Bifidobacterium breve M-16V alters the gut microbiota to alleviate OVA-induced food allergy through IL-33/ST2 signal pathway
Na Li and Yi Yu contributed equally to this study.
Abstract
There has been a marked increase in life-threatening food allergy (FA). One hypothesis is that changes in bacterial communities may be key to FA. To better understand how gut microbiota regulates FA in humans, we established a mouse model with FA induced by ovalbumin. We found that the mice with FA had abnormal bacterial composition, accompanied by increased immunoglobulin G, immunoglobulin E, and interleukin-4/interferon-γ, and there existed a certain coherence between them. Interestingly, Bifidobacterium breve M-16V may alter the gut microbiota to alleviate the allergy symptoms by IL-33/ST2 signaling. Our results indicate that gut microbiota is essential for regulating FA to dietary antigens and demonstrate that intervention in bacterial community regulation may be therapeutically related to FA.
Abbreviations
-
- Bb
-
- Bifidobacterium breve
-
- CAE
-
- chloroacetate esterase
-
- CFU
-
- colony-forming units
-
- FA
-
- food allergy
-
- IECi
-
- intestinal epithelial cell
-
- IFN-γ
-
- interferon-γ
-
- i.p.
-
- intraperitoneal
-
- IL
-
- interleukin
-
- OVA
-
- ovalbumin
-
- qPCR
-
- quantitative PCR
-
- sIgE
-
- specific IgE
-
- sIgG
-
- specific IgG
-
- ST2
-
- suppression of tumorigenicity 2
-
- Th1
-
- T-helper 1
-
- Th2
-
- T-helper 2
-
- Th17
-
- T-helper 17
-
- Tregs
-
- regulatory T cells
1 INTRODUCTION
Over the past two decades, public attention has improved in response to the significant increasing prevalence of food allergy (FA). The FA has emerged as a life-threatening disease worldwide with gastrointestinal damage (Yu, Freeland, & Nadeau, 2016), affecting 1.1–10.4% of children (National Academies of Sciences, 2016). Despite the evidence for the true prevalence of FA is complex with inadequate or inconsistent data, many healthcare professionals believe that an actual increase in FA has occurred and that this is unlikely to be solely due to increased awareness and better diagnostic tools.
The microbial composition in the gut is associated with the risk of FA (Ling et al., 2014). The decrease of bacterial diversity and imbalance of intestinal flora in infancy is related to food sensitivities, which indicates that changes in the intestinal flora may play a role in FA (Azad et al., 2015). Comprehensive analysis of intestinal flora structure can provide some unique insights into the study of microbiome-associated pathogenic mechanisms.
The immune response of the body is closely regulated by the immune system under physiological conditions. T cell subsets and cytokines may play an important role in regulating immune response and maintaining immune balance to suppress FA (Abdel-Gadir et al., 2019; Chiang et al., 2018; Mondoulet et al., 2019). Interleukin (IL)-33 is an alarm cytokine that exerts an important action on Th2 immune response in allergic diseases. IL-33 activated by allergen proteases can rapidly induce allergic airway inflammation after exposure to allergens (Cayrol et al., 2018). It is found that the IL-33/suppression of tumorigenicity 2 (ST2) pathway may involve the occurrence and development of allergic diseases through analysis of patient samples and the study of mouse models (Artis & Spits, 2015; Liew, Girard, & Turnquist, 2016). Besides, the occurrence of FA symptoms is closely related to the presence of specific antibodies for food antigen and abnormal levels of immunoglobulin E (IgE) in the serum (Berin et al., 2018).
Probiotics are defined as active microorganisms that have a positive impact on body health when administered in appropriate amounts (Marinelli, Tenore, & Novellino, 2017). The study has shown that probiotics can modulate the host immune system or the composition of gut microbiota, effectively alleviating allergy symptoms (Diesner et al., 2016). Bifidobacterium breve (Bb) is an important part of human intestinal microorganisms and is also one of the earliest beneficial and the dominant bacteria community in the intestines of infant. After oral administration, Bb can colonize in the intestines and participate in mucosal immune response, which plays a vital role in maintaining human health (Nowak-Wegrzyn, Szajewska, & Lack, 2017). Bb exerted immune regulation and antiallergic effects on BALB/c mice by reducing IL-4 levels and activating Tregs (Ren et al., 2018).
