al., 2016; Liu et al., 2018).
Interestingly, the use of bispecific anti-CD3, anti-CD19 (Blinatumomab) antibodies in these patients increased the expression of inhibitory molecules in leukemic blasts (Feucht et al., 2016). The presence of these inhibitory molecules resulted in resistance to Blinatumomab, suggesting the use of immune checkpoint blockers in combination with Blinatumomab (Feucht et al., 2016). Therefore, T-cell exhaustion is associated with response to treatment as well as post-transplant relapse in patients with B-ALL. The drawback of the studies, however, is that they have not examined T-cell exhaustion on tumor-specific T cells. Besides T cells, NK cells seem to have a critical role in ALL eradication through DNAM-1, NKp30, and NKp46 signals. Hence, the decrease in such activatory receptors on NK cells as well as low expression of their ligands in leukemic blasts cause NK cell dysfunction and cancer progression (Pende et al., 2005). The mechanism and role of NK cell exhaustion in B-cell-derived leukemia such as ALL and CLL are less clear than myeloid leukemia (Mattias Carlsten & Järås, 2019).
3.2 Chronic lymphocytic leukemia
CLL cells also employ various mechanisms of adaptive resistance to evade antitumor immune responses, including the induction of T/NK cell exhaustion through binding PD1, TIM3, and other inhibitory receptors on the surface of T cells to their ligands expressed by CLL cells and myeloid-derived suppressor cells (Jitschin et al., 2014; Ramsay, Clear, Fatah, & Gribben, 2012). The amount of NK cells with low NKp30 and high TIM3 expression in the peripheral blood of patients with CLL is reported to be increased and associates with poor prognosis (Hadadi et al., 2019). CD4+ and CD8+ effector T cells in advanced-stage patients with CLL showed an exhausted phenotype (McClanahan et al., 2015; Taghiloo et al., 2017) with functional deficits in proliferation, immunologic synapse formation, and cytotoxicity. These functional defects are mainly due to decreased expression of cytoskeletal genes that play an important role in the formation of immunological synapses and vesicle trafficking (Ramsay et al., 2008). Despite exhaustion derived from chronic viral infections, T-cell exhaustion in CLL is partial, and the inflammatory cytokine secretion in these cells is not diminished (Riches et al., 2013). A possible cause of this partial exhaustion is the differences in the affinity of antigens that lead to exhaustion. Consistent stimulation of T cells with high-affinity viral antigens leads to complete T-cell exhaustion, whereas tumor antigens in CLL are usually self-antigens with low affinity, which induces partial exhaustion in T cells. Another explanation for partial exhaustion of T cells in CLL, especially for cytokine secretion, is that the presence of cytokines such as IFN-γ and TNF-α also have protumoral effects, so the tumor may preferentially not induce this functional defect in exhausted T cells (Buschle et al., 1993; Digel et al., 1989; Riches et al., 2013). Given the high susceptibility of patients with HSCT to chronic viral infections, one of the critical questions arose is that the T-cell exhaustion following HSCT might be due to chronic viral infection rather than tumor microenvironment. To answer this question, a study showed that CD8+ T-cell exhaustion in patients with CLL did not correlate with positive or negative serologic status of CMV infection and rejected the mere causative role of CMV infection in T-cell exhaustion (Riches et al., 2013). This finding has also been demonstrated in patients with AML (Noviello et al., 2019). However, the answer to this question needs further investigation of other viral infections and other hematologic malignancies. Using a CLL mouse model, it is shown that blocking the PD1/PDL1 pathway restore the antitumor effects of CD4+ and CD8+ T cells and subsequently reduces tumor burden (McClanahan et al., 2015). Blocking PD1 by the Pidilizumab antibody in the clinic has been shown to be safe and clinically effective such that it increases the proportion of antitumor immune cells in patients with CLL (Berger et al., 2008). Interestingly, Ibrutinib, a Bruton's tyrosine kinase covalent inhibitor approved for relapsed/refractory patients with CLL, besides inhibiting signaling of kinases, can also reverse T-cell exhaustion by reducing PD1/PDL1 expression (Kondo et al., 2018).
