NLRC3 inhibits PDGF-induced PASMCs proliferation via PI3K-mTOR pathway
Abstract
Few studies about nucleotide-oligomerization domain-like receptor subfamily C3 (NLRC3) in PASMCs have been conducted. This research aimed to investigate the role of NLRC3 on platelet-derived growth factor (PDGF)-induced proliferation of pulmonary artery smooth muscle cells (PASMCs) and its underlying mechanism. We found that the proliferation of PASMCs stimulated with PDGF decreased when phosphoinositide 3-kinase (PI3K) or mammalian target of rapamycin (mTOR) inhibitors pretreatment. Overexpression of NLRC3 inhibited the proliferation of PASMCs and the phosphorylation of PI3K and mTOR while knocking down NLRC3 reversed this effect. Targeted to PI3K or mTOR can also reverse the effect of NLRC3. Activation of PI3K increased the phosphorylation of mTOR while inhibition of PI3K reduced it. Our data suggest that PDGF can induce abnormal proliferation of PASMCs, and NLRC3 suppresses activation of the PI3K-mTOR signaling thus inhibits PASMCs proliferation. These findings unveiled the effect of NLRC3 as an inhibitor of the PI3K-mTOR pathway mediating protection against PASMCs proliferation.
Abbreviations
-
- ANOVA
-
- one-way analysis of variance
-
- BSA
-
- bovine serum albumin
-
- CCK-8
-
- Cell Counting Kit-8
-
- DMEM
-
- Dulbecco's modified Eagle's medium
-
- FBS
-
- fetal bovine serum
-
- GFP
-
- green fluorescent protein
-
- LV-NLRC3
-
- lentivirus NLRC3
-
- LVCON307
-
- negative control lentivirus
-
- mTOR
-
- mammalian target of rapamycin
-
- NLR
-
- nucleotide-binding domain and leucine-rich repeats
-
- NLRC3
-
- nucleotide-oligomerization domain (NOD)-like receptor subfamily C3
-
- NOD
-
- nucleotide-oligomerization domain
-
- PASMCs
-
- pulmonary arterial smooth muscle cells
-
- PDGF
-
- platelet-derived growth factor-BB
-
- PH
-
- pulmonary hypertension
-
- PI3K
-
- phosphoinositide 3-kinase
-
- RT-qPCR
-
- real-time quantitative polymerase chain reaction
-
- RVSP
-
- right ventricular systolic pressure
-
- SMCs
-
- smooth muscle cells
-
- TU
-
- transducing units
1 BACKGROUND
Pulmonary hypertension (PH) is a refractory disease with poor prognosis. Pulmonary vascular remodeling, which is characterized by thickening of the vascular wall, is considered to play a key role in the progress of PH (Leopold & Maron, 2016; Thenappan, Ormiston, Ryan, & Archer, 2018; Tuder, 2017). Abnormal proliferation of pulmonary artery smooth muscle cells (PASMCs) leads to pulmonary vascular stenosis or occlusion, which is an important mechanism of pulmonary vascular remodeling (Kwapiszewska et al., 2012; Satoh et al., 2018; Tajsic & Morrell, 2011; Zurlo et al., 2018). The mechanism of these effects is not yet fully understood.
Phosphoinositide 3-kinase (PI3K) is a serine/threonine-protein kinase that can be activated by many growth factors and cytokines. Most malignant diseases are closely related to abnormal PI3K signals, including cardiovascular diseases and tumors (Ghigo, Laffargue, Li, & Hirsch, 2017). Many studies have confirmed the abnormal expression of PI3K in various human cancer tissues (Engelman, 2009; Fruman & Rommel, 2014; Liu, Cheng, Roberts, & Zhao, 2009). Histological examination showed that in PH specimens, pulmonary vascular remodeling is based on the proliferation and migration of PASMCs which is caused by disorders of PI3K signals (Zha et al., 2019). In vivo, inhibition of PI3K/mammalian target of rapamycin (mTOR) pathway can negatively regulate the proliferation and migration of smooth muscle cells (SMCs), thereby suppressing vascular remodeling and inhibiting the occurrence and progression of PAH (Shah et al., 2015; Tang et al., 2018; ten Freyhaus et al., 2011; Yuan et al., 2013). It has been shown that platelet-derived growth factor (PDGF) stimulated PASMCs proliferation was related to PI3K activation (Song et al., 2016). However, the exact relationship between the upstream and downstream targets of this pathway and the proliferation of PASMCs remains unclear.
