Emerging landscape of circular RNAs as biomarkers and pivotal regulators in osteosarcoma
Abstract
Osteosarcoma represents the most prevailing primary bone tumor and the third most common cancer in children and adolescents worldwide. Among noncoding RNAs, circular RNAs (circRNAs) refer to a unique class in the shape of a covalently closed continuous loop with neither 5′ caps nor 3′-polyadenylated tails, which are generated through back-splicing. Recently, with the development of whole-genome and transcriptome sequencing technologies, a growing number of circRNAs have been found aberrantly expressed in multiple diseases, including osteosarcoma. circRNA are capable of various biological functions including miRNA sponge, mediating alternatives, regulating genes at posttranscriptional levels, and interacting with proteins, indicating a pivotal role of circRNA in cancer initiation, progression, chemoresistance, and immune response. Moreover, circRNAs have been thrust into the spotlight as potential biomarkers and therapeutic targets in osteosarcoma. Herein, we briefly summarize the origin and biogenesis of circRNA with current knowledge of circRNA in tumorigenesis of osteosarcoma, aiming to elucidate the specific role and clinical implication of circRNAs in osteosarcoma.
Abbreviations
-
- A-to-I
-
- adenosine-to-inosine
-
- ABCB1
-
- ATP binding cassette subfamily B member 1
-
- ADAR1
-
- adenosine deaminase acting on RNA 1
-
- Akt
-
- protein kinase B
-
- ALP
-
- alkaline phosphatase
-
- AUC
-
- area under curve
-
- c-FLIP
-
- cellular FLICE-inhibitory protein
-
- CCNE1
-
- cyclin E1
-
- CDK
-
- cyclin-dependent kinase
-
- ceRNAs
-
- competing endogenous RNAs
-
- CHI3L1
-
- chitinase 3-like 1
-
- circRNA
-
- circular RNA
-
- ciRNAs
-
- circular intronic RNAs
-
- CIs
-
- confidence intervals
-
- CRC
-
- colorectal cancer
-
- CREB3
-
- cAMP responsive element binding protein 3
-
- DFS
-
- disease-free survival
-
- DM
-
- distant metastasis
-
- ecirRNAs
-
- exonic circRNAs
-
- ECM
-
- extracellular matrix
-
- EGFR
-
- epidermal growth factor receptor
-
- EIciRNAs
-
- exon-intron circRNAs
-
- eIF4A3
-
- eukaryotic initiation factor 4A3
-
- EMT
-
- epithelial–mesenchymal transition
-
- ESCC
-
- esophageal squamous cell carcinoma
-
- EVs
-
- extracellular vesicles
-
- EZH2
-
- enhancer of zeste 2 polycomb repressive complex 2 subunit
-
- FOXC2
-
- forkhead box C2
-
- FOXF1
-
- forkhead box F1
-
- GBM
-
- glioblastoma multiforme
-
- HaCaT
-
- human keratinocyte
-
- HCC
-
- hepatocellular carcinoma
-
- HNSCC
-
- neck squamous cell carcinoma
-
- HOXB2
-
- homeobox B2
-
- HRs
-
- hazard ratios
-
- IL6R
-
- interleukin 6 receptor
-
- K-M
-
- Kaplan-Meier
-
- KLF2
-
- kruppel like factor 2
-
- LDH
-
- lactate dehydrogenase
-
- LEF1
-
- lymphoid enhancer binding factor 1
-
- lncRNAs
-
- long oncoding RNAs
-
- LNM
-
- lymph node metastasis
-
- LSD1
-
- LSD1 zinc finger family protein
-
- MBL
-
- musclblind
-
- MCL1
-
- myeloid cell leukaemia 1
-
- MDM2
-
- MDM2 proto-oncogene
-
- MDR1
-
- multidrug resistance protein 1
-
- miRNA
-
- microRNA
-
- MRE
-
- miRNA response elements
-
- MSCs
-
- mesenchymal stem cells
-
- mTOR
-
- mammalian target of rapamycin
-
- ncRNAs
-
- noncoding RNAs
-
- NSCLC
-
- non-small cell lung cancer
-
- OS
-
- overall survival
-
- PDAC
-
- pancreatic ductal adenocarcinoma
-
- PFS
-
- progression-free survival
-
- PI3K
-
- phosphatidylinositol 3-kinase
-
- PI3KCD
-
- phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit delta
-
- Pol II
-
- polymerase II
-
- PTEN
-
- phosphatase and tensin homolog
-
- QKI
-
- quaking
-
- qRT-PCR
-
- quantitative reverse transcription polymerase chain reaction
-
- RAF1
-
- Raf-1 proto-oncogene, serine/threonine kinase
-
- RBPs
-
- RNA-binding proteins
-
- RNA-seq
-
- RNA-sequencing
-
- ROC
-
- receiver operating characteristic
-
- RUVBL1
-
- RuvB like AAA ATPase 1
-
- siRNAs
-
- small interfering RNAs
-
- STAT3
-
- signal transducer and activator of transcription 3
-
- TGFB2
-
- transforming growth factor beta 2
-
- TNBC
-
- triple-negative breast cancer
-
- TNM
-
- tumor-node-metastasis
-
- U1 snRNP
-
- U1 small nuclear ribonucleoprotein (U1 snRNP)
-
- YAP1
-
- Yes1 associated transcriptional regulator
-
- YB1
-
- Y-box binding protein 1
-
- YTHDF2
-
- YTH N6-methyladenosine RNA binding protein 2
1 INTRODUCTION
Osteosarcoma is the most prevalent primary malignant bone tumor among children, adolescents, and young adults (Saraf, Fenger, & Roberts, 2018). It is characterized by high aggressiveness with rapid progression and great distant metastatic potential (S. Q. Li, Tu, et al., 2019; Z. Li, Dou, Liu, & He, 2017; Otoukesh, Boddouhi, Moghtadaei, Kaghazian, & Kaghazian, 2018; X. Ren, Tu, Tang, Ma, & Li, 2018). Currently, due to insufficiency in early-stage diagnosis and effective therapeutic approaches, the 5-year survival and prognosis for osteosarcoma patients remain largely unimproved, especially for patients with multi-drug resistance, recurrence, or lung metastasis (C. Wang, Ren, Zhao, Wang, & Wang, 2018). The disappointing results in multi-centered collaborative trials motivated researchers' efforts to focus more on the fundamental pathophysiology of osteosarcoma (Saraf et al., 2018). Therefore, there is an urgent need to identify novel clinically relevant prognostic biomarkers and explore the underlying molecular mechanisms in carcinogenesis to develop more effective strategies for the treatment of osteosarcoma (G. J. S. Tan, Gerrand, & Rankin, 2019; Tu, He, Chen, & Li, 2019).
Noncoding RNAs (ncRNAs) are defined as functional RNAs which are destined to be transcribed, instead being translated. As over 95% of the human genome belongs to ncRNAs, they encompass manifold families, such as microRNA (miRNA), long noncoding RNAs (lncRNAs), small interfering RNAs (siRNAs/RNAi) and circular RNAs (circRNAs), to name a few (Consortium, 2012). Among them, circRNAs are a novel and fascinating class. Different from linear RNAs, circRNAs shape a covalently closed continuous loop with neither 5′ caps nor 3′ polyadenylated tails, which makes them more stable and not readily degraded by endonuclease (L. L. Chen & Yang, 2015). circRNAs were first discovered in RNA viruses and subsequently in the eukaryotes via electron microscopy in 1970s (Capel et al., 1993; Cocquerelle, Mascrez, Hetuin, & Bailleul, 1993; Sanger, Klotz, Riesner, Gross, & Kleinschmidt, 1976). Previously, circRNAs were considered as a “junk molecule” or “splicing noise” in organisms and did not attract much attention among researchers. Recently, due to the rapid advancement and widespread use of reliable high-throughput RNA-sequencing (RNA-seq) technologies and bioinformatics approaches, an important role of circRNAs has been increasingly recognized in gene regulation at the posttranscriptional level. Moreover, circRNAs are widely expressed and highly evolutionarily conserved in mammals. Emerging studies have revealed that circRNAs are capable of various molecular functions in gene regulation, such as interacting with proteins, mediating alternatives, and acting as competing endogenous RNAs (ceRNAs) to bind with miRNAs and regulate target genes.
Recently, circRNAs are being found to function in multiple disorders progression (Memczak et al., 2013; Salzman, Chen, Olsen, Wang, & Brown, 2013), such as aging (Knupp & Miura, 2018), osteoarthritis (H. Z. Li, Lin, Xu, Lin, & Lu, 2018; Z. B. Zhou et al., 2019), and cancer (Cui et al., 2019; Fu, Jiang, Li, Hu, & Guo, 2018; X. Huang, Zhang, et al., 2019; Q. Zhong, Huang, Wei, & Wu, 2019). Of note, circRNAs are reported to exert diverse functions in cancer tumorigenesis (S. Meng, Zhou, et al., 2017), metastasis (Shi et al., 2019; Wei et al., 2019) and even chemoresistance (W. Huang, Yang, et al., 2019). Characteristics including high stability, abundance and tissue-specific expression indicate that circRNAs may serve as ideal biomarkers in multiple cancers for clinical treatment and research (J.-F. Li & Song, 2017). In this review, we briefly summarize the current knowledge regarding biogenesis of circRNA and their clinical implication in osteosarcoma.