4 LYMPHOMA
4.1 Hodgkin's lymphoma
Hodgkin's lymphoma (HL) accounts for 15–25% of all lymphomas with a 30% relapse after treatment (Ansell, 2018; Ghasemi et al., 2020). Malignant cells in HL are able to suppress antitumor responses using various strategies, one of which is the induction of exhaustion in T and NK cells through engagement of PD1 and PDL1 expressed on the surface of tumor cells and tumor-associated macrophages (TAMs; Cader et al., 2018; Carey et al., 2017; Vari et al., 2018; Yamamoto et al., 2008). PDL1+ TAMs preferentially surround tumor cells, creating an immune-suppressive niche that suppresses PD1+ T and NK cells (Vari et al., 2018). Therefore, the high presence of TAMs in patients is associated with poor prognosis (Carey et al., 2017). Recently, the role of inhibitory receptors such as CD200R, B- and T-lymphocyte attenuator, and LAG3 expressed on HL infiltrated T cells and their ligands (CD200, herpesvirus entry mediator, and major histocompatibility complex Class II, respectively) on HL cells has been reported in T-cell exhaustion (Péricart et al., 2018; Wein et al., 2017).
Another mechanism of immunosuppression in the tumor microenvironment reported in some hematologic malignancies, as well as HL, is the production of adenosine from ATP by CD39 and CD73 ectonucleotidases. Adenosine binds to its inhibitory receptors, including A2 adenosine receptor, on the surface of functional T cells, making them exhausted (Ghalamfarsa et al., 2019; Kazemi et al., 2018). HL tumor cells are also capable of indirectly inducing T-cell exhaustion by driving T cells toward regulatory T cells (Tregs; Wein et al., 2017; Zeiser, Robson, Vaikunthanathan, Dworak, & Burnstock, 2016). Therapeutic strategies have generally focused on blocking PD1 and suggested that the use of anti-PD1 antibody can interfere with the interaction of NK and T cells with TAMs, preventing the induction of exhausted phenotype in these cells (Goodman, Patel, & Kurzrock, 2017). The significant clinical efficacy of nivolumab and pembrolizumab, together with their safety and tolerability, led to the approval of using these monoclonal antibodies by the Food and Drug Administration in refractory and recurrent patients with HL (Table 1). However, the presence of a wide range of inhibitory receptors demonstrates the importance of combined blocking to achieve better results (Ansell, 2018; Armand et al., 2016; Chen et al., 2017; Gholami et al., 2019; von Keudell & Younes, 2019; Younes & Ansell, 2016).
| Blocked immune checkpoint | Disease/drug | Phase | Result | Clinical trial code | Reference | |
|---|---|---|---|---|---|---|
| Myeloid linage leukemia and MDS | PD1 | AML-MDS/nivolumab | I/II | CR: 77% | NCT02464657 | Ravandi et al. (2017) |
| MRD negative: 18/34 patients in CR | ||||||
| AML-MDS/nivolumab | II | 12-month CR: 71% | NCT02532231 | Kadia et al. (2018) | ||
| 12-month OS: 86% | ||||||
| 18-month OS: 67% | ||||||
| AML-MDS/nivolumab | II | ORR: 33% | NCT02397720 | Daver et al. (2018) | ||
| CR: 22% | ||||||
| PR: 1.4% | ||||||
| SD: 8.5% | ||||||
| AML-MDS/nivolumab | II | ORR: 69% | NCT02530463 | Garcia-Manero et al. (2016) | ||
| CR:18% | ||||||
| 1-year OS: 25% | ||||||
| AML-MDS/pembrolizumab | II | ORR: 42% | NCT02768792 | Zeidner et al. (2018) | ||
| CR: 35% | ||||||
| PR: 45% | ||||||
| MRD: 5/9 patients in CR | ||||||
| CTLA4 | AML-MDS/ipilimumab | II | ORR: 30% | NCT02530463 | Garcia-Manero et al. (2018) | |
| CR: 14% | ||||||
| 1-year OS: 45% | ||||||
| Lymphoid linage leukemia | PD1 | CLL/nivolumab | II | ORR: 66% | NCT02420912 | Jain et al. (2016) |
| CR: 0% | ||||||
| CLL/pembrolizumab | II | ORR: 16% | NCT02332980 | Ding et al. (2016) | ||
| CR: 4% | ||||||
| PR: 12% | ||||||
| SD: 24% | ||||||