The nucleotide-oligomerization domain (NOD)-like receptor subfamily C3 (NLRC3) is a newly discovered member of the NLR family that has not been fully understood. NLRC3 is located in the cytoplasm and contains a nucleotide-binding domain encoded by one large exon and 14 leucine-rich repeat sequences encoded by several exons. The N-terminal does not meet any current domain definition. It has been proved that NLRC3 can regulate many pathophysiological processes, such as substrate metabolism, protein synthesis, cell proliferation, and apoptosis (Conti et al., 2005; Karki et al., 2016). The analysis of human colorectal cancer data showed that the expression of NLRC3 gene in colorectal cancer tissues was significantly lower than that in healthy tissues. Colitis-related colorectal neoplasms were induced in mice by intraperitoneal injection of azomethane for 80 days. The expression of NLRC3 in tumors was significantly lower than that in nontumors. Immunohistochemical results showed that the numbers of Ki67 and proliferating cell nuclear antigen-positive cells in an intestinal recess of NLRC3−/− mice increased significantly. Further studies showed that the proliferation of colon cells overexpressing NLRC3 was significantly reduced compared with the control cells. Primary NLRC3−/− fibroblasts proliferate faster than wild-type fibroblasts (Karki et al., 2016; Leavy, 2017).
Studies have shown that NLRC3 can downregulate the expression of mTOR thus inhibit the proliferation of colorectal cancer cells (Karki, Malireddi, Zhu, & Kanneganti, 2017). Intestinal cells lacking NLRC3 could not inhibit the activation of PI3K-mTOR signals, therefore, they could not inhibit cell proliferation and stem cell-derived organoid formation. Our previous study has concluded that compared to the control group, serum NLRC3 concentration in PH patients was significantly decreased (Zha et al., 2018).
Based on the theory above, we speculate that NLRC3 may inhibit the proliferation of PASMCs via inhibiting the activation of PI3K-mTOR signals. NLRC3 is a newly discovered member of the NOD family. There are few studies of NLRC3 in PASMCs have been conducted. PDGF, which is a classical stimulator, can promote the abnormal proliferation of PASMCs. In this study, we first evaluate the expression of NLRC3 and activation of PI3K/mTOR pathway in PASMCs exposed to PDGF, and then examined the effects of NLRC3 on PDGF-induced proliferation in PASMCs, further explored its mechanisms.
2 MATERIAL AND METHODS
2.1 Cell culture and experiments
Male Sprague-Dawley rats aged 6–8 weeks were euthanized with pentobarbital sodium (100 mg/kg i.p.). The distal pulmonary arteries were rapidly removed after the corneal reflex disappears. The middle layer of arteries was separated, and the PASMCs were extracted by enzymatic digestion. PASMCs were cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco, Grand Island, NY), supplemented by 1% streptomycin, 1% penicillin, and 20% fetal bovine serum (GiBCO) in 5% CO2 incubator. Every 2–3 days the culture medium was replaced.
Morphologic appearance by phase-contrast microscopy and immunofluorescence with an anti-α-smooth muscle cell antibody was used to identify PASMCs. PASMCs between Passages 3 and 8 from different isolations were used to perform independent replicate experiments. PDGF (96-100-14b-10, purity >95%, supplied by Peprotech) and 740YP (CAS 1236188-16-1, purity greater than 95%, supplied by Selleck) were dissolved in phosphate-buffered saline (PBS). All the rats received humane care in compliance with the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health. The procedure was approved by the Institutional Animal Care and Use Committee of Xiangya Hospital, Central South University (Hunan, China). The approval reference number is SYXK (Xiang) 2016-0002. T3, 2-(4-morpholinyl)-8-phenyl-chromone (LY294002), and sodium pentobarbital were purchased from Sigma-Aldrich (St. Louis, MO). PDGF (Peprotech; PBS as a vehicle) was applied to stimulate the proliferation of PASMCs. 740YP (Selleck; PBS as a vehicle) was used as a PI3K agonist and Ly294002 (Sigma; dimethyl sulfoxide [DMSO] as a vehicle) as a PI3K inhibitor. 3BDO was applied as an mTOR agonist while Rapamycin (Cell Signaling Technology; DMSO as a vehicle) as an mTOR inhibitor.
2.2 Lentivirus transfection
GeneChem Biomedical Co. Ltd. (Shanghai, China) was commissioned to construct and purify green fluorescent protein (GFP) tagged-lentivirus (LV-NLRC3 and negative control lentivirus (LVCON307). The titers were 5E+8 and 6E+8 transducing units (TU)/ml. Transfection was achieved using a Transfection Kit (GeneChem Biomedical Co. Ltd.) according to the manufacturer's protocol. LV-NLRC3 and LVCON307 were dissolved in PBS. Establish a stable transfected cell line is based on our previous study.