2 BIOGENESIS
Unlike canonical splicing, circRNAs are generated through back splicing, which is completed through transcription or after transcription (Iyer et al., 2015; X. Wang et al., 2008). cirRNAs are mainly classified into three categories based on the components (Figure 1): exonic circRNAs (ecirRNAs), exon-intron circRNAs (EIciRNAs), and circular intronic RNAs (ciRNAsf; X. Meng, Li, et al., 2017). Formation of ecircRNAs involves nonsequential back splicing where a downstream splice donor of pre-mRNA covalently links with an upstream splice acceptor. Two models, lariat-driven circularization and intron pairing-driven circularization, are widely recognized for the synthesis of cirRNAs. The former requires close binding of the downstream 5′ splice site and the upstream 3′ splice site to form a lariat intermediate consisting of several exons and introns. Then, the flanking introns are removed through internal splicing completely or incompletely to produce ecirRNAs or ElciRNAs respectively. Differently, the latter involves reverse complementary base-pairing across exon-flanking introns. This process induces “head-to-tail” splicing by promoting a hairpin formation and bringing the 5′ and 3′ termini of an exon into spatial proximity. The 200 bp upstream or downstream back-splicing site usually possesses canonical complementary ALU repeats (Jeck et al., 2013). ciRNA generation depends on conserved motifs containing a 7-nt GU-rich element at the 5′ splicing site and an 11-nt C-rich element at the 3′-branchpoint site following off-branch (Y. Zhang et al., 2013). The resulting sequence circle is covalently looped through a 2′, 5′-phosphodiester bond at the junction site, while the tail stretching from the end of the intron to the branch point is removed to produce stable ciRNAs.
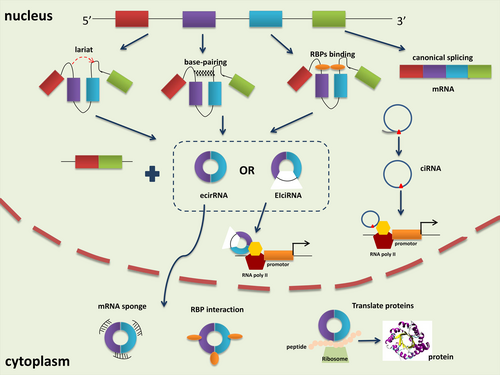
Additionally, RNA-binding proteins (RBPs) participate in circRNAs biogenesis as trans-factors. Musclblind (MBL), Quaking (QKI) and adenosine deaminase acting on RNA 1 (ADAR1) are representative RBPs. For instance, QKI can promote ecircRNA formation by bridging the 5′ splice site closer to the upstream 3′ splice site within the flanking introns (Conn et al., 2015). Similarly, MBL drives the circularization of circMBL by binding specifically to its own pre-mRNA (Ashwal-Fluss et al., 2014). Conversely, the function of the RNA-editing enzyme ADAR1 in circRNA formation is correlated with its Adenosine-to-Inosine (A-to-I) editing capability, by specifically targeting dsRNA pairing structures and catalyzing the conversion of A to I, destabilizing RNA paring and inhibiting back-splicing (Ivanov et al., 2015). The recently discovered RBPs, including RBM20, FUS and HNRNPL, exhibit RNA splicing and editing function in the formation of circRNAs (Errichelli et al., 2017; Fei et al., 2017; Khan et al., 2016).
2.1 Biological functions
circRNAs are abundant and conserved between species, but present specificity to tissues, developmental stages or cell types (Jeck et al., 2013). The functions of circRNAs are consistent with their characteristics of distribution. circRNAs can regulate gene expression through various regulatory modes. However, only a handful of studies have unveiled the biological function of circRNAs (Figure 1), which remain largely unknown.
2.1.1 miRNA sponge
miRNA are well recognized regulators of gene expression by negatively modulating mRNAs expression. circRNAs, containing miRNA response elements (MREs), could exhibit posttranscriptional regulation as miRNA sponges to further regulate the expression of miRNA targets. CicrRNAs can bind to and compete for the 3′-untranslated region of mRNAs, which is also the target site for miRNA. Specifically, the CDR1/ciRS-7 circRNA harbours more than 70 conserved miR-7 target sites, and therefore regulates the expression of miR-7 target mRNAs as a sponge for miR-7 (Memczak et al., 2013). Similarly, the circular transcript of the male sex-determining gene Sry contains 16 sites for miR-138 (Hansen et al., 2013). In another study, circ-ITCH, a renowned anti-oncogenic circRNA in cancer progression, could directly bind to miR-22 to repress tumor cell viability, migration and invasion, but facilitate apoptosis in osteosarcoma (C. Ren et al., 2019). Moreover, circRNAs have been proved to perform this function even with limited miRNA-binding sites (Jeck & Sharpless, 2014). The advantage for the role of circRNA as a miRNA sponge is the lack of poly(A) tails and 5′ termini, which enables them to escape deadenylation, decapping and degradation caused by miRNA association (Huntzinger & Izaurralde, 2011).
2.1.2 Alternative splicing and cis/trans regulation
Primarily located in the nucleus, ciRNAs and ElciRNAs are implicated to function at the transcriptional level (Y. Zhang et al., 2013). EIcircRNAs can interact with U1 small nuclear ribonucleoprotein (U1 snRNP) and RNA polymerase II (Pol II) via U1 snRNA-binding sites and perform cis-regulation functions, which promotes a positive feedback loop on gene transcription (Z. Li et al., 2015). Moreover, cirRNAs, like ci-ankrd52, ci-MCM5, and ci-SIRT7 regulate the transcription of their parent genes via the cis-regulatory effects of RNA Pol II (Y. Zhang et al., 2013). Meanwhile, some circRNAs also trans-function on gene expression by competing with linear splicing. Their back-splicing might produce circRNAs and the corresponding linear RNAs. For instance, the biosynthesis of circMbl is influenced by the competition for the MBL binding sites with MBL pre-mRNA linear splicing (Ashwal-Fluss et al., 2014).
2.1.3 Interaction with RBPs
Many circRNAs have dynamic interactions with multiple RBPs for various purposes. Some circRNAs might bind, store, sort or sequester RBPs to particular subcellular locations, or compete with RBPs to affect the expression of their targets. For example, circ-foxo3 could retard cell-cycle progression by forming a ternary complex with p21 and Cdk2 proteins (W. W. Du et al., 2016). The interactions of circ-Amotl1 with c-myc, STAT3 have been demonstrated in tumorigenesis and wound healing (Q. Yang, Du, et al., 2017; Z. G. Yang, Awan, et al., 2017). The competition between a cirRNA and its cognate mRNA was proposed as circPABPN1 can compete with PABPN1 to bind to HuR, thus repressing the translation of PABPN1 mRNA (Abdelmohsen et al., 2017).
2.1.4 Protein translation
The exceptional capacity of circRNAs is that they can be translated into proteins, which is inconsistent with the original definition. Initial findings revealed that synthetic exonic circRNAs with internal ribosome entry sites could be translatable both in vivo and in vitro. Later, Legnini et al. (2017) screened differentially expressed circRNAs in Duchenne muscular dystrophy by RNA-seq. They discovered that circ-ZNF609, which is involved in myogenesis, can encode protein in a splicing-dependent and cap-independent manner (Legnini et al., 2017). Pamudurti et al. (2017) demonstrated the discovery of numerous circRNA-translated proteins or peptides by performing ribosome footprinting experiments in Drosophila brains. They proved that ribosome-bound circRNAs can use the start codon of the hosting mRNA.
3 circRNAs IN OSTEOSARCOMA
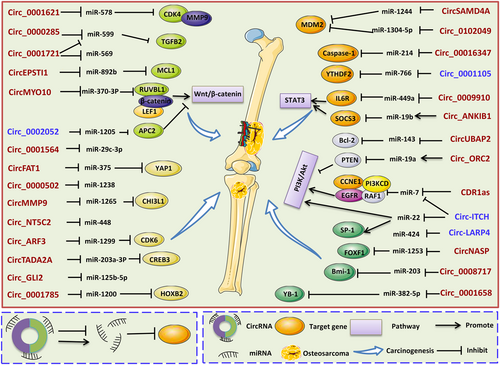
With the advancement of high-throughput sequencing, massive circRNAs are discovered to be abnormally expressed in cancers and have become a new focus of studies. More recently, the emerging circRNAs are upregulated or deregulated in osteosarcoma when compared with noncancerous tissues. Thus, circRNAs may be involved in various aspects of osteosarcoma initiation and development. In the following sections, we demonstrate the pivotal role of circRNAs in the regulation of hallmarks in carcinogenesis and their potential underlying molecular mechanisms (Tables 1, 2 and Figure 2).