This study aimed to examine the relationships among gut microbiota, immune function, and FA, and evaluate the potential of an oral exposure to Bb to relieve allergy symptoms in a mouse model of FA. Based on the literatures and results, we hypothesized that Bb M-16V may alter the gut microbiota to alleviate ovalbumin (OVA)-induced FA through the IL-33/ST2 signal pathway (Figure 1). The results of this study seek to provide a new basis for the application of Bb M-16V in FA and reveal the vital function of different intestinal microbiota composition after exposure to allergen in regulating T cell responses to intestinal allergic reactivity.
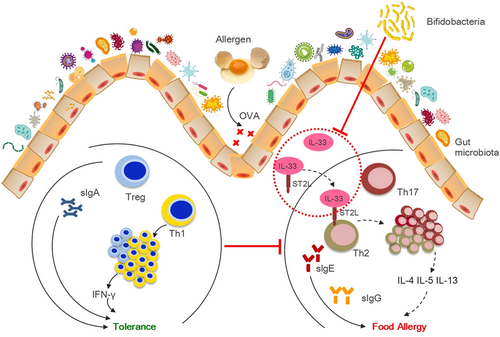
2 MATERIALS AND METHODS
2.1 Animal
The experimental procedures were approved by the Animal Ethical Committee. BALB/c mice aged 3 weeks (SPF) were obtained and housed in the Animal Research Center of the Ruijin Hospital, Shanghai Jiao Tong University School of Medicine with a 12-hr light/12-hr dark cycle in 23 ± 2°C.
2.2 Ethics statement
The procedure of animal was approved by the Ruijin Hospital, School of Medicine, Shanghai Jiao Tong University. Animal study was carried out in accordance with the Guide for Care and Use of Laboratory Animals of National Research Council (SYXK [Shanghai 2011–0113]).
2.3 Sensitization and challenge
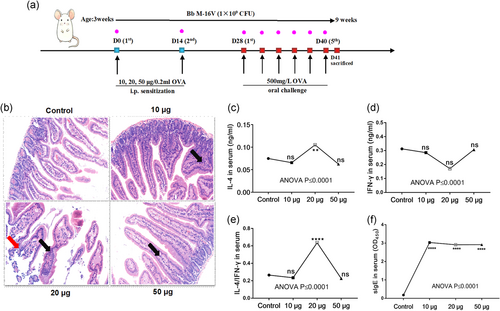
The FA were induced by OVA in mice, as previously described (Kim et al., 2016), with some modification. Briefly, to determine the optimal OVA concentration inducing FA, the mice were sensitized with 10, 20, and 50 μg/0.2 ml OVA (grade V; Sigma-Aldrich, St. Louis, MO) by intraperitoneal injection adsorbed on aluminum potassium sulfate adjuvant (A-7210, Sigma-Aldrich) on Days 0 and 14, respectively. From Day 28, 500 mg/L OVA in saline was administered orally to challenge every 3 days, five times altogether. The mice were killed on the first day after the final challenge (Figure 2a).
 inflammatory cell infiltration
inflammatory cell infiltration  exfoliated villous epithelial cells
exfoliated villous epithelial cells2.4 Probiotic supplements
Bb M-16V (Dipro; AB-BIOTICS, S.A, SPAIN) were provided by MIHC Science Laboratory, DERBY CARE MEDICAL TECHNOLOGY CO., LIMITED. Mice in the probiotic group received Bb M-16V orally (1 × 107 CFU/g formula per mouse daily; Figure 2a).
2.5 Histology
The intestinal tissue was fixed by 4% paraformaldehyde, embedded in paraffin, and sections of paraffin were stained with chloroacetate esterase. Tissue sections were counted by investigators blinded to the sample identity in 10 high power fields (HPFs) at 400 times magnification.