| Median OS: 10.7 months | ||||||
| Richter's syndrome/pembrolizumab | II | ORR: 44% | NCT02332980 | Ding et al. (2016) | ||
| CR: 33% | ||||||
| SD: 11% | ||||||
| CTLA4 | CLL/ipilimumab | I | CR: 23% | NCT01822509 | Assi et al. (2018) | |
| PR: 9% | ||||||
| Tumor burden decrease: 27% | ||||||
| Hodgkin Lymphoma | PD1 | Nivolumab | I | ORR: 87% | NCT01592370 | Ansell et al. (2015) |
| CR: 17% | ||||||
| PR: 70% | ||||||
| SD: 13% | ||||||
| 24-week PFS: 86% | ||||||
| Nivolumab | II | ORR: 68% | NCT02181738 | Timmerman et al. (2016) | ||
| CR: 8% | ||||||
| PR: 60% | ||||||
| Median PFS: 14.8 months | ||||||
| 12-month OS: 94.9% | ||||||
| 12-month PFS: 54.6% | ||||||
| Nivolumab | I/II | ORR: 100% | NCT02572167 | Herrera et al. (2016) | ||
| CR: 50% | ||||||
| Nivolumab | II | ORR:81.3% | JapicCTI-142755 | Maruyama et al. (2017) | ||
| CR:23.5% | ||||||
| 6-month PFS: 60% at | ||||||
| Pembrolizumab | Ib | ORR: 65% | NCT01953692 | Armand et al. (2016) | ||
| CR: 16% | ||||||
| PR: 48% | ||||||
| 24-week PFS: 69% | ||||||
| 52-week PFS: 46% | ||||||
| Pembrolizumab | II | ORR: 69%, | NCT02453594 | Chen et al. (2017) | ||
| CR: 22.4% | ||||||
| OS: 99.5 | ||||||
| 6-month PFS: 72.4% | ||||||
| ≥6 months’ response: 75.6% | ||||||
| Sintilimab | II | ORR: 80.4 | NCT03114683 | Shi et al. (2019) | ||
| CR: 34% | ||||||
| 6-month PFS: 77.6% | ||||||
| CTLA4 | Ipilimumab | I | ORR: 67% | NCT01896999 | Prendergast et al. (2014) | |
| CR: 42% | ||||||
| Median PFS: 0.74 years | ||||||
| PD1/CTLA4 | Nivolumab + ipilimumab | I | ORR: 82% | NCT01896999 | Diefenbach et al. (2018) | |
| CR: 68% | ||||||
| Non-Hodgkin lymphoma | PD1 | DLBCL/nivolumab | Ib | OR: 36% | NCT01592370 | Lesokhin et al. (2016) |
| PR: 18% | ||||||
| CR: 18% | ||||||
| SD: 27% | ||||||
| Median PFS: 7 weeks | ||||||
| DLBCL/nivolumab | II | ORR: 10% | NCT02038933 | Ansell et al. (2019) | ||
| OS: 12.2 | ||||||
| PFS: 1.9 | ||||||
| FL/nivolumab | Ib | ORR: 40% | NCT01592370 | Lesokhin et al. (2016) | ||
| CR: 10% | ||||||
| PR: 30% | ||||||
| SD: 60% | ||||||
| T-NHL/nivolumab | Ib | CR: 0 | NCT01592370 | Lesokhin et al. (2016) | ||
| PR:17% | ||||||
| SD:43% | ||||||
| Median PFS: 10 weeks | ||||||
| PMBCL/pembrolizumab | Ib | ORR: 44% | NCT01953692 | Zinzani et al. (2018) | ||
| CR: 11% | ||||||
| PR: 33.3% | ||||||
| DLBCL/pembrolizumab | Ib | ORR: 41% | NCT01953692 | Zinzani et al. (2017) | ||
| SD: 35% | ||||||
| DLBCL/pembrolizumab | II | ORR:44% | NCT02332980 | Ding et al. (2017) | ||
| CR: 11% | ||||||
| OS: 10.7 | ||||||
| PFS: 5.4 | ||||||
| NHL-Sézary syndrome/pembrolizumab | II | ORR: 33% | NCT02243579 | Khodadoust et al. (2016) | ||
| CR: 0 | ||||||
| PR: 33% | ||||||
| 12-month PFS: 69% | ||||||
| NHL-mycosis fungoides/pembrolizumab | II | ORR: 50% | NCT02243579 | Khodadoust et al. (2016) | ||
| CR: 17% | ||||||
| PR: 33% | ||||||
| DLBCL/pidilizumab | II | ORR: 51% | NCT00532259 | Armand et al. (2013) | ||
| CR: 34% | ||||||
| SD: 37% | ||||||
| PFS: 72 | ||||||
| FL/pidilizumab | II | ORR: 66% | NCT00904722 | Westin et al. (2014) | ||
| CR: 52% | ||||||
| SD: NA | ||||||
| PFS: 18.8 months | ||||||
| CTLA4 | NHL/ipilimumab | I | ORR: 11% | NCT00089076 | Ansell et al. (2009) | |
| CR: 5.6% | ||||||
| PR: 5.6% | ||||||
| Multiple myeloma | PD1 | Nivolumab | I | ORR: 4% | NCT01592370 | Lesokhin et al. (2016) |
| CR: 0 | ||||||
| PR: 4% | ||||||
| SD: 63% | ||||||
| PFS: 10 weeks | ||||||
| Pembrolizumab | I | ORR: 76% | NCT02036502 | San Miguel et al. (2015) | ||
| PR: 56% | ||||||
| Pembrolizumab | II | ORR: 56% | NCT02289222 | A. Z. Badros et al. (2016) | ||
| PR: 29% | ||||||
| 6-month OS: 56% | ||||||
| Median PFS: 57 days | ||||||
| Pidilizumab | I/II | ORR: 33% | NCT02077959 | Efebera et al. (2015) | ||
| CR: 0% | ||||||