| circRNA (alias) | Gene symbol | Chromosome location | miRNAs/gene targets | Involved signaling pathway | Carcinogenetic roles |
|---|---|---|---|---|---|
| circ_0000285 (Z. Zhang, Pu, et al., 2019) | HIPK3 | chr11: 33362513–33363232 | miR-599/TGFB2 | – | Proliferation, migration |
| circ_0000502 (H. Qi et al., 2018) | – | chr13: 108895879–108903664 | miR-1238 | – | Proliferation, apoptosis, migration, invasion |
| circ_0001564 (Y. Z. Song & Li, 2018) | CANX | chr5: 179132679–179137066 | miR-29c-3p | – | Proliferation, cell cycle arrest, apoptosis |
| circ_0001621 (Ji et al., 2020) | CASP8AP2 | chr6:90556280–90566918 | miR-578/CDK4, MMP9 | – | Proliferation, migration |
| circ_0001658 (L. Wang, Wang, et al., 2019) | ARID1B | chr6: 157357968–157406039 | miR-382-5p/YB-1 | – | Proliferation, migration, invasion, apoptosis |
| circ_0001721 (L. Li, Guo, et al., 2019) | CDK14 | chr7: 90355880–90356126 | miR-569, miR-599 | – | Proliferation, apoptosis, tumor growth |
| circ_0001785 (S. Li, Pei, et al., 2019) | ELP3 | chr8: 28013458–28019595 | miR-1200/HOXB2 | PI3K/Akt and Bcl-2 | Proliferation, apoptosis |
| circ_0007534 (B. Li & Li, 2018) | DDX42 | chr17: 61869771–61877977 | – | Akt/GSK-3β, Bcl-2/caspase-3 | Proliferation, invasion, apoptosis |
| circ_0008717 (circABCB10) (X. Zhou, Natino, et al., 2018) | ABCB10 | chr1: 229665945–229678118 | miR-203/Bmi-1 | – | Proliferation, migration, invasion, apoptosis |
| circ_0009910 (Deng et al., 2018) | MFN2 | chr1: 12049221–12052747 | miR-449a/IL6R | JAK1/STAT3 | Proliferation, cell cycle arrest, apoptosis |
| circ_001569 (circ_0000677) (H. Zhang, Yan, et al., 2018) | ABCC1 | chr16: 16101672–16162159 | – | Wnt/β-catenin | Proliferation, colon formation, Chemoresistance |
| circ_0016347 (H. Jin, Zhang, & Wang, 2017) | KCNH1 | chr1: 211092981–211192598 | miR-214/caspase-1 | – | Proliferation, invasion, metastasis |
| circ_0081001 (Kun-Peng, Chun-Lin, et al., 2018) | CYP51A1 | chr7: 91755566–91756945 | - | – | – |
| circ_0102049 (Y. Jin, Li, et al., 2019) | ATL1 | chr14: 51079976–51081229 | miR-1304-5p/MDM2 | – | Proliferation, apoptosis, migration, invasion |
| circ_ANKIB1 (Y. X. Du et al., 2019) | ANKIB1 | chr7 | miR-19b/SOCS3 | STAT3 | Proliferation, apoptosis, invasion |
| circ_ARF3 (Gao et al., 2020) | ARF3 | chr12 | miR-1299/CDK6 | – | Cell growth |
| CDR1as (ciRS-7/circ_0001946) (B. Xu, Yang, et al., 2018) | CDR1 | chrX: 139865339–139866824 | miR-7/EGFR, CCNE1, PI3KCD, RAF1 | EMT | Cell vitality, migration, apoptosis, cell cycle arrest |
| circEPSTI1 (circRNA_000479) (X. Tan et al., 2019) | EPSTI1 | chr13 | miR-892b/MCL1 | – | Cell proliferation, migration |
| circFAT1 (G. Liu, Huang, et al., 2018) | FAT1 | chr4 | miR-375/YAP1 | – | Cell growth, migration, invasion, apoptosis |
| circ_GLI2 (J.-F. Li & Song, 2017) | GLI2 | chr2: 121708818–121713006 | miR-125b-5p | – | Proliferation, migration, invasion |
| circLRP6 (Zheng et al., 2019) | LRP6 | chr12 | LSD1, EZH2, KLF2, APC | – | Proliferation, migration, invasion, apoptosis, cell cycle |
| circMMP9 (Pan et al., 2019) | MMP9 | chr20 | miR-1265/CHI3L1 | – | Proliferation, migration, invasion |
| circMYO10 (J. Chen, Liu, et al., 2019) | MYO10 | chr5 | miR-370-3p/RUVBL1, β-catenin, LEF1 | – | – |
| circNASP (circ_0092340) (L. Huang et al., 2018) | NASP | chr1: 46079180–46079500 | miR-1253/FOXF1 | – | Proliferation, cell cycle progression, invasion, metastasis |
| circ-NT5C2 (circ_0092509) (X. Liu et al., 2017; Nie et al., 2018) | NT5C2 | – | miR-448 | – | Proliferation, invasion, apoptosis |
| circ_ORC2 (X. Li, Sun, et al., 2019) | ORC2 | chr2 | miR-19a/PTEN | Akt | Proliferation, apoptosis, invasion |
| circPVT1 (circ_0001821) (Kun-Peng, Chun-Lin, et al., 2018; Kun-Peng, Xiao-Long, & Chun-Lin, 2018; Kun-Peng, Xiao-Long, Lei, et al., 2018; Yan et al., 2020; Y. P. Liu et al., 2020) | PVT1 | chr8: 128902834–128903244 | ABCB1; miR-526b/FOXC2; miR-205-5p/c-FLIP | EMT | Chemoresistance, proliferation, migration, invasion |
| CircSAMD4A (circ_101356/circ_0004846) (Yanbin & Jing, 2019) | SAMD4A | chr14: 55168779–55169298 | miR-1244/MDM2 | – | Proliferation, cells stemness features |
| circTADA2A (circ_0043278) (Y. Wu et al., 2019) | TADA2A | chr17: 35797838–35800763 | miR-203a-3p/CREB3 | – | Proliferation, migration, invasion |
| circUBAP2 (H. Zhang et al., 2017) | UBAP2 | chr9 | miR-143/Bcl-2 | – | Cell growth, apoptosis |
- Abbreviations: ABCB1, ATP binding cassette subfamily B member 1; Akt, protein kinase B; CCNE1, cyclin E1; CDK, cyclin-dependent kinase; c-FLIP, cellular FLICE-inhibitory protein; CHI3L1, chitinase 3 like 1; CREB3, cAMP responsive element binding protein 3; EGFR, epidermal growth factor receptor; EMT, epithelial–mesenchymal transition; EZH2, enhancer of zeste 2 polycomb repressive complex 2 subunit; FOXC2, forkhead box C2; FOXF1, forkhead box F1; HOXB2, homeobox B2; IL6R, interleukin 6 receptor; KLF2, kruppel like factor 2; LEF1, lymphoid enhancer binding factor 1; LSD1, LSD1 zinc finger family protein; MCL1, myeloid cell leukaemia 1; MDM2, MDM2 proto-oncogene; PI3K, phosphatidylinositol 3-kinase; PI3KCD, phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit delta; PTEN, phosphatase and tensin homolog; RAF1, Raf-1 proto-oncogene, serine/threonine kinase; RUVBL1, RuvB like AAA ATPase 1; STAT3, signal transducer and activator of transcription 3; TGFB2, transforming growth factor beta 2; YAP1, Yes1 associated transcriptional regulator; YB1, Y-box binding protein 1.
| circRNA (alias) | Gene symbol | Chromosome location | miRNAs/gene targets | Involved signaling pathway | Carcinogenetic roles |
|---|---|---|---|---|---|
| circ_0001105 (J. Yang et al., 2020) | SP140 | chr2:231155174–231253348 | miR-766/YTHDF2 | – | Cell viability, invasion |
| circ_0002052 (Z. Wu et al., 2018) | PAPPA | chr9: 118969734–118997916 | miR-1205/APC2 | Wnt/β-catenin | Proliferation, migration, invasion, apoptosis |
| circ_HIPK3 (circ_0000284) (Xiao-Long et al., 2018) | HIPK3 | chr11: 33307958–33309057 | – | – | Proliferation, migration, invasion |
| circ-ITCH (C. Ren et al., 2019; H. Li, Lan, et al., 2020) | ITCH | chr20 | miR-22/SP-1 miR-7/EGFR | PTEN/PI3K/Akt - | Viability, proliferation, apoptosis, migration invasion |
| circ-LARP4 (Y. Hu et al., 2019) | LARP4 | chr12 | miR-424 | – | Chemo-sensitivity |
- Abbreviations: Akt, protein kinase B; APC2, APC regulator of WNT signaling pathway 2; EGFR, epidermal growth factor receptor; PI3K, phosphatidylinositol 3-kinase; PTEN, phosphatase and tensin homolog; SP-1, Sp1 transcription factor; YTHDF2, YTH N6-methyladenosine RNA binding protein 2.

3.1 circRNA profiles in osteosarcoma
Xi et al. (2019) utilized RNA-seq to screen differentially expressed circRNAs between tumor and adjacent paracanceous tissues from three osteosarcoma patients. Overall, they identified 259 dysregulated circRNAs in osteosarcoma, including 132 circRNAs upregulated and 127 downregulated. Of note, expression of circ_24831 and circ_32279 were found significantly decreased, while levels of circ_20403 and circ_2137 were remarkably increased (Xi et al., 2019). Besides this, X. Liu, Zhong, Li, and Shan (2017) demonstrated 785 distinguished differently expressed circRNAs with two-fold change in osteosarcoma tissue compared with nontumor tissue by circRNA microarray analysis. Furthermore, Kun-Peng, Xiao-Long, Lei, et al. (2018) reported the circRNAs expression profiles of three paired multi-drug resistant and sensitive osteosarcoma cell lines by RNA-seq, including 57 upregulated and 23 downregulated circRNAs. Validation of reliability of RNA-seq results was further evaluated by quantitative reverse transcription polymerase chain reaction (qRT-PCR). Notably, the most upregulated circRNA, circ_0004674, was significantly elevated in both chemoresistant osteosarcoma tissues and cell lines and predicted poor prognosis of cancer patients. Taken together, lots of circRNAs with aberrant expression have been found in osteosarcoma by next generation RNA-seq or microarray. Further functional experiments were performed to unveil their biological functions in tumor initiation and progression of osteosarcoma. The extensive roles of circRNAs in osteosarcoma are described as follows.