2.3 Small interfering RNA (siRNA) transfection
GenePharma Co. Ltd. (Shanghai, China) was commissioned to construct and purify the NLRC3 sequence-specific or nontargeting siRNA (Si-NLRC3 or Si-Neg). LipofectamineTM 2000 reagent (Invitrogen, Carlsbad, CA) was applied for the transfection. PASMCs between Passages 3 and 8 were cultured until they reached 30–50% confluence. At first, diluted the siRNA and LipofectamineTM 2000 in DMEM, respectively, and then incubated them at 37℃ for 5 min. Second, mixed siRNA with LipofectamineTM 2000 and then incubated it at 37℃ for 20 min. At last, added the mixture of siRNA and LipofectamineTM 2000 into the culture medium of PASMCs. Then, PASMCs were cultured for 48 hr at 37℃ (in 5% CO2 incubator). The transfection effect of siRNA was determined using western blotting.
2.4 Cell Counting Kit-8 (CCK-8) assay
PASMCs proliferation was measured by CCK-8 assay (B34302; Biotool) as our previous study described. Briefly, PASMCs (3–10 × 103 per well) were seeded and cultured in 96-well culture plates. After that, PASMCs were exposed to diverse culture conditions and subsequently treated with 10 μl CCK-8 solution for 3 hr. The absorbance of the plate was measured at a dual-wavelength of 450 nm by a spectrophotometer microplate reader. At last, a multimode detector (DTX880; Beckman Coulter, Danvers, MA) was used to perform the colorimetric analysis.
2.5 Immunofluorescent staining
PASMCs seeded on glass coverslips were gently rinsed with PBS three times and then fixed with 4% paraformaldehyde for 15–30 min at 37℃. After that, PASMCs were gently rinsed three times with PBS, and subsequently blocked and permeabilized in 0.3% Triton X-100% and 2% bovine serum albumin for 1 hr at 37℃. Then PASMCs were incubated with primary antibody (p-mTOR; #2971; Cell Signaling Technology) overnight at 4℃. Next, PASMCs were gently rinsed three times with PBS and incubated with secondary antibody (1:200; Vector) for 2 hr at 37℃. And then rinsed PASMCs with PBS again, incubated PASMCs with streptavidin–horseradish peroxidase (Alexa-Fluor-594-conjugated streptavidin; Jackson, Immunoresearch) in the dark field away from light for 1 hr at 37℃. Finally, PASMCs were rinsed and incubated with 4′,6-diamidino-2-phenylindole dihydrochloride (5 μg/ml; Sigma-Aldrich) in the dark field away from light for 3–5 min at 37℃. Rinsed the PASMCs with DDW and acquired images using a fluorescence microscope (Eclipse; Nikon, Japan).
2.6 Real-time quantitative polymerase chain reaction (RT-qPCR)
TRIzol reagent (Invitrogen) was used to extract total RNA. Calculated the volume and quality of the sample RNA, and then performed reverse transcription reactions using a PrimeScriptTM RT Reagent Kit (TaKaRa, Shiga, Japan). For real-time PCR amplification, complementary DNAs were amplified using SYBRV R Premix Ex TaqTM II (Tli RNaseH Plus; TaKaRa) and 0.4 mmol each primer pair in an ABI 7500 real-time PCR system (Applied Biosciences, Foster City, CA). RT-qPCR was achieved according to the manufacturer's protocol. Data quantitation was calculated using the 2−ΔΔCt method, with glyceraldehyde 3-phosphate dehydrogenase gene expression as an internal control. The primer sequences were as follows: NLRC3 (forward: 5′-CTGGGAAGGGCAGTCAAG; reverse: 5′-TGCCTCTGTATCCTTGAGTC).
2.7 The western blotting
PASMCs were lysed in radioimmunoprecipitation assay buffer containing 1 mmol/L phenylmethanesulfonyl fluoride (Beyotime, Jiangsu, China). BCA Protein Assay kits (Beyotime) were used to measure protein concentrations. Quantitative proteins were separated by 5–15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels (Beyotime) and transferred to polyvinylidene difluoride membranes with 0.2-µm or 0.45-µm pores (Millipore). Then membranes were rinsed with tween in tris buffer saline (TBST) three times and blocked in 5% nonfat dry milk for 2 hr at room temperature. Rinsed with TBST three times and incubated the membranes with primary antibodies overnight at 4℃. After that, the membranes were washed and incubated with secondary antibodies for 2 hr at 37℃, and treated with Super Signal West Pico Chemiluminescent Substrate (Pierce, Rockford, IL) for blot detection. Gel documentation system (Bio-Rad, Hercules, CA) was used to analyze the protein bands. Primary antibodies were used as follows: NLRC3 (1:1,000; #PAB19298; Abnova); β-actin (1:2,000; #8457; Cell Signaling), Tyr199-phosphorylated PI3K (#4228S; Cell Signaling Technology), t-mTOR (#2972; Cell Signaling Technology), p-mTOR (#2971; Cell Signaling Technology).