2.6 ELISA experiments
Cytokine and immunoglobulin levels in serum were assayed by ELISA, using a kit (Cusabio Biotech, Wuhan, China) following the manufacturer's use instructions. Quantitative sandwich enzyme immunoassay was used in this assay. This assay employs the qualitative enzyme immunoassay technique. This experiment has high sensitivity and sensitivity for the detection of serum samples in mice. No significant cross-reaction or interference was observed in the relationship between detection index and their analogs.
2.7 Quantitative polymerase chain reaction
Gene expression was detected by quantitative polymerase chain reaction (qPCR). In brief, RNA lysis buffer homogenized the samples of small intestine tissue. Total RNA was extracted using TRIzol reagent (Qiagen, Hilden, Germany). Complementary DNA (cDNA) was prepared using the kit of iScript™ cDNA synthesis (Promega, Madison, WI). qPCR was done with ABI prism 7900 sequence detection system (Application Binary Interface). Total IL-33 and ST2 messenger RNA levels were measured by qPCR using appropriate primers. IL-33 (F: 5′-GGTGTGGATGGGAAGAAGCTG-3-G R: 5′-GAGGACTTTTTGTGAAGGACG-3′) and ST2 (F: 5′-CAAGTAGGACCTGTGTGCCC-3′ and R: 5′-CGTGTCCAACAATTGACCTG-3′). The gene expression levels were standardized and calculated with the method for relative quantification normalized by glyceraldehyde-3-phosphate dehydrogenase gene expression.
2.8 16S rRNA gene amplification and sequencing
The fecal samples of mouse were collected and stored at −80°C. The DNA extraction of samples was performed using the kit of MagAttract PowerSoil DNA Isolation (Qiagen, Hilden, Germany). The V4-V5 variable region of the 16S rRNA gene was amplified, standardized, pooled, and sequenced by the Illumina MiSeq platform using 2 × 250 cycles.
2.9 Quantification and statistical analysis
All experiments were conducted by using randomly assigned mice. Data analysis was performed with GraphPad Prism 7.0. One-way analysis of variance was used to analyze the results. The sparse canonical correlation analysis was applied to identify the relationship between immunological variables and gut microbiota taxa. All data are presented as mean ± standard error of the mean. The p values of lower than .05 were considered significant: ∗p ≤ .05, ∗∗p ≤ .01, ∗∗∗p ≤ .001, ∗∗∗∗p ≤ .0001.
3 RESULTS
3.1 The determination of optimal OVA concentration inducing FA
The avirulent food allergens, such as OVA, may cause oral sensitization in mice with anaphylaxis that occurs in IgE-dependent and T-helper 1/T-helper 2 (Th1/Th2) imbalance manner (Noah et al., 2019; O'Konek et al., 2018).
The results showed that the intestinal tissue structure was intact and there was no obvious inflammatory change in the control group. In 10 and 50 µg OVA groups, a small amount of inflammatory cell infiltration was observed. In 20 µg OVA group, intestinal mucosa was destroyed, villous epithelial cells were not neatly arranged or exfoliated, and a large number of inflammatory cells such as lymphocytes and eosinophils were observed to infiltrate (Figure 2b).
SlgE was significantly increased in all the OVA-sensitized groups, suggesting the occurrence of anaphylaxis in mice (Figure 2c). The upregulation of IL-4 and nonsignificant change of interferon-γ (IFN-γ) resulted in the increase of IL-4/IFN-γ ratio in the 20 µg OVA group (Figure 2d-f).
Twenty microgram OVA-sensitized, but not 10 and 50 µg OVA-sensitized, mice had anaphylaxis on the oral challenge with OVA, suggesting that the FA model by 20 µg OVA is more suitable for probiotic therapy studies.
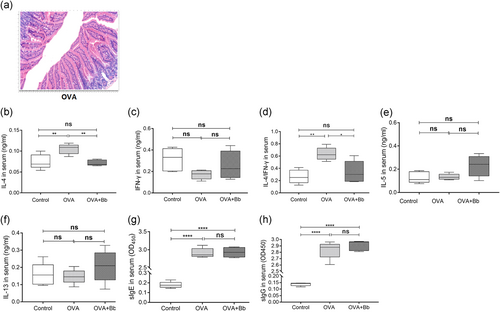
3.2 The immune effect of Bb M-16V16V on the OVA-induced FA mouse model
While different doses of OVA were used to induce FA, corresponding Bb M-16V treatment groups were also assigned. We selected OVA (20 µg)-sensitized and probiotic (Bb M-16V)-intervened mice as research objects to further explore the therapeutic role of probiotics on FA.