3.2 Osteosarcoma-related circRNAs
3.2.1 Upregulated circRNAs in osteosarcoma
circ_ANKIB1
circ_ANKIB1 was first found enriched in schwann cells and could regulate their proliferation in response to peripheral nerve injury (Mao et al., 2019). Recently, Y. X. Du, Guo, Pan, Liang, and Li (2019) found that circ_ANKIB1 expression was upregulated in osteosarcoma cells. miR-19b is a well-appraised oncogene in osteosarcoma progression (Y. X. Du et al., 2019). circ_ANKIB1 could act as an miR-19b sponge to promote its expression, suppress the downstream gene SOCS3 as well as activate the STAT3 pathway, and in turn enhance cell proliferation, invasion and inhibit apoptosis.
circ_ARF3
circ_ARF3 was found overexpressed in osteosarcoma tissues and cell lines. A functional assay revealed that circ_ARF3 could decoy miR-1299 and upregulate CDK6 to promote osteosarcoma cell growth, implying it may be a possible target for osteosarcoma treatment (Gao, Yuan, Hu, & Liu, 2020).
CDR1as
circRNA CDR1as, also known as ciRS-7, functions as an oncogenic or suppressive regulator in multiple cancer progression and chemoresistance, such hepatocellular carcinoma (HCC; Su et al., 2019), ovarian cancer (Zhao, Ji, Wang, He, & Li, 2019), bladder cancer (Yuan et al., 2019), and nasopharyngeal carcinoma (Q. Zhong et al., 2019). Similarly, through systematic and comprehensive pan-cancer analysis, CDR1as was proved to play a specific role in alternation of the tumor microenvironment by regulation of the TGF-β pathway and extracellular matrix (ECM)-receptor interaction (Zou et al., 2019). B. Xu, Li, Fan, and Wu (2018) reported that CDR1as expression levels in osteosarcoma tissues were significantly higher compared to noncancerous bone tissues. Additionally, overexpression of CDR1as was positively correlated with a larger tumor size, Enneking stage, and DM, while negatively associated with a tumor suppressor—miR-7 (B. Xu, Yang, et al., 2018). In vitro assay demonstrated that suppression of CDR1as downregulated miR-7 expression and subsequently induced cell apoptosis and G1/S cell cycle arrest and impaired cell viability and migration in osteosarcoma cell lines (MG63 and U2OS). Besides this, in vivo experiments showed that inhibition of CDR1as was able to induce tumor regression. Moreover, the oncogenic role of CDR1as could be abolished by the miR-7 inhibitor. Further study confirmed that CDR1as acted as a sponge of miR-7 and thereby regulated target genes, such as EGFR, CCNE1, PI3KCD, and RAF1. In addition, CDR1as could upregulate N-cadherin and downregulate E-cadherin to promote the epithelial–mesenchymal transition (EMT).
circEPSTI1
circEPSTI1, also named as circRNA_000479, was first found upregulated in triple-negative breast cancer (TNBC) by circRNA microarray (B. Chen et al., 2018). Later, circEPSTI1 was reported overexpressed in ovarian cancer (J. Xie et al., 2019). In addition, circEPSTI1 could affect cancer progression and function as ceRNA to decoy miR-4753/6809-BCL11A in TNBC (B. Chen et al., 2018) or the miR-942/EPSTI1 axis in ovarian cancer (J. Xie et al., 2019), respectively. Consistently, circEPSTI1 expression was remarkably increased in osteosarcoma tissues when compared with the paired adjacent normal counterparts (X. Tan et al., 2019). In vitro assay revealed that circEPSTI1 inhibition impaired cancer cell proliferation and migration. Furthermore, dual luciferase reporter indicated that circEPSTI1 could directly sponge miR-892b and regulate the expression of MCL1 (X. Tan et al., 2019).
circFAT1
circFAT1 originates from exon 2 of the FAT1 gene. G. Liu, Huang, et al. (2018) found significant upregulation of circFAT1 in osteosarcoma tissues and cell lines. Functional assay showed that circFAT1 inhibition distinctly repressed cell migration, invasion and tumorigenesis in vitro and decreased osteosarcoma growth in vivo (G. Liu, Huang, et al., 2018). Mechanistically, circFAT1 could act as a molecular sponge of miR-375 and thereby upregulate the expression of Yes-associated protein 1 (YAP1). In addition, inhibition of miR-375 could rescue attenuation of the tumor-promoting effect induced by circFAT1 knockdown, indicating a circFAT1/miR-375/YAP1 axis in regulation of tumorigenesis in osteosarcoma.
circ_GLI2
J.-F. Li and Song (2017) found that circ_GLI2 was markedly overexpressed in osteosarcoma tissues compared to nontumor counterparts. Silence of circ_GLI2 by siRNA could significantly inhibit the cell growth, migration, and invasion capacity in vitro. Bioinformatics analysis and luciferase report assay further predicted that circ_GLI2 could abundantly bind to a well annotated tumor suppressor in osteosarcoma—miR-125b-5p. Subsequently, functional assay confirmed the oncogenic role of circ_GLI2 in osteosarcoma patients by negatively targeting miR-125b-5p.
circLRP6
Arsenic is a toxic metalloid that can induce carcinogenesis under long-term exposure by modulating EMT and malignant transformation (Bai, Lei, Huang, Jiang, & Zhou, 2019). A previous study confirmed that human keratinocyte (HaCaT) cells exposed to arsenite resulted in upregulation of circLRP6 (Xue et al., 2018). circLRP6 could increase expression of ZEB1 and subsequently induce EMT via sponging miR-455 to promote malignant transformation, suggesting a unique role in arsenite-induced tumorigenesis (Xue et al., 2018). circLRP6 expression was observed significantly elevated in osteosarcoma tissues as measured by qRT-PCR (Zheng et al., 2019). Moreover, compared with osteosarcoma patients with low circLRP6 expression, those with high expression were predicted a shortened OS and disease-free survival (DFS; Zheng et al., 2019). In vitro, knockdown of circLRP6 promoted osteosarcoma cell apoptosis, induced G0/G1 cell cycle arrest, and repressed proliferative, migratory and invasive rates. A further mechanical assay revealed that circLRP6 exerted its oncogenic role in osteosarcoma via binding to LSD1 and EZH2, and then impaired the expression of KLF2 and APC (Zheng et al., 2019).
circMMP9
circMMP9, generated from exons 12 and 13 of MMP9 mRNA, was initially screened and identified as an oncogene in glioblastoma multiforme (GBM) by microarray in 2018 (R. Wang, Zhang, et al., 2018). Transfection with the circMMP9 overexpression vector promoted GBM cell proliferation, migration and invasion capacities. Eukaryotic initiation factor 4A3, an important component of RNA splicing, could bind to the upstream region of the MMP9 transcript, induce circMMP9 cyclization, and increase its expression in GBM (R. Wang, Zhang, et al., 2018). Recently, circMMP9 was found markedly upregulated in osteosarcoma tissues (Pan, Hu, Chen, & Zhang, 2019), and its knockdown in osteosarcoma cells could suppress cell proliferation, migration and invasion, but enhance cellular apoptosis. Located in the cytoplasm, circMMP9 could directly sponge miR-1265 and hence target a well-known oncogene-CHI3L1, indicating the circMMP9/miR-1265/CHI3L1 axis in regulating osteosarcoma progression (Pan et al., 2019). A higher expression of circMMP9 (Pan et al., 2019), predicted unfavorable clinicopathological parameters including unfavorable prognosis, advanced tumor stage, larger tumor size and DM in osteosarcoma patients.
circMYO10
As a novel circRNA generated by back-splicing of MYO10, circMYO10 was found generally upregulated in osteosarcoma tissues comparing with paired chondroma (J. Chen, Liu, et al., 2019). Further functional investigations demonstrated that circMYO10 enhanced cell proliferation and EMT both in vitro and in vivo. Mechanistically, miR-370-3p could abrogate the oncogenic phenotype of circMYO10, suggesting that circMYO10 may promote chromatin remodeling by sponging miR-370-3p and regulating RUVBL1 (J. Chen, Liu, et al., 2019). Moreover, circMYO10 was found to increase the transcriptional activity of the β-catenin/LEF1 complex, and thus induce osteosarcoma aggressiveness (J. Chen, Liu, et al., 2019).
circ_ORC2
circ_ORC2 was found highly expressed in osteosarcoma cell lines (X. Li, Sun, et al., 2019). Mainly located in the cytoplasm, circ_ORC2 could accelerate osteosarcoma cell growth and invasion by regulating miR-19a/PTEN and Akt pathway (X. Li, Sun, et al., 2019).
circNASP
The expression of circNASP was dramatically upregulated in osteosarcoma tissue when compared with adjacent normal control. Knockdown of circNASP significantly impaired cancer cell proliferation, cell cycle progression and invasion. Mechanism analysis demonstrated that circNASP could serve as a sponge of miR-1253 and subsequently target FOXF1 in osteosarcoma cells, contributing to malignant behaviors of cancer cells (L. Huang, Chen, Pan, & Yu, 2018).
circ-NT5C2
Nie, Zhao, Guo, Wang, and Ye (2018) explored the expression profile of circ-NT5C2 in osteosarcoma tissue samples and matched normal tissues by qRT-PCR, and the results showed that circ-NT5C2 was conspicuously increased in cancer tissues. Besides this, X. Liu et al. (2017) also validated the upregulated expression of circ-NT5C2 in 52 pairs of osteosarcoma tissues and cell lines. Consistently, overexpressed levels of circ-NT5C2 in serum and tissues were correlated with lower OS, DFS or progression-free survival (PFS; Nie et al., 2018; Zhu et al., 2019), implicating a potential role as prognostic biomarker and therapeutic target in osteosarcoma patients. Moreover, silence of circ-NT5C2 impaired cell proliferation, invasion and induced apoptosis in vitro, and inhibited tumor growth in vivo. Bioinformatics and luciferase reporter assays further revealed that circ-NT5C2 promoted osteosarcoma tumorigenesis by sponging miR-448 (X. Liu et al., 2017).
circPVT1
circPVT1, also known as circ6 (Memczak et al., 2013), originates by circulation of exon 3 of the PVT1 gene and was first termed as senescence-associated circRNA (Verduci et al., 2017). The expression level of circPVT1 was significantly elevated in dividing fibroblasts but reduced in senescent cells (Panda et al., 2017). Downregulation of circPVT1 in proliferating fibroblasts induced senescence and reduced proliferation by selectively regulating let-7 activity (Panda et al., 2017). Later, circRNA was screened and identified as a proliferative factor in gastric cancer (J. Chen et al., 2017). Subsequent studies further confirmed the oncogenic role of circPVT1 in several carcinomas, including head and neck squamous cell carcinoma (Verduci et al., 2017), acute lymphoblastic leukemia (ALL; Gaffo et al., 2019; J. Hu, Han, et al., 2018), non-small cell lung cancer (NSCLC; S. Qin, Chang, Yuan, Huang, & Qiu, 2019), colorectal cancer (CRC; Z. Wang, Su, Xiang, Zhao, & Qin, 2019), GBM (G. Chi, Yang, Xu, & Liu, 2020) and esophageal carcinoma (R. Zhong, Chen, Mo, Li, & Zhang, 2019).