2.8 Statistical analysis
All statistical analysis was conducted with the GraphPad Prism 6.0 (GraphPad, Inc., San Diego, CA). For each determination, the experiments were repeated at least three times independently. Data were presented as the mean ± standard deviation. Unpaired Student t tests were used for comparisons of parameters between two groups. One-way analysis of variance was performed to compare the parameters among three groups. Comparisons of multiple groups were conducted with Student–Newman–Keuls tests. The p values <.05 were regarded as statistically significant.
3 RESULTS
3.1 The optimum concentration and time that PDGF stimulates PASMCs proliferation
We first explored the optimal exposure concentration and time to understand the effect of PDGF on the proliferation of PASMCs. First, PASMCs were stimulated with PDGF at different concentrations (0–60 ng/ml) for 24 hr, and PASMCs proliferation was measured with CCK-8 test. The results showed that PDGF stimulated the proliferation of PASMCs dose dependently in the range of 0–30 ng/ml. However, the proliferation of PASMCs decreased gradually after 30 ng/ml, suggesting that drug cytotoxicity of PDGF appeared. Therefore, according to the analysis of the results, we believed that the concentration of 30 ng/ml was the optimum stimulating concentration (Figure 1a).
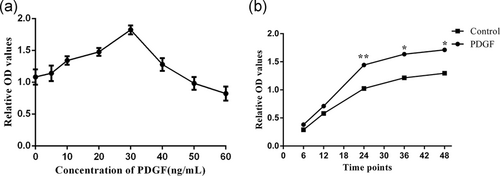
Second, we incubated PASMCs with PDGF at a concentration of 30 ng/ml to find out the appropriate stimulation time. We observed the results at different time points (0, 6, 12, 24, 36, and 48 hr). Compared with the control group, PASMCs in PDGF group proliferated significantly from 12 to 24 hr. The proliferation rate of both groups decreased after 24 hr. However the proliferation rate of PASMCs in PDGF group was still higher than that of control group (p < .01, Figure 1b). These results suggested that stimulating PASMCs with PDGF by the concentration of 30 ng/ml for 24 hr is an effectively intervention.
3.2 PDGF-induced proliferation of PASMCs is associated with activation of PI3K-mTOR pathway
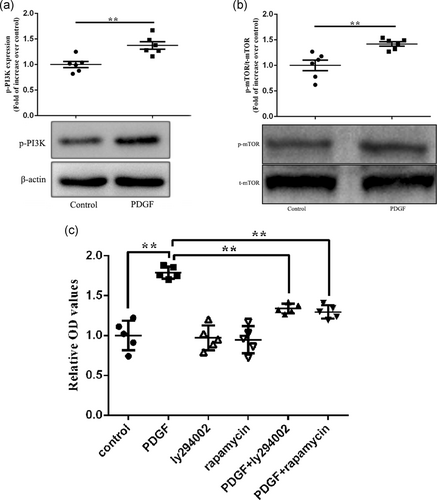
To investigate the mechanisms that PDGF-induced proliferation of PASMCs, the protein expressions of p-PI3K, p-mTOR, and t-mTOR were checked. The expression of p-PI3K, p-mTOR, and t-mTOR were found in both groups. It was found that the expression of p-PI3K and p-mTOR in PDGF group was significantly higher than that in control group (p < .01, Figure 2a,b). That was to say, the phosphorylation of PI3K and mTOR of PASMCs in PDGF group increased significantly. PI3K and mTOR were frequently activated. In PASMCs stimulated by PDGF, we observed not only obvious proliferation, but also increased phosphorylation levels of PI3K and mTOR. Is this a concomitant phenomenon or a direct correlation? We then conducted an intervention study with Ly294002 (inhibited the activation of PI3K) and rapamycin (inhibited the activation of mTOR). Selected appropriate drug concentrations based on our preliminary experimental results. PASMCs were randomly divided into six groups (control group, PDGF group, Ly294002 group, rapamycin group, PDGF + Ly294002 group, and PDGF + rapamycin group). PASMCs in PDGF group, Ly294002 and rapamycin groups were treated with PDGF, Ly294002, or rapamycin alone. PASMCs in PDGF + Ly294002 group or PDGF + rapamycin group were pretreated with Ly294002 or rapamycin for 2 hr, and then were exposed to PDGF for 24 hr (PASMCs in control group were added with the same amount of PBS). The results showed that Ly294002 or rapamycin alone had no significant effect on cell proliferation. However, PDGF induced abnormal PASMCs proliferation, and PASMCs proliferation was inhibited to some extent after PI3K inhibitor was given. Similarly, the proliferation of PASMCs could be inhibited by mTOR inhibitors (Figure 2c). This study confirms that PDGF-induced PASMCs proliferation is directly related to activation of PI3K and mTOR.