| SD: 33% | ||||||
| CTLA4 | Ipilimumab | I | ORR: 32% | NCT01822509 | Ansell et al. (2015) | |
| CR: 23% | ||||||
| PR: 9% | ||||||
| SD: 27% |
- Abbreviations: AML, acute myeloid leukemia; CLL, chronic lymphoblastic leukemia; CR, complete response; CTLA4, cytotoxic T-lymphocyte-associated protein-4; DLBCL, diffuse large B-cell lymphoma; FL, follicular B-cell lymphoma; MDS, myelodysplastic syndrome; MRD, minimal residual disease; NHL, non-Hodgkin's lymphoma; ORR, overall response rate; PD1, programmed cell death protein-1; PFS, progression-free survival; PMBCL, primary mediastinal large B-cell lymphoma; PR, partial response; SD, stable disease.
Generally, chronic viral infection, especially Epstein–Barr virus (EBV), is highly prevalent in lymphomas and is one of the causes of T-cell exhaustion. Up to 90% of patients with HL in developing countries are positive for EBV infection, which shows higher levels of exhaustion in their T cells resulting from chronic viral inflammation (Carbone & Gloghini, 2018; Johnson et al., 2015).
4.2 Non-Hodgkin's lymphomas
Diffuse large B-cell lymphoma (DLBCL) is the most invasive and frequent type of B-cell lymphoma, accounting for about one-third of non-Hodgkin's lymphomas (NHLs; Menon, Pittaluga, & Jaffe, 2012). PD1 has been reported to be expressed on 40–70% of immune cells in DLBCL microenvironment including T and NK cells (Ahearne et al., 2014; Cohen et al., 2017; Fang et al., 2017; Kiyasu et al., 2015; Kwon et al., 2016; Muenst, Hoeller, Willi, Dirnhofer, & Tzankov, 2010). Although the expression of PD1 on NK cells is reported in DLBCL, the role of NK cell exhaustion in HL is more prominent than in DLBCL (Vari et al., 2018). In normal and noncancerous conditions, PD1 expression is found on follicular THs of the germinal center (GC) in lymph nodes; and therefore, PD1+ tumor-infiltrating lymphocytes (TILs) are abundant in GC type of DLBCL (Kiyasu et al., 2015; Song, Park, & Uhm, 2019). Interestingly, studies on DLBCL correlate high PD1+ TIL counts with good prognosis of disease that contradicts reports of solid tumors in which tumor-specific PD1+ TILs reflect poor prognosis (Ahearne et al., 2014; Carreras et al., 2009; Fang et al., 2017; J. R. Kim et al., 2013; Kiyasu et al., 2015; Kwon et al., 2016; Tavakoli et al., 2019; Thompson et al., 2006; Wahlin et al., 2010). This finding suggests that the expression of PD1 on T cells might be indicative of an active T-cell response against tumor; therefore, PD1 may act as a positive prognostic factor in DLBCL (Carreras et al., 2009; Song et al., 2019; Wahlin et al., 2010). However, this interpretation can be true as long as the majority of PD1 on T cells does not bind to PDL1 on the tumor cell surface. Tumor cells are able to induce PDL1 expression on their surface through different mechanisms, including genetic anomalies, chromosomal alterations, and Janus kinase–signal transducer and activator of transcription-dependent pathways resulting from EBV signaling or inflammatory cytokines such as IFN-γ (Blank et al., 2004; Green et al., 2010, 2012; Lin, Lo, & Wu, 2010; Mathas et al., 2002; Spranger et al., 2013). PDL1 expression on the surface of tumor cells is strongly associated with malignant B-cell survival and proliferation, indicating invasive features and poor prognosis in DLBCL (Hu et al., 2017; Kiyasu et al., 2015; Siddiqi et al., 2016; Xing et al., 2016). Therefore, PDL1 in DLBCL seems to be a better marker than PD1 to determine the prognosis. Phases I and II clinical trials on blocking PD1/PDL1 axis using anti-PD1 showed promising results and low-side effects in relapsed/refractory patients with DLBCL and clinical trials on anti-PDL1 are ongoing (reviewed in Song et al., 2019) and listed in Tables 1 and 2).