Three independent studies (Kun-Peng, Xiao-Long, & Chun-Lin, 2018; Y. P. Liu, Wan, Long, Tian, & Zhang, 2020; Yan et al., 2020) observed obvious upregulated expressions of circPVT1 in the osteosarcoma tissues, serums and chemoresistant cell lines, significantly associated with unfavorable prognosis of patients with osteosarcoma. Currently, alkaline phosphatase (ALP) and lactate dehydrogenase (LDH) are the common serum markers in diagnosis of osteosarcoma. Surprisingly, serum circPVT1 (Kun-Peng et al., 2018; AUC 0.871) was more reliable to separate osteosarcoma from control individuals than ALP (0.673) but not LDH (0.852), indicating it may serve as a better diagnostic biomarker than ALP with both enhanced sensitivity and specificity.
In vitro study showed a multifaceted role of circPVT1 in regulating carcinogenesis. There were studies demonstrating that knockdown of circPVT1 could inhibit cell proliferation, migration and invasion through regulation of miR-526b/FOXC2 (Yan et al., 2020), miR-205-5p/c-FLIP and EMT process (Y. P. Liu et al., 2020). ATP-binding cassette sub-family B member 1 (ABCB1) is a key driver of drug resistance in cancers by encoding multidrug resistance protein 1 (MDR1) and conferring resistance to cytotoxic and targeted chemotherapy (Robey et al., 2018; X. Wang, Qiao, et al., 2019; Williams, Amaral, Simeoni, & Somervaille, 2019). Another study found that circPVT1 was capable of alleviating the resistance to cisplatin and doxorubicin of osteosarcoma cell lines via downregulating the expression of ABCB1 (Kun-Peng et al., 2018). Taken together, circPVT1 may serve as a promising prognostic biomarker in osteosarcoma and its restoration may be a successful strategy in treating osteosarcoma.
circSAMD4A
circSAMD4 exhibited upregulated expression levels in osteosarcoma tissues when compared with adjacent normal tissues (Yanbin & Jing, 2019). Functional experiments revealed that circSAMD4A could promote cell proliferation and stemness features. Moreover, circSAMD4A was found readily sponged to miR-1244 and regulated target gene MDM2 (Yanbin & Jing, 2019).
circTADA2A
circTADA2A was found upregulated in both osteosarcoma tissues and cell lines (Y. Wu et al., 2019). In vitro, circTADA2A knockdown inhibited cell proliferation, migration and invasion. In vivo, circTADA2A inhibition could attenuate tumorigenesis and metastasis (Y. Wu et al., 2019). Furthermore, a mechanistic assay showed that circTADA2A promotes the oncogenic phenotype through regulation of miR-203a-3p/CREB3 axis (Y. Wu et al., 2019).
circUBAP2
H. Zhang et al. (2017) reported for the first time that circUBAP2 expression was significantly increased in osteosarcoma tissues, as compared to matched controls, and correlated with cancer progression and prognosis. Functional assay showed that cicrUBAP2 overexpression induced cell growth while suppressing apoptosis both in vitro and in vivo. A mechanism study revealed that circUBP2 could inhibit expression of miR-143, and thereby enhance the expression and function of target gene—Bcl-2.
circ_0000285
circ_0000285 was found aberrantly expressed in bladder cancer (B. J. Chi et al., 2019), and laryngocarcinoma (S. Qin, Zhao, et al., 2019). More recently, H. Zhang, Shen, et al. (2019) identified that circ_0000285 was also highly expressed in osteosarcoma by analyzing the GSE96964 data set. The uppregulated expression of circ_0000285 in osteosarcoma cell lines was validated by qRT-PCR. In laryngocarcinoma, circ_0000285 could function as an oncogene by activating the wnt/β-catenin pathway (J. B. Qin, Chang, et al., 2019). However, in osteosarcoma, circ_0000285 could promote proliferation and migration via binding miR-599 and targeting TGFB2 gene (Z. Zhang, Pu, et al., 2019).
circ_0000502
H. Qi, Sun, Jiang, and Li (2018) demonstrated that circ_0000502 was overexpressed in osteosarcoma specimens and cell lines. Moreover, circ_0000502 (Y. Qi, Zhang, Wang, & Yao, 2018)******* predicted unfavorable prognosis in osteosarcoma patients, as illustrated by the Kaplan-Meier (K-M) curve. Both in vitro and in vivo assay showed that cicr_0000502 effectively triggered cell proliferation, migration and invasion, but suppressed cell apoptosis in osteosarcoma. Furthermore, the tumor promoting effect of circ_0000502 may be partially dependent on regulation of miR-1238, revealing circ_0000502 could exert its oncogenic role by sponge miR-1238.
circ_0000885
When compared with patients with benign bone tumors or healthy control, patients with osteosarcoma had higher serum levels of circ_0000885. Moreover, those with advanced Enneking stage (IIB and III) had an even higher expression of circ_0000885 in tissues and serums comparing with early-stage osteosarcoma (Zhu et al., 2019).
circ_0001564
circ_0001564, located at 5q35.3, is the transcriptional product of the CANX gene. By screening circRNAs expression profiles using microarray analysis, Li et al. identified circ_0001564 was significantly upregulated in osteosarcoma tissues and cell lines. Knockdown of circ_0001564 by siRNA impaired cell proliferation, promoted apoptosis and induced cell cycle arrest in G0/G1 phase in osteosarcoma cell lines (HOS and MG-63). Furthermore, circ_0001564 may directly bind with miR-29c-3p and thereby play an oncogenic role in osteosarcoma, as predicted by bioinformatics analysis and confirmed by dual-luciferase reporter assay (Y. Z. Song & Li, 2018).
circ_0001621
circ_001621 is a newly discovered circRNA with upregulated expression in osteosarcoma (Ji, Shan, Shen, & He, 2020). Patients with high circ_001621 expression tend to have shorter survival time. In vitro and in vivo assays demonstrated that circ_001621 could enhance cell proliferation and migration by directing targeting miR-578 and in turn promoting CDK4 and MMP9 expression (Ji et al., 2020).
circ_0001658
circ_0001658 was previously found to be markedly differentially expressed with a two fold change between gastric cancer and normal tissue (Jiang & Shen, 2019). Similarly, compared with normal control, osteosarcoma tissues displayed a remarkable higher expression of circ_0001658 (L. Wang, Wang, Su, & Zhao, 2019). Furthermore, enforced expression of circ_0001658 could impede apoptosis and facilitate the proliferative and metastasis potential of osteosarcoma cells via modulating miR-382-5p/YB-1 axis (L. Wang, Wang, et al., 2019).
circ_0001721
circ_0001721 is located at chr7: 90355880-90356126 and generated from the CDK14 gene (L. Li, Guo, et al., 2019). L. Li et al. (2019) demonstrated that expression of circ_0001721 was upregulated in both osteosarcoma samples and cell lines compared to non-cancer tissues. Moreover, elevated circ_0001721 (L. Li, Guo, et al., 2019) was strikingly associated with clinical severity, revealing its potential prognostic role in osteosarcoma patients. Functional assay further showed that circ_0001721 could facilitate cancer cell progression partly owing to its suppression of miR-569 and miR-599 (X. Zhou, Natino, et al., 2018).
circ_0001785
circ_0001785 was found aberrantly expressed in plasma specimens from breast cancer patients compared to healthy controls by microarray assay and qRT-PCR (Yin et al., 2018). Further in-depth study found that the circulating circ_0001785 level was significantly correlated with histological grade, tumor-node-metastasis (TNM) stage and distal metastasis (DM), and had an even better diagnostic accuracy than conventional biomarkers, including CEA and CA15-3 (Yin et al., 2018). S. Li, Pei, et al. (2019) demonstrated that circ_0001785 was overexpressed in osteosarcoma cell lines compared with normal human osteoblasts. circ_0001785 inhibition impeded tumor cell proliferation and facilitated cell apoptosis. In mechanism, circ_0001785 could competitively sponge to miR-1200, and further upregulate target gene-HOXB2 (S. Li, Pei, et al., 2019). Moreover, PI3K/Akt and Bcl-2 signaling pathways were also involved in the regulatory network of circ_0001785 in osteosarcoma (S. Li, Pei, et al., 2019).
circ_0007534
Upregulated irc_007534 with oncogenic properties in vitro and in vivo was reported consecutively in multiple human cancers, including CRC (R. Zhang, Xu, Zhao, & Wang, 2018), glioma (G. F. Li, Li, Yao, & Zhuang, 2018), NSCLC (Y. Qi, Zhang, Wang, & Yao, 2018, 2018), breast cancer (L. Song & Xiao, 2018), osteosarcoma (B. Li & Li, 2018), pancreatic ductal adenocarcinoma (PDAC; Hao et al., 2019) and cervical cancer (Rong, Gao, Yang, & Guo, 2019). In 2018, B. Li and Li (2018) demonstrated that circ_0007534 was found to be distinctly overexpressed in 57 osteosarcoma tissues compared to corresponding normal controls.