3.3 NLRC3 mediates the expression of PI3K and mTOR in PASMCs exposed to PDGF
To investigate the role of NLRC3 in PDGF-induced proliferation of PASMCs, overexpression of NLRC3 was conducted in this research. We constructed lentivirus tagged with GFP and observed PASMCs directly under microscope. No green fluorescence was observed in PASMCs (Control group) which were not transfected with lentivirus. Under fluorescence microscopy, PASMCs were spindle-shaped and well adherent-grew with GFP fluorescence in both groups transfected with LVCON307 or LV-NLRC3 (Figure 3a). To further confirm the results, we performed RT-qPCR and western blot (WB) analysis. RT-qPCR and WB showed that the expressions of NLRC3 messenger RNA (mRNA) and protein were positive in PASMCs of control group, and the expressions of NLRC3 mRNA and protein in PASMCs of LVCON307 group did not change significantly compared to that of control group. However, the expressions of NLRC3 mRNA and protein in PASMCs of LV-NLRC3 group were upregulated significantly compared to that of control group (p < .01, Figure 3b,c). These results confirmed the successful transfection of lentivirus.
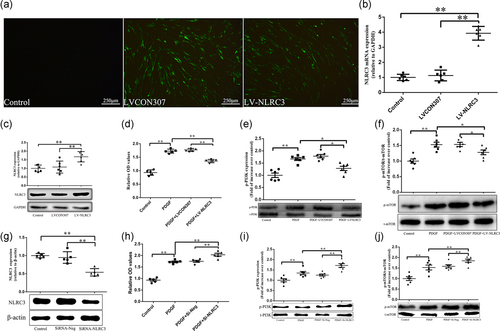
To understand the effect of NLRC3 on the proliferation of PASMCs, we exposed PASMCs transfected with lentivirus to PDGF. Through the CCK-8 experiment, we found that the proliferation of PASMCs exposure to PDGF (PDGF group) was more obvious than that in the control group. PASMCs in PDGF + LVCON307 group also proliferated significantly when exposed to PDGF. There was no significant difference between PDGF group and PDGF + LVCON307 group. However, the proliferation of PASMCs in PDGF + LV-NLRC3 group was lower than that in PDGF group and PDGF + LVCON307 group (p < .01, Figure 3d).
NLRC3 has been supposed to inhibit the proliferation of tumor cells by negatively regulating PI3K pathway. In this study, the phosphorylation of PI3K and mTOR after NLRC3 intervention was examined. The results showed that the protein expressions of p-PI3K and p-mTOR were significantly upregulated after PDGF-BB stimulation (in PDGF group and PDGF + LVCON307 group). The protein expressions of p-PI3K and p-mTOR were no significant difference between PDGF group and PDGF + LVCON307 group. After overexpression of NLRC3, the protein expressions of p-PI3K and p-mTOR in PDGF + LV-NLRC3 group were lower than that in PDGF group and PDGF + LVCON307 group (Figure 3e,f).
To further confirm it, next, we constructed the NLRC3-specific siRNA to understand the role of NLRC3 in PDGF-induced PASMCs proliferation and its relationship with PI3K and mTOR. As shown in Figure 3g, transfection of siRNA-NLRC3 reduced the NLRC3 level to 46.05% of control in PASMCs. PASMCs in PDGF + siRNA-Neg group proliferated significantly when exposed to PDGF. The proliferation of PASMCs between PDGF group and PDGF + siRNA-Neg group was no significant difference. However, the proliferation of PASMCs in PDGF + siRNA-NLRC3 group was higher than that in PDGF group and PDGF + siRNA-Neg group (p < .01, Figure 3h).
The protein expressions of p-PI3K and p-mTOR were upregulated in PDGF and PDGF + siRNA-Neg group compared to control group. However, when silencing NLRC3, the protein expressions of p-PI3K and p-mTOR in PDGF + siRNA-NLRC3 group were higher than that in PDGF and PDGF + siRNA-Neg group (p < .01, Figure 3i,j).