| Blocked immune checkpoint | Disease/drug | Phase | Completion date | Clinical trial code | |
|---|---|---|---|---|---|
| Myeloid leukemia | PD1 | AML/pembrolizumab | Early phase I | February 2020 | NCT02981914 |
| AML/pembrolizumab | II | October 2020 | NCT02708641 | ||
| AML-MDS/nivolumab | II | October 31, 2020 | NCT02532231 | ||
| AML-MDS/nivolumab | I/II | January 2021 | NCT02464657 | ||
| AML/MDS/nivolumab | II | April 30, 2021 | NCT02397720 | ||
| AML/pembrolizumab | II | July 2021 | NCT02845297 | ||
| AML-MDS/nivolumab | II | August 31, 2021 | NCT02275533 | ||
| AML-MDS/pembrolizumab | I | October 2021 | NCT03286114 | ||
| AML/pembrolizumab | II | December 31, 2021 | NCT02771197 | ||
| MDS/nivolumab | II | September 2022 | NCT02530463 | ||
| AML-MDS/nivolumab | II | February 2023 | NCT03417154 | ||
| AML-MDS/nivolumab | I | July 2023 | NCT02846376 | ||
| AML-MDS/nivolumab | II/III | August 1, 2023 | NCT03092674 | ||
| AML/pembrolizumab | II | September 2025 | NCT02768792 | ||
| PDL1 | AML-MDS/durvalumab | II | September 30, 2020 | NCT02775903 | |
| CML-MDS/Atezolizumab | I/II | November 2, 2021 | NCT02935361 | ||
| TIM3 | AML-MDS/MBG453 | I | April 1, 2021 | NCT03066648 | |
| Lymphoid leukemia | PD1 | CTCL/pembrolizumab | I | December 2020 | NCT03240211 |
| B-ALL/pembrolizumab | I | October 2021 | NCT03286114 | ||
| B-ALL/pembrolizumab | II | August 2023 | NCT03160079 | ||
| PD1 + CTLA4 | B-ALL/nivolumab + ipilimumab | I | November 5, 2021 | NCT02879695 | |
| CTLA4 | B-ALL, CLL/ipilimumab | I | July 2029 | NCT00586391 | |
| PDL1 | CLL/durvalumab | I/II | July 8, 2022 | NCT02733042 | |
| Hodgkin lymphoma | PD1 | Nivolumab | I/II | December 2020 | NCT02572167 |
| Nivolumab | II | December 2020 | NCT03004833 | ||
| Nivolumab | II | March 27, 2022 | NCT02927769 | ||
| Nivolumab | III | July 27, 2023 | NCT03138499 | ||
| Nivolumab | II | August 7, 2024 | NCT03233347 | ||
| Pembrolizumab | I | June 10, 2022 | NCT01953692 | ||
| Pembrolizumab | I | August 2023 | NCT02875067 | ||
| Pembrolizumab | 1/2 | September 1, 2022 | NCT02332668 | ||
| Pembrolizumab | Early phase 1 | February 2020 | NCT02981914 | ||
| Pembrolizumab | II | September 2020 | NCT03226249 | ||
| Pembrolizumab | II | February 2020 | NCT03077828 | ||
| Pembrolizumab | II | June 2020 | NCT03179917 | ||
| PD1/2 | CA-170 | I | January 2020 | NCT02812875 | |
| PD1 + CTLA4 | Nivolumab + ipilimumab | I | December 31, 2020 | NCT02408861 | |
| Nivolumab + ipilimumab | I/II | January 13, 2022 | NCT01592370 | ||
| Nivolumab + ipilimumab | I/II | March 20, 2025 | NCT01896999 | ||
| PDL1 | Atezolizumab | I/II | October 17, 2020 | NCT02729896 | |
| Avelumab | II | August 2021 | NCT03046953 | ||