Consistently, circ_0007534 expressions were also significantly upregulated in osteosarcoma cell lines (HOS, Sao2, MG63 and U2OS) when compared with hFOB1.19. Besides this, enhanced expressions of circ_0007534 (B. Li & Li, 2018) were positively correlated with tumor size and worse differentiation, but not gender, age, grade, and metastasis. Functional studies further demonstrated that circ_0007534 knockdown by siRNA hindered osteosarcoma cell proliferation and facilitated apoptosis both in vitro and in vivo. Additionally, circ_0007534 may facilitate osteosarcoma progression via Akt/GSK-3β pathway.
circ_0008717
X. Zhou, Natino, et al. (2018) identified circ_0008717 was conspicuously overexpressed in osteosarcoma tissues. circ_0008717 knockdown prevented cell proliferation, migration and invasion, while facilitating cell apoptosis in osteosarcoma cell lines. CircRNA chip further identified miR-203 and Bmi-1 as the circRNA-associated miRNA and target gene, respectively. Conversely, the tumor promoting effect of circ_0008717 could be abrogated by miR-203 mimics or Bmi-1 silence (X. Zhou, Natino, et al., 2018).
circ_0009910
The circ_0009910 expression level was overexpressed in osteosarcoma cells, and knockdown of circ_0009910 led to inhibition of cell proliferation and cell cycle arrest as well as apoptosis in osteosarcoma cells. In terms of mechanism, circ_0009910 was identified as a sponge to miR-449a to upregulate the target gene-IL6R, thus contributing to the tumorigenesis of osteosarcoma. Additionally, the JAK1/STAT3 signaling pathway was found to be interacting with the circ_0009910/miR-449a/IL6R axis, indicating an important role in cancer development (Deng et al., 2018).
circ_001569
H. Xie et al. (2016) showed that circ_001569 was highly expressed in CRC in 2016. circ_001569 acted as a positive regulator in CRC cell proliferation and invasion by modulating miR-145 and targeting genes, including E2F5, BAG4, and FMNL2 (H. Xie et al., 2016). Moreover, the upregulated expression patterns of circ_001569 were validated in NSCLC (Ding, Yao, Lu, Gong, & Zhang, 2018), breast cancer (J. H. Xu, Wang, & Xu, 2019) and HCC (H. Liu, Xue, et al., 2018). In addition to ceRNA, circ_001569 could promote cancer cell promotion or metastasis by activating wnt/β-catenin and the PI3K/Akt signaling pathway (Ding et al., 2018; J. H. Xu et al., 2019). circ_001569 was found to be obviously overexpressed in 36 osteosarcoma tissues compared to adjacent normal controls (H. Zhang, Yan, Lang, & Zhuang, 2018). Gain- and loss-of-function assays revealed that knockdown of circ_001569 by siRNA markedly rendered cell proliferation, while overexpression of circ_001569 induced resistance to cisplatin by the wnt/β-catenin pathway in osteosarcoma.
circ_0016347
H. Jin, Zhang, and Wang (2017) found that circ_0016347 expression levels were distinctly elevated in osteosarcoma tissues compared to matched adjacent nontumor tissues from six patients as measured by qRT-PCR. Consistently, circ_0016347 expressions were markedly enhanced in osteosarcoma cell lines (MG-63, Saos-2) when compared with normal osteoblasts. Moreover, knockdown of circ_0016347 by siRNA obviously restricted cell proliferation, invasion and metastasis of osteosarcoma cells in vitro. By contrast, in vivo study showed that overexpression of circ_0016347 remarkably increased the tumor sizes and numbers of pulmonary metastasis tumors in mice injected with osteosarcomas cell lines. Moreover, circ_0016347 could act as a sponge of miR-214 and upregulate caspase-1 expression, indicating an inflammation-related mechanism in the carcinogenesis of osteosarcoma.
circ_0081001
Kun-Peng, Chun-Lin, Jian-Ping, and Lei (2018) identified circ_0081001 as a significantly upregulated circRNA in osteosarcoma by RNA-seq in three paired chemoresistant and chemosensitive cancer cell lines, tissues, and serums.
Intriguingly, receiver operating characteristic (ROC) analysis showed that circ_0081001 (Kun-Peng, Chun-Lin, et al., 2018) can serve as an independent biomarker, even better than ALP and LDH, for distinguishing an osteosarcoma case from controls. More importantly, in addition to the promise as a stable biomarker, serum circ_0081001 expression may be adopted for dynamically monitoring and accurately reflecting the condition changes of osteosarcoma patients, especially in response to chemotherapy and lung metastasis (Kun-Peng, Chun-Lin, et al., 2018).
circ_0102049
circ_0102049 was found upregulated in both osteosarcoma specimens and cells (Y. Jin, Li, Zhu, & Liu, 2019). The expression of circ_0102049 (Y. Jin, Li, et al., 2019) was remarkably associated with poor prognosis, clinical severity, including WHO grade, tumor size and pulmonary metastasis in osteosarcoma, suggesting these circRNAs may be utilized as clinical prognostic biomarkers. Gain/loss of function assays showed that circ_0102049 attenuated cell apoptosis but enhanced proliferation, migration and invasion. Bioinformatics and dual-luciferase reporter assays revealed that circ_0102049 function as a ceRNA to bind miR-1304-5p and increased expression of MDM2 in osteosarcoma progression (Y. Jin, Li, et al., 2019).
circRNA_100876
circRNA_100876 was found remarkably elevated in NSCLC tissues when compared with adjacent normal counterparts (Yao et al., 2017). A significant link between circRNA_100876 overexpression and detrimental clinical characteristic in NSCLC was established, as determined by shortened overall survival (OS), advanced tumor staging, and lymph node metastasis (LNM; Yao et al., 2017). Moreover, depletion of circRNA_100876 could contribute to inhibition of proliferation and metastasis in esophageal squamous cell carcinoma (ESCC; Cao, Chen, Yan, Li, & Huang, 2018) and breast cancer (C. Y. Yang, Zhang, He, & Wang, 2019), indicating a positive correlation between circRNA_100876 levels and carcinogenesis. circRNA_100876 was highly expressed in osteosarcoma, and its knockdown markedly suppressed tumor proliferation, induced apoptosis and G2/M cell cycle arrest (J. Jin, Chen, et al., 2019). Furthermore, circRNA_100876 was demonstrated to negatively modulate miR-136 in osteosarcoma (J. Jin, Chen, et al., 2019).
3.2.2 Downregulated circRNAs in osteosarcoma
circ_HIPK3
circ_HIPK3, a stably downregulated circRNA in osteosarcoma tissues, plasmas and cell lines, was screened and identified by Xiao-Long, Kun-Peng, and Chun-Lin (2018) via qRT-PCR. A statistically significant correlation between circ_HIPK3 expression and advanced clinical pathological features including Enneking stage (p = .042) and lung metastasis (p = .036), rather than age, gender and tumor location, was also demonstrated (Xiao-Long et al., 2018). Further survival analysis showed that lower expression of circ_HIPK3 significantly associated with poorer OS and prognosis of osteosarcoma patients, with the sensitivity and specificity of 0.56 and 0.84 respectively (Xiao-Long et al., 2018).
Besides this, functional assays revealed that upregulated circ_HIPK3 strikingly inhibited cell proliferation, migration and invasion potential in vitro, as detected by CCK-8, colony formation, wound healing and cell invasion assays, respectively. In contrast, circ_HIPK3 was highly expressed in glioma and knockdown of circ_HIPK3 in U87 and U251 cells hindered their proliferative and invasive capacities through absorption of miR-124-3p to destabilize STAT3 expression (D. Hu & Zhang, 2019).
circ-ITCH
circ-ITCH, located on chromosome 20q11.22, is a circRNA derived from several exons of ITCH and has been well-documented as a tumor suppressor in numerous malignancies (Y. Li, Ge, Xu, & Jia, 2019; X. Y. Xu, Zhou, et al., 2018), such as prostate cancer (E. Huang, Chen, & Yuan, 2019; X. Wang, Wang, Wu, & Bai, 2019), ovarian cancer (Luo, Gao, & Sun, 2018a, 2018b), TNBC (S. T. Wang, Liu, et al., 2019), bladder cancer (C. Yang, Yuan, et al., 2018), papillary thyroid cancer (PTC; M. Wang, Chen, Ru, & Cong, 2018), and melanoma (X. Y. Xu, Zhou, et al., 2018).