3.4 The effect of NLRC3 on the proliferation of PASMCs stimulated by PDGF is related to PI3K and mTOR
We have found that NLRC3 affects PDGF-induced PASMCs proliferation and the expression of PI3K and mTOR. To clarify the exact relationship between NLRC3 and PI3K/mTOR in PDGF-induced proliferation of PASMCs, different interventions were conducted on PASMCs (PBS, PDGF, PDGF + LV-NLRC3, PDGF + LV-NLRC3 + 740YP, and PDGF + LV-NLRC3 + 3BDO). 740YP is a PI3K phosphor-peptide activator with cell permeability. 3BDO is a butyrolactone derivative that can activate mTOR signaling pathway. We found that overexpression of NLRC3 inhibited PDGF-induced proliferation of PASMCs. Both PI3K activator 740YP and mTOR activator 3BDO reversed this effect. As shown in the figure, PDGF stimulation increased the optical density (OD) value of PASMCs from 0.4 (in control group) to 1.2. When PASMCs overexpressed NLRC3 and then stimulated with PDGF, the OD value decreased to 0.7. Exposure of PASMCs overexpressing NLRC3 to PDGF and 740YP increased OD values to 1.0. Exposure of PASMCs overexpressing NLRC3 to PDGF and 3BDO, the OD value increased to 1.1. Activation of PI3K or mTOR could affect the inhibitory effect of NLRC3 on PASMCs proliferation stimulated by PDGF (Figure 4a).
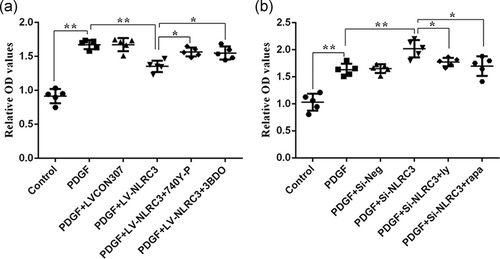
To further demonstrate our results, we knocked down NLRC3 from PASMCs and then intervened with PI3K and mTOR inhibitors. As shown in the figure, after NLRC3 knocking down, the OD value of PASMCs stimulated with PDGF in PDGF + siRNA-NLRC3 group increased from 1.8 to 2.2. Exposure of PASMCs knockdown NLRC3 to PDGF and PI3K inhibitors made OD value decreased to 1.6. After exposing NLRC3 knockdown PASMCs to PDGF and mTOR inhibitors, OD value decreased to 1.5 (Figure 4b).
3.5 NLRC3 inhibits PASMCs proliferation through PI3K-mTOR pathway, mTOR is a downstream target of PI3K
Previous results confirmed that NLRC3 affects the proliferation of PASMCs stimulated by PDGF and is related to PI3K and mTOR. To clarify the upstream and downstream relationship between PI3K and mTOR, we detected the phosphorylation of mTOR after PI3K intervention. We found that overexpression of NLRC3 can downregulate the increase of p-mTOR in PDGF-induced PASMCs, and PI3K agonists can reverse this effect. The expression of p-mTOR in PASMCs was significantly upregulated by PDGF stimulation compare to that in control group. After overexpression of NLRC3, the expression of p-mTOR in PDGF + LV-NLRC3 group was lower than that in PDGF group (p < .01). However, after exposure of PASMCs overexpressing NLRC3 to PDGF and 740YP, the downward trend of p-mTOR expression in PDGF + LV-NLRC3 + 740YP group was reversed (Figure 5b).
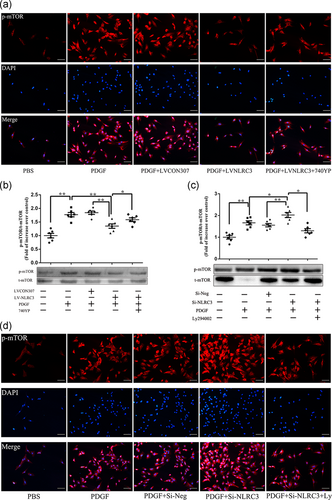
Immunofluorescence detection also found the same trend. In control group, the red fluorescence of p-mTOR was sparse and dim. After PDGF stimulation, the red fluorescence intensity increased significantly. When overexpression of NLRC3, the red fluorescence of p-mTOR in PDGF + LV-NLRC3 group decreased than that in PDGF group, but after 740YP stimulation, the red fluorescence increased and became bright in PDGF and 740YP. These data confirmed that NLRC3 inhibits the activation of PI3K and mTOR in PASMCs stimulated by PDGF, and activation of PI3K could upregulate p-mTOR (Figure 5a).