| Durvalumab | I/II | July 8, 2022 | NCT02733042 | ||
| PD1 + LAG3 | Cemiplimab + REGN3767 | I | October 4, 2021 | NCT03005782 | |
| Pembrolizumab + MK-4280 | I/II | December 18, 2025 | NCT03598608 | ||
| LAG3 | BMS-986016 + BMS-936558 | I/II | May 15, 2020 | NCT02061761 | |
| Sym022 | I | August 2020 | NCT03489369 | ||
| MGD013 | I | August 2022 | NCT03219268 | ||
| Sym021 | I | November 2020 | NCT03311412 | ||
| TIM3 | Sym023 | I | September 2020 | NCT03489343 | |
| Non-Hodgkin lymphoma | PD1 | FL, BL, DLBCL, LPL, MCL, MZL/nivolumab | I/II | April 30, 2020 | NCT03015896 |
| NHL, FL, DLBCL, PMBCL/pembrolizumab | I | June 10, 2022 | NCT01953692 | ||
| NHL/pembrolizumab | II | August 2023 | NCT02875067 | ||
| NHL/pembrolizumab | Early phase 1 | February 2020 | NCT02981914 | ||
| DLBCL/pembrolizumab | II | September 14, 2020 | NCT02576990 | ||
| FL/nivolumab | I | January 31, 2028 | NCT03121677 | ||
| PDL1 | DLBCL/atezolizumab | I/II | August 2033 | NCT02926833 | |
| DLBCL/atezolizumab | I/II | April 17, 2020 | NCT02596971 | ||
| FL/atezolizumab | I/II | November 10, 2020 | NCT02631577 | ||
| DLBCL/durvalumab | II | August 2023 | NCT03212807 | ||
| DLBCL/durvalumab | II | December 2021 | NCT03241017 | ||
| DLBCL/durvalumab | II | March 13, 2023 | NCT03003520 | ||
| DLBCL/atezolizumab | II | June 2023 | NCT03422523 | ||
| DLBCL/atezolizumab | I | September 8, 2023 | NCT03630159 | ||
| T-cell lymphoma/durvalumab | I/II | February 2021 | NCT03161223 | ||
| PD1+CTLA | NHL/nivolumab + ipilimumab | I/II | January 13, 2022 | NCT01592370 | |
| PD1 + LAG3 | NHL/pembrolizumab + MK-4280 | I/II | December 18, 2025 | NCT03598608 | |
| Multiple myeloma | PD1 | Pembrolizumab | I | February 24, 2020 | NCT02036502 |
| Pembrolizumab | III | September 30, 2020 | NCT02579863 | ||
| Pembrolizumab | I | June 10, 2022 | NCT01953692 | ||
| Nivolumab | III | December 8, 2022 | NCT02726581 | ||
| PD1 + CTLA4 | Nivolumab + ipilimumab | I/II | January 13, 2022 | NCT01592370 |
- Abbreviations: AML, acute myeloid leukemia; B-ALL, B-cell acute lymphoblastic leukemia; BL, Burkitt lymphoma; CLL, chronic lymphoblastic leukemia; CML, chronic myeloid leukemia; CTCL, cutaneous T-cell lymphoma; CTLA4, cytotoxic T-lymphocyte-associated protein-4; DLBCL, diffuse large B-cell lymphoma; FL, follicular B-cell lymphoma; LAG3, lymphocyte-activation gene-3; LPL, lymphoplasmacytoid lymphomas; MCL, Mantle cell lymphoma; MDS, myelodysplastic syndrome; MZL, marginal zone lymphoma; NHL, non-Hodgkin's lymphoma; PD1, programmed cell death protein-1; PDL1, PD1 ligand; PMBCL, primary mediastinal large B-cell lymphoma; TIM3, T-cell immunoglobulin and mucin-domain containing-3.