Interestingly, studies on circ-ITCH showed a discrepancy with regard to the expression and function in osteosarcoma. C. Ren et al. (2019) found circ-ITCH expression also declined in both osteosarcoma tissues and cells. A functional assay demonstrated that circ-ITCH overexpression promoted cell apoptosis, while inhibiting cell viability, proliferation, migration and invasion. The phosphatidylinositide 3-kinase/protein kinase B/mammalian target of rapamycin (PI3K/Akt/mTOR) pathway is a major determinant in carcinogenesis by sustaining energy homeostasis via regulation of important biological processes, such as the Warburg effect (Courtnay et al., 2015) and autophagy (O'Donnell, Massi, Teng, & Mandala, 2018; Z. Xu et al., 2020; X. Zhang, Wang, et al., 2019). Importantly, the PI3K/Akt/mTOR axis could promote tumor metastasis by activating EMT (X. Zhang, Wang, et al., 2019). Mechanically, circ-ITCH acted as a miR-22 sponge, and consecutively regulated the PTEN/PI3K/Akt axis as well as the SP-1 signaling pathway. However, a more recent study performed by H. Li, Lan, Liao, Tang, and Yang, (2020) revealed an upregulated expression pattern of circ-ITCH in osteosarcoma cells. Besides this, circ-ITCH could facilitate the tumor cell growth, migration and invasion by attenuating the inhibitory effect of miR-7 on EGFR expression (H. Li, Lan, et al., 2020). Therefore, more efforts should be made to further elucidate the role of circ-ITCH in osteosarcoma.
circ-LARP4
circ-LARP4 is another newly identified circRNA with a downregulated expression patterns in osteosarcoma tissues (Y. Hu et al., 2019). Expression of circ-LARP4 was negatively associated with the Enneking stage of osteosarcoma patients (Y. Hu et al., 2019), and positively correlated with prolonged DFS and OS (Y. Hu et al., 2019). Besides this, circ-LARP4 could be used to distinguish tumor tissues from noncancerous control with an area under curve (AUC) of 0.829 (95% CI: 0.762-0.859; Y. Hu et al., 2019). In vitro assay showed that overexpression of circ-LARP4 could enhance the tumor cell chemo-sensitivity to cisplatin and doxorubicin rather than methotrexate, via sponging miR-424 (Y. Hu et al., 2019).
circ_0001105
circ_0001105 was found significantly lowly expressed in osteosarcoma tissues, and Kaplan-Meier showed its low expression was correlated with unfavorable survival of osteosarcoma patients. Moreover, overexpression of circ_0001105 could impede tumor cell viability as well as invasion via modulating the miR-766/YTHDF2 axis both in vitro and in vivo (J. Yang et al., 2020).
circ_0002052
Z. Wu, Shi, and Jiang (2018) demonstrated that circ_0002052 expression levels were markedly downregulated in osteosarcoma tissues and cell lines. In vitro assays showed that circ_0002052 overexpression could inhibit cell proliferation, migration and invasion, but induce apoptosis in osteosarcoma. Consistently, in vivo experiments revealed that ectopic expression of circ_0002052 suppressed osteosarcoma cell growth. Mechanistically, circ_0002052 could act as a molecular sponge of miR-1205 and inhibit target gene APC2 and subsequently suppress the activation of wnt/β-catenin pathway. Worthy of note, circ_0002052/miR-the 1205/APC2/wnt/β-catenincirc_0002052/miR-1205/APC2/wnt/β-catenin axis may be a therapeutic target for osteosarcoma therapy.
3.3 circRNAs indicating chemoresistance
Development of drug resistance remains the major problem in treatment failure and poor prognosis in osteosarcoma. It is reported that most osteosarcoma patients may develop DM if exhibiting chemoresistance (Saraf et al., 2018). Accordingly, it is of great importance to reveal the molecular pathways contributing to metastasis and chemotherapy in osteosarcoma. A growing number of investigations have revealed the role of circRNA in chemoresistance of osteosarcoma. For instance, cisplatin and doxorubicin treatment in osteosarcoma is facing challenges. Overexpression of circ-LARP4 could sensitize tumor cells to cisplatin and doxorubicin by acting as an miR-424 sponge (Y. Hu et al., 2019). Kun-Peng, Chun-Lin, et al. (2018) identified circ_0081001 as a significantly upregulated circRNA in osteosarcoma by RNA-seq in three paired chemoresistant and chemosensitive cancer cell lines (MG63, KHOS, U2OS), tissues and serums. Meanwhile, by adopting RNA-seq and qRT-PCR validation, Kun-Peng, Xiao-Long, Lei et al. (2018) proved that circ_0004674 was significantly upregulated in osteosarcoma chemoresistant cells and tissues, indicating circ_0004674 may serve as a candidate target gene in chemoresistance. Besides this, Y. Hu et al. (2019) recruited 72 osteosarcoma patients with Enneking Stage IIA-IIB, and found that high expression of circ-LARP4 indicated a better tumor cell necrosis rate to adjuvant chemotherapy. Considering certain circRNAs are closely correlated with chemoresistant osteosarcoma, dynamic analysis of abnormally expressed circRNAs in different sensitivities to chemotherapy may provide novel avenues for future therapy in osteosarcoma.
3.4 Exosomal-circRNAs in osteosarcoma
Exosomes refers to a class of membrane-bound extracellular vesicles (EVs) released by most cell types with sizes ranging from 30 to 100 nm (Shi et al., 2019). Exosome could facilitate intercellular communication by transferring active components, including DNA, RNA, protein and lipid from the donor cells to recipient cells (Tu et al., 2019). Recently, increasing evidence showed that circRNA are identified for enrichment and stability in exosomes (Y. Wang, Liu, et al., 2019), and could be delivered to recipient cells to affect their protein function and control the state of gene expression (Shi et al., 2019; R. Zhou, Chen, et al., 2018). Therefore, circRNA-enriched exosomes are involved in multiple biological processes of tumor progression, particularly metastasis and chemoresistance. For example, Lu et al. (2020) showed that circ-RanGAP1 was overexpressed in plasma exosomes from gastric cancer patients, resulting in enhanced migration and invasion ability of cancer cells. Besides this, Cdr1as was downregulated in serum exosomes from ovarian cancer patients who succumb to cisplatin resistance (Zhao et al., 2019).
Recently, S. Li, Pei, et al. (2020) identified a significantly reduced circRNA, circ_0000190, in both EVs and osteosarcoma tissues. EVs circ_0000190 could be used to distinguish osteosarcoma patients from healthy controls with accurate diagnostic value. Moreover, EVs-encapsulated circ_0000190 could be transported from normal cells to tumor cells, and thereby impede the migration, proliferation and invasion potential of osteosarcoma cells both in vitro and in vivo, implying capacity for therapeutics (S. L i, Pei, et al., 2020). More investigations are still warranted to further explore the innovations of exosomal-circRNA in the diagnostic and prognostic implications of osteosarcoma (Z. Li, Chen, Hu, & Jiang, 2019).
3.5 circRNAs in immune response in osteosarcoma
The immune system consists of multiple components and mediators, which can monitor, protect against, and regulate various exogenous pathogens to maintain internal homeostasis (X. Chen, Yang, et al., 2019). The immune response mainly comprises immune surveillance and defense (X. Chen, Yang, et al., 2019), based on the function of immunuocytes. In addition to proteins, circRNAs are also implicated to be novel candidates in regulation of immunocytes in immune response, such as macrophages, neutrophils, CD8+T cells and cells in tumor microenvironments (X. Chen, Yang, et al., 2019). Thus, circRNAs could play an indispensable role in the occurrence and progression of various immune diseases including tumor. Several mechanisms have been found involved in the role and function of circRNAs in mediating tumor immunity: First, it is not surprising to speculate that circRNAs could contribute to immune regulation via circRNA-miRNA-mRNA network, as the relationship between miRNA and immunity has already been well-documented (L. Yang, Fu, et al., 2018). Second, circRNAs could trigger immune response by directly interacting with proteins (L. Yang, Fu, et al., 2018), such as MDM2, to regulate its stability as well as antitumor immunity (Z. Xu, Li, Fan, & Wu, 2018). Third, some circRNAs may control the innate immune response by activation of immunocytes (X. Chen, Yang, et al., 2019). Fourth, circRNAs may be incorporated into EVs and transferred between tumor cells and immunocytes to regulate intercellular communication as possible tumor antigens (X. Chen, Yang, et al., 2019).
Currently, studies on the role and function of circRNAs in osteoimmunology are relatively scarce. B7-H3 (CD276), a membrane protein of B7 family members and costimulatory molecular, serves as a critical negative regulator in immune response in osteosarcoma by facilitating tumor cells to escape from immunosurveillance (He & Li, 2019; L. Wang, Zhang, Kang, Zhang, & Zhang, 2019). A previous study showed an abnormal expression pattern of B7-H3 in osteosarcoma, which may partially contribute to its immune escape, cell growth and invasive ability (L. Wang, Kang, et al., 2018; L. Wang, Zhang, et al., 2019). Through circRNA screening, L. Wang, Zhang, et al., 2019) identified that circ0021347 was markedly downregulated in osteosarcoma and may be a potential downstream target gene of B7-H3. Intriguingly, the detailed molecular mechanism of the crosstalk between circ0021347 with B7-H3 in osteosarcoma progression remains unknown and requires further exploration.
Though our knowledge of circRNAs in immune response still remains preliminary, it is worthy of further exploration as circRNAs have rich potential in tumor immunity and could be considered as biomarkers and effective immunotherapeutic targets. Moreover, the mechanisms by which differentially expressed circRNAs regulate immunity in osteosarcoma merit deeper elucidation.