Next, we knocked down NLRC3 on PASMCs and intervened with inhibitors of PI3K and mTOR. We found that the expression of p-mTOR in PASMCs stimulated by PDGF after NLRC3 knocking down was higher than that in PDGF group (p < .05). When PASMCs knocked down NLRC3 were exposed to PDGF and PI3K inhibitors in PDGF + siRNA-NLRC3 + PI3K inhibitors, the upward trend of p-mTOR expression was reversed (p < .05, Figure 5c).
Through Immunofluorescence, we found that the red fluorescence of p-mTOR increased and brightened after knocking down NLRC3 in PDGF + Si-NLRC3 group compared to that in PDGF group, but darkened and decreased after adding PI3K inhibitors in PDGF + siRNA-NLRC3 + PI3K inhibitors compared to that in PDGF + siRNA-NLRC3 group (Figure 5d).
4 DISCUSSION
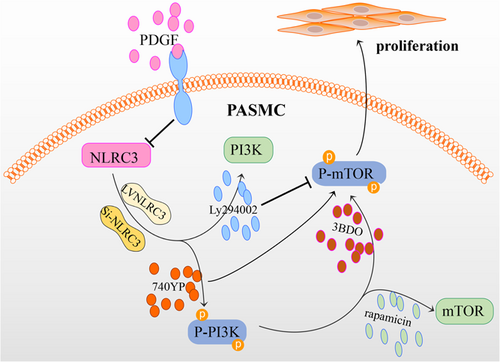
According to this study, NLRC3 downregulated and PASMCs proliferated significantly after PDGF stimulated. Therefore, we speculated that proliferation of PASMCs after PDGF stimulation might be related to the downregulation of NLRC3. we conclude that PDGF stimulates the proliferation of PASMCs via activating PI3K-mTOR pathway, which is associated with the downregulation of NLRC3. NLRC3 inhibits the activation of PI3K-mTOR pathways, thereby inhibiting the proliferation of PASMCs induced by PDGF (Figure 6). This research provides important insights into the PASMCs proliferation stimulated by PDGF and present a new idea that NLRC3 may prevent PH by inhibiting PASMCs proliferation.
The abnormal proliferation of PASMCs leads to the thickness of the medial membrane, the decrease of lumen diameter and even occlusion of pulmonary arterioles (Dai et al., 2018b; Guignabert & Dorfmuller, 2017; Shi et al., 2018). PASMCs proliferation has been proved to be an important pathological feature of PH (Bourgeois et al., 2019; Cheng, Wang, Li, & He, 2017; Weiss et al., 2019; Zucker et al., 2019). Pathological examination revealed that the pulmonary arterioles of patients with idiopathic PH was markedly thickened, which was mainly due to the proliferation of PASMCs (Thompson & Lawrie, 2017). The increase of pulmonary artery pressure and pulmonary vascular resistance was significantly correlated with the increase of the media thickness in pulmonary arterioles (Cheng et al., 2017; McMurtry et al., 2004; Paulin et al., 2014). In vivo, researches have shown that inhibiting the proliferation of PASMCs can decrease pulmonary artery pressure and pulmonary vascular resistance, thus delaying or even reversing pulmonary vascular remodeling in PH.
As one member of the PDGF family, numerous studies have shown that PDGF-BB and its downstream pathways are dysfunctional in PAH. PDGF-BB can stimulate the proliferation of PASMCs, promote contraction of pulmonary vascular smooth muscle, and regulate pathophysiological process in pulmonary vascular remodeling (Dai et al., 2018a; Kovacs et al., 2019). The expression of PDGF in pulmonary arterioles is significantly increased in MCT or hypoxia-induced PAH animal models. Similarly, the plasma PDGF level in PAH patients increases significantly, and the upregulation of PDGF-BB is also observed in pulmonary tissues and pulmonary arterioles in PH patients. Animal experiments have shown that imatinib, an inhibitor of PDGF, can inhibit PASMCs proliferation, reduce pulmonary artery pressure, decrease pulmonary vascular remodeling and alleviate right ventricular hypertrophy, thereby weakening or even reversing the process of PH (Thomas, Ciuclan, Hussey, & Press, 2013). Our results showed that PASMCs proliferated significantly after 24 hr stimulation with PDGF-BB at the concentration of 30 ng/ml (Figure 1).