Follicular B-cell lymphoma (FL) is the second most common type of NHL characterized by large numbers of T cells in the tumor microenvironment (Dave et al., 2004; Glas et al., 2007). Intriguingly, studies have shown that the use of IL-12 in combination with rituximab reduces the response rate in patients with FL. Prolonged exposure of T cells to IL-12 makes these cells to become exhausted with IFN-γ-dependent expression of TIM3 and functional defects correlated with poor prognosis in patients with follicular lymphoma (Yang et al., 2012).
Primary mediastinal large B-cell lymphoma (PMBCL) is a progressive B-cell lymphoma, comprising 2–3% of NHLs, of which 30% are refractory to treatment (Campo et al., 2011). The role of T-cell exhaustion in disease progression and the presence of inhibitory receptors such as PD1 have made these patients candidates for anti-PD1 therapy (Lees, Keane, Gandhi, & Gunawardana, 2019). The beneficial effects of Pembrolizumab in 50% reduction of the relapse risk in PMBCL led to the approval of this drug for the treatment of these patients (Armand et al., 2018; Zinzani et al., 2017). Clinical trials are currently ongoing to block other immune checkpoints as well as the combination of this treatment with chemotherapy or CD19-specific CAR-T-cell therapy in patients who did not respond well to CD19–CAR-T cells (Campo et al., 2011; Table 2).
Mantle cell lymphoma (MCL) accounts for approximately 6–8% of all B lymphomas with the worst prognosis among lymphomas (Cheah, Seymour, & Wang, 2016). There are conflicting reports on the expression of PD1/PDL1 in the malignant B-cell population of patients with MCL; however, PDL1 expression on MCL cells is able to inhibit tumor-specific T proliferation/function (Menter, Bodmer-Haecki, Dirnhofer, & Tzankov, 2016; Xerri et al., 2008; Xu-Monette, Zhou, & Young, 2018). Moreover, in vitro and in vivo studies showed that blocking or knocking down PDL1 in MCL cells effectively improved T-cell responses, and the sensitivity of the tumor cells to T-cell-mediated killing was reinduced (L. Wang et al., 2013).
T-cell lymphomas comprise about 10% of all lymphatic neoplasms, which have a worse prognosis than B lymphomas (L. Wang et al., 2013). Adult T-cell leukemia/lymphoma (ATLL) is a T-cell malignancy that develops after a long latency period of human T-cell leukemia virus Type 1 (HTLV1) infection. Despite therapeutic advances, the prognosis of ATLL remains poor and the 3-year overall survival is only 24% (Tsukasaki et al., 2007; Yasunaga & Matsuoka, 2007). HTLV1 infection, like other chronic viral infections, causes T-cell exhaustion and dysfunction (Shimauchi et al., 2007). Increased PD1 expression on HTLV1-specific CTLs and CD4+ T cells has been reported in asymptomatic HTLV1 carriers and patients with ATLL (Kozako et al., 2009; Shimauchi et al., 2007). These studies indicate that HTLV1-specific CTLs play an important role in suppressing the proliferation of HTLV1-infected and transformed T cells and thereby preventing ATLL development (Arnulf et al., 2004; Kannagi et al., 2005). Intriguingly, in individuals carrying all three HTLV1, CMV, and EBV viruses, HTLV1-specific T cells showed greater exhaustion than those specific for either CMV or EBV, confirming the broad immunosuppression seen in patients with ATLL (Heß, 2017). PD1/PDL1 pathway blockade in these studies increased T-cell responses and reversed their dysfunctional state (Kozako et al., 2009; Shimauchi et al., 2007). Noteworthy, CTLA4 overexpression is not reported in PD1+ HTLV1-specific CD8+ T cells (Arnulf et al., 2004; Hirata et al., 2006).
PD1/PDL1 expression in other T-cell lymphomas including anaplastic lymphoma kinase-positive anaplastic large-cell lymphoma, nasal-type extranodal NK/T-cell lymphoma, cutaneous T lymphoma, and angioimmunoblastic T lymphoma have also been reported (Goodman et al., 2017; W. Y. Kim et al., 2016; Xerri et al., 2008) but clinical trials in this area is low with contradictory results (Chan, Khong, & Kwong, 2016; Kwong et al., 2017; Lesokhin et al., 2016).