4 CONCLUSIONS AND PERSPECTIVES
Osteosarcoma still remains one of the most lethal tumors despite modernized surgical and radiotherapy treatment. More efficient treatment options are in urgent need in the future. As a newly appreciated class of endogenous RNAs, circRNA has been shown to be dramatically dysregulated in osteosarcoma. The most extensively investigated mechanism for circRNA in osteosarcoma is to act as an miRNA molecular sponge, and thereby regulate the downstream target genes and signaling pathways (Figure 3). In addition, circRNAs are reported to exert other intriguing functions, such as regulating alternative splicing, bind and sequester RBP, and acting as a posttranscriptional regulator, indicating their vital roles in multiple disorders. Of note, circRNAs participate in diverse biological process in osteosarcoma progression, including proliferation, apoptosis, migration, invasion, maintenance of stemness, chemoresistance and EMT (R. Wang et al., 2018). These studies provided novel insights for understanding of pathogenesis of osteosarcoma and the therapeutic potential of these circRNA in cancer treatment.
Besides this, circRNAs widely exist in transcriptomes. Unlike linear RNA, circRNA are comparatively more resistant to exonucleases (including RNase R), deadenylation and cap removal, which is attributed to the lack of accessible ends (H. Zhang, Shen, et al., 2019). More important, circRNAs are highly evolutionarily conserved and may be expressed in tissue-specific and stage-specific manners. Due to higher abundance, stability and specificity, circRNAs could serve as much more credible diagnostic and prognostic biomarkers in cancers. The diagnostic value of circRNAs in osteosarcoma has been demonstrated in a number of studies, as listed in Table 3. Furthermore, numerous circRNAs have been found to participate in predicting the prognosis of osteosarcoma (Table 4). Collectively, circRNAs are proposed as novel interventional biomarkers for osteosarcoma.
| circRNAs | Year | Expression | Sample sources | AUC | Sensitivity | Specificity | Cut-off | References |
|---|---|---|---|---|---|---|---|---|
| circ_0000885 | 2019 | Upregulated | Serum | 0.783 (vs. healthy control); 0.714 (vs. benign bone tumor) | – | – | – | K. Zhu et al. (2019) |
| circ_0008717 | 2018 | Upregulated | Tissue | 0.782 | 0.8 | 0.7333 | 0.97 | X. Zhou, Natino, et al. (2018) |
| circ_0081001 | 2018 | Upregulated | Serum | 0.898 | – | – | – | Kun-Peng, Chun-Lin et al. (2018) |
| CDR1as | 2018 | Upregulated | Tissue | 0.857 | – | – | 1.613 | B. Xu et al. (2018) |
| circ_HIPK3 | 2018 | Downregulated | Plasma | 0.783 | 0.56 | 0.84 | 29.3 | Xiao-Long et al. (2018) |
| circ-LARP4 | 2019 | Downregulated | Tissue | 0.829 | – | – | – | Y. Hu et al. (2019) |
| circLRP6 | 2019 | Upregulated | Tissue | 0.8742 | – | – | – | S. Zheng et al. (2019) |
| circ-NT5C2 | 2017 | Upregulated | Tissue | 0.753 | – | – | – | X. Liu et al. (2017) |
| circPVT1 | 2018 | Upregulated | Serum | 0.871 | – | – | – | Kun-Peng et al. (2018) |
- Abbreviation: AUC, area under curve.
| circRNAs | Year | Expression | Other clinical parameters | Sample sources | Sample size | Survival | Follow-up months | HRs and 95% CIs for OSa | References |
|---|---|---|---|---|---|---|---|---|---|
| circ_0000502 | 2018 | Upregulated | WHO grade, tumor size | Tissue | 63 | OS | 60 | 2.292 (1.245–4.220) | H. Qi et al. (2018) |
| circ_0000885 | 2019 | Upregulated | Enneking stage, lung metastasis | Tissue | 50 | OS | <60 | 2.458 | K. Zhu et al. (2019) |
| circ_0001721 | 2019 | Upregulated | WHO grade, tumor size | Tissue | 52 | OS | 60 | 1.928 (1.012–3.673) | L. Li, Guo, et al. (2019) |
| circ_0002052 | 2018 | Downregulated | – | Tissue | 54 | OS, PFS | <60 | – | Z. Wu et al. (2018) |
| circ_0007534 | 2018 | Upregulated | Tumor size, differentiation grade | Tissue | 57 | OS | 60 | 2.046 (1.058–3.956) | B. Li and Li (2018) |
| circ_0008717 | 2018 | Upregulated | TNM stage, pulmonary metastasis | Tissue | 45 | OS, PFS | <80 | 3.505 (1.287–5.221) | X. Zhou, Natino, et al. (2018) |
| circ_001569 | 2018 | Upregulated | DM, TNM stage, chemoresistance | Tissue | 36 | – | – | – | H. Zhang, Yan, et al. (2018) |
| circ_0081001 | 2018 | Upregulated | Enneking stage, lung metastasis, chemoresistance | Tissue | 82 | OS | 60 | 3.122 | Kun-Peng, Chun-Lin et al. (2018) |
| circ_0102049 | 2019 | Upregulated | Tumor size, lung metastasis | Tissue | 76 | OS | 60 | 1.929 (1.093–3.402) | Y. Jin, Li, et al. (2019) |
| CDR1as | 2018 | Upregulated | Enneking stage, lung metastasis, tumor size | Tissue | 38 | – | – | – | B. Xu et al. (2018) |
| circ_HIPK3 | 2018 | Downregulated | Enneking stage, lung metastasis | Tissue | 82 | OS, | 60 | – | Xiao-Long et al. (2018) |
| circ-LARP4 | 2019 | Downregulated | Enneking stage, histological response | Tissue | 72 | OS, DFS | 120 | – | Y. Hu et al. (2019) |
| circLRP6 | 2019 | Upregulated | Tumor size, lung metastasis | Tissue | 50 | OS, DFS | >100 | 1.44 (1.21–1.78) | S. Zheng et al. (2019) |
| circMMP9 | 2019 | Upregulated | TNM stage, tumor size, DM | Tissue | 51 | OS | 60 | – | Pan et al. (2019) |
| circNASP | 2018 | Upregulated | Enneking stage, tumor size, lung metastasis | Tissue | 39 | – | – | – | L. Huang et al. (2018) |
| circ-NT5C2 | 2017/2018 | Upregulated | Enneking stage, lung metastasis, chemotherapy | Tissue | 52/170 | OS, DFS | 60 | 2.133 (1.037–4.037) | X. Liu et al. (2017) and Nie et al. (2018) |
| circPVT1 | 2018 | Upregulated | Enneking stage, chemoresistance, lung metastasis | Tissue | 80 | OS | 60 | – | Kun-Peng et al. (2018) |
- Abbreviations: CI, confidence interval; DFS, disease-free survival; DM, distant metastasis; HR, hazard ratio; OS, overall survival; PFS, progression-free survival; TNM, tumor-node-metastasis.
- a Results from multivariate analysis were adopted if available.
However, it is worth noting that several issues remained in exploration of circRNA in disease progression. First, the biogenesis and the factors controlling the circulation of circRNA still remain largely elusive (X. Wang & Fang, 2018). Secondly, only a few circRNAs have been assigned functional roles or clinical relevance, while the vast majority remain unexplored and need further investigation (W. Hu, Bi, et al., 2018). Thirdly, discrepant results remain in certain circRNA's expression patterns and functions in osteosarcoma (H. Li, Lan, et al., 2020; C. Ren et al., 2019), which may be attributed to the small sample size or detection method. It is critical to further validate the precise role of each circRNA in osteosarcoma and explore the underlying mechanisms related to the circRNA. Fourthly, it is also of great importance to understand the upper stream regulation of circRNA as well. As mentioned above, B7-H3 is a negative regulator in osteoimmunology. A recent study showed that B7-H3 knockdown could modulate circ0021347 and thereby affect osteosarcoma malignancy (L. Wang, Zhang, et al., 2019). Comprehensive understanding of the circRNA regulatory network in OS could provide us with more information to utilize circRNAs' potential in clinical application. Fifthly, most of the circRNA was detected in cells of tissue RNA samples from biopsy or surgery, while few studies are done in noninvasive samples, like serum, plasma, urine or even saliva (Z. Li, Chen, et al., 2019; X. Wang & Fang, 2018). Like liquid biopsy, detection of circRNAs in noninvasive samples is worth trying as it may be more acceptable to patients and can enable the possibility of disease surveillance through repeated detection (Z. Li, Chen, et al., 2019). Sixthly, given the fact that circRNAs may act as possible targets in osteosarcoma, more consideration should be put on efficient and accurate delivery with a long-term sustained effect as well as minimized immune rejection (X. Wang & Fang, 2018). The exosome is regarded as an important cargo carrier mediating cell-to-cell interaction with high efficacy and limited side-effects. Recently, their role in clinical application in diagnosis and innovative treatment has emerged (Bai et al., 2019). Of particular interest is that engineered exosomes preloaded with anti-oncogenic circRNAs may be a potential therapeutic option for osteosarcoma. Moreover, in addition to tumor-derived exosomal circRNA, the role of those originating from other cells in the tumor microenvironment, such as mesenchymal stem cells, adipocytes and macrophages, also deserves further investigation and may shed light on the underlying mechanisms involved in osteosarcoma.
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (81902745), the Natural Science Foundation of Hunan Province, China (2018JJ3716, 2018JJ3759), the China Scholarship Council (201806375067, 201806375068), the Fundamental Research Funds for the Central Universities of Central South University (2017zzts231), and Central South University Innovative Program for Undergraduates (20190034020002).
CONFLICTE OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
C. T. and J. H. contributed to drafting the review and figures. L. Q., X. R., and C. Z. contributed to the linguistic revision. Z. D., K. Y., and W. W. contributed to the text proofing. Q. L. and Z. L. contributed to designing and composing the review. All authors reviewed and approved the final version of manuscript as submitted.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