It has been corroborated that in various types of tumors, the PI3K/mTOR pathway is abnormally activated, leading to uncontrolled cell proliferation. The proliferation of many kinds of cells, including cancer and normal cells, is related to the activation of mTOR. The PI3K/mTOR signaling pathway has been confirmed by many investigators to play a key role in the regulation of PASMCs proliferation and the progression of PH. It is reported that the proliferation and apoptosis of PASMCs are related to PI3K/mTOR pathway in PH. Previous studies have pointed out that PI3K and its downstream kinase are significantly activated in PASMCs stimulated by PDGF-BB. Inhibition of PI3K/mTOR pathway can inhibit PDGF and serotonin-induced proliferation of vascular SMCs. PDGF can cause excessive proliferation of PASMCs. Rapamycin or Akt inhibitors can block Akt/mTOR signals and attenuate the abnormal proliferation of PASMCs (Houssaini et al., 2013). Some investigators found that Knockout of smooth muscle-specific mTOR in mice and then exposed these mice to chronic hypoxia condition, the hemodynamic measurement such as RVSP and Fulton index were significantly lower, and histological analysis such as pulmonary artery wall thickness was significantly attenuated than that in wide mice (Shah et al., 2015; Tang et al., 2018). Our study also observed significant activation of PI3K/mTOR signals in PASMCs stimulated by PDGF-BB (Figure 2).
The decrease of NLRC3 is related to the proliferation of different kinds of cells. Inhibiting the proliferation of tumor cells by NLRC3 is associated with PI3K/mTOR signal suppression. LY294002, an inhibitor of PI3K, inhibits the ability of intestinal stem cells to proliferate into organoid-like organs (Karki et al., 2016; Karki et al., 2017). In our study, we find that after PDGF-BB stimulating, the expression of NLRC3 is downregulated in PASMCs significantly. Overexpression of NLRC3 significantly decrease PI3K/mTOR expression in PDGF-BB stimulated PASMCs (Figure 3a–e). Knockdown of NLRC3 significantly increase PI3K/mTOR expression in PDGF + siRNA-NLRC3 group compared with PDGF-BB stimulated PASMCs (Figure 3f–i). 740YP (an agonist of PI3K) could increase the phosphorylation of mTOR and promote the proliferation of PASMCs overexpressing NLRC3 to a certain extent, while PI3K inhibitor LY294002 could reverse this trend (Figures 4, 5). Therefore, we speculate that NLRC3/PI3K/mTOR pathway plays a critical role in PASMCs proliferation. Downregulation of NLRC3 induced by PDGF-BB may lead to PI3K activation, phosphorylation of mTOR, and ultimately abnormal proliferation of PASMCs.
Although we have shown that NLRC3 can inhibit the proliferation of PASMCs, our study has some limitations. The first limitation is that we just performed PASMCs isolated from rats. The pathogenesis of PH is still unclear. Apart from PASMCs, it is also related to a variety of other vascular cell types, especially PAEC and fibroblasts are also critical to the pathogenesis of PH (Gao, Chen, & Raj, 2016). We also know that the use of human PASMCs can better avoid racial differences. Meanwhile, if we can take PASMCs from patients with PH for supplementary experiments, the conclusion will be more reliable.
Another limitation is that we only used one stimulator (PDGF) to stimulate cell proliferation. Whether NLRC3 can inhibit the proliferation of PASMCs induced by various factors warrants further study.
This study elucidates the main targets of NLRC3 regulation. Compared with previous studies, NLRC3 inhibits PDGF-induced cell proliferation via PI3K/mTOR pathway. Our current and previous researches both demonstrated that NLRC3 can inhibit PDGF-induced proliferation of PASMCs, which means that NLRC3 may be a new target for PH.
5 CONCLUSIONS
In conclusion, NLRC3 affects the proliferation of PASMCs stimulated by PDGF and is directly related to PI3K-mTOR pathway, mTOR is the direct downstream of PI3K. The mechanism is related to the regulation of PI3K-mediated mTOR phosphorylation by NLRC3 (Figure 6).
ACKNOWLEDGMENTS
This work was funded by the National Natural Science Foundation of China (81570050, 81571382, 81873416) and Major National Science and Technology Projects (2017ZX0930401405).
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
L. H. Z. and Z. X. Y. carried out the study, analyzed the data, and wrote the manuscript. M. Q. Z. and S. L. contributed equally to this work, Z. X. Y. designed the research. Z. X. Y. and J. Z. helped in funding the project. Y. L. T. and S. H. G. performed the research. All authors have reviewed this manuscript and approved it for publication.
ETHICS STATEMENT
All experiments were performed according to the European Communities Council Directive of 24 November 1986 (86/609/EEC). All the experimental protocols with rats have been approved by the institutional ethics committee of Xiangya Hospital, Central South University (Changsha, Hunan, China).
Open Research
DATA AVAILABILITY STATEMENT
All data during this study are available on request.