TAMs also have high expression of PDL1/2 in almost all cases of ATLL, FL, and DLBCL. PDL1+ TAMs increase the infiltrated Tregs and suppress antitumor response in the tumor microenvironment (Horlad et al., 2016; Webb, Milne, Kroeger, & Nelson, 2016). On the other hand, the overexpression of PDL1 in macrophages and dendritic cells increases the clinical response of these patients to anti-PDL1 treatment (Herbst et al., 2014).
5 MULTIPLE MYELOMA
MM is an incurable plasma cell malignancy that is generally confined to the bone marrow (Hardiman et al., 2017). Although autologous transplantation is a standard treatment for MM, relapse is still one of the major post-transplant problems in this disease (Attal et al., 1996; Child et al., 2003; Martinez-Lopez et al., 2011). Immune escape through inducing the exhaustion has a critical role in relapse of MM after HSCT. CD8+ T cells in MM express exhaustion markers, including PD1, CTLA4, CD160, and TIGIT, and their capacity for proliferation, degranulation, and IFN-γ production is drastically reduced (Minnie et al., 2018). In contrast, IL-10 secretion from these cells increases, which is related to the expression of PD1 and TIGIT (Guillerey et al., 2018; Minnie et al., 2018). Like many malignancies, T-cell exhaustion at the site of MM, that is BM, is more severe than the peripheral blood, indicating the role of the tumor microenvironment in inducing the exhausted phenotype and also it has been shown that the extent to which the T cells are exhausted is strongly associated with post-HSCT disease progression. Blocking of inhibitory molecules in the mouse model of MM led to post-transplant disease control (Guillerey et al., 2018; Minnie et al., 2018). It has also been shown that NK cells in patients with MM have a high PD1 expression, which leads them exhausted through the engagement of PDL1 on MM cells. Expectedly, PD1 blockade increases NK cell antitumor function against PDL1+ MM cells (Benson et al., 2010). Notably, the use of Lenalidomide, a conventional medication for MM, reduces the expression of PDL1 on the surface of MM cells and enhances the effect of PD1 blocking (Benson et al., 2010). These findings suggest the possibility of using immune checkpoint blockers to reactivate T/NK cells and prevent relapse in MM (A. Badros et al., 2017).
5.1 Perspective
The promising results in the completed clinical trials (Table 1) and the growing number of ongoing clinical trials (Table 2) emphasize the role of immune exhaustion in the progression of hematologic malignancies as well as indicating the bright future of combining the immune checkpoint blockers with other treatments such as HSCT. One major concern about targeting inhibitory pathways to treat post-transplant relapse is the increased risk of GVHD. The mouse model has shown that PD1/PDL1 blockade improves GVL but also accelerates GVHD (Blazar et al., 2003). Unlike solid tumors where targeting CTLA4 rather than PD1 causes autoimmune-like symptoms such as diarrhea, pneumonia, and thyroid injury, the administration of anti-CTLA4 in early clinical trials of malignancies that relapsed after allogeneic HSCT did not exacerbate GVHD (Bashey et al., 2009; Lühder, Höglund, Allison, Benoist, & Mathis, 1998; H. B. Wang et al., 2001). However, whenever conducting clinical trials and administering immune checkpoint blockers after allogeneic HSCT, the incidence of GVHD indicates drug toxicity in which the dose of the drug or treatment modality should be changed. Future studies should select the appropriate population of patients who respond best. For example, the failure of a clinical trial in the treatment of MCL patients with Nivolumab was due to inadequate or low PD1/PDL1 expression in selected patients (Xu-Monette et al., 2018). Studies should also compare these treatments with current and standard therapies to better judgment of their efficacy. Moreover, because of the high potential of hematologic malignancies to become drug-resistant (Heß, 2017), early initiation of treatment with checkpoint blockers should be rapidly considered in these patients.
ACKNOWLEDGMENT
The authors would like to thank all members of the Hematopoietic Stem Cell Research Center.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
A. H. designed, supervised, evaluated, and approved the manuscript. S.P. design the outline and clinically interpreted the opinions. M. H. K. searched the literature, discussed the immunological aspects, wrote the manuscript, prepared the figure, and participated in tables preparation. E. R. and R. C. evaluated and discussed the hematologic aspects. M. G. searched clinical trials and prepared the tables. All authors approved the final version of the manuscript.