M-16V significantly improved the inflammation of small intestine promoted by OVA and reduced the infiltration of inflammatory cells (Figure 3a). The increase of IL-4 and IL-4/IFN-γ induced by OVA was significantly inhibited, but the impact on IL-5 and IL-13 was not obvious (Figure 3b-f). Moreover, Bb M-16V had no significant inhibitory effect on the sIgE and sIgG induced by OVA. It indicated that Bb M-16V may have an immunomodulatory effect on FA (Figure 3g,h).
 inflammatory cell infiltration
inflammatory cell infiltration  exfoliated villous epithelial cells
exfoliated villous epithelial cells3.3 The effect of oral Bb M-16V treatment on IL-33 and ST2 expression in OVA-sensitized mice
IL-33/ST2 may be an alarm in cytokine with important roles in allergic diseases, such as asthma and atopic dermatitis (AD) (Cayrol et al., 2018; Galand et al., 2016), regardless of association with Type 2 immunity. We used OVA-sensitized mice to verify the role of IL-33/ST2 in this model. Compared with the control group, the IL-33 expression increased in the OVA-sensitized (OVA) group and the same results were observed in the probiotic-intervened (OVA + Bb) group (Figure 4a). Interestingly, ST2 expression had not significantly changed in the OVA group and was significantly inhibited in the OVA + Bb group (Figure 4b).
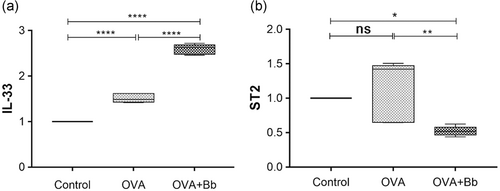
These results suggest that IL-33 plays a crucial part in the FA elicited by OVA, accompanied by the production of sIgE antibody and antigen-driven Th2 cytokines in sensitized mice. Whereas administration of Bb M-16V during FA showed no significant inhibitory effect on IL-33. Meanwhile, The ST2 blockade induced by Bb M-16V not only affected the systemic Th2 response but also significantly reduced the severity of allergic reactions. However, the alleviation of FA by Bb M-16V is not associated with IgE-mediated immune response.
3.4 The oral administration of Bb M-16V improved the structure of gut microbiota during OVA sensitization
After discussing the immune effects of Bb M-16V, we used 16S rRNA gene amplification and sequencing technology to detect the effects of Bb M-16V on the gut microbiota of OVA-induced FA mice.
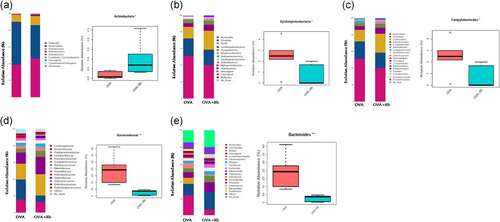
The gut microbiota was analyzed from different taxonomic levels. At the phylum, class, order, and family level, Actinobacteria, Epsilonproteobacteria, Campylobacterales, and Bacteroidaceae composition of OVA and OVA + Bb groups showed significant differences, respectively (Figure 5a-d). In addition, at the genus level, the average abundance of Bacteroides in the OVA group was higher, while Bb M-16V significantly reduced the composition of Epsilonproteobacteria (Figure 5e).
3.5 Search for the biomarkers of microbiota in the feces of mice with FA and Bb M-16V intervention
The results and differences in taxa caused by Bb M-16V are seen in the LEfSe analysis (Figure 6a). After the Bb M-16V treatment, the abundance of Actinobacteria was higher than before the treatment at the phylum level. At the class level, Bb M-16V increased Actinobacteria and Erysipelotrichia. The abundances of Bifidobacteriales, Coriobacteriales, and Erysipelotrichales were enriched by Bb M-16V at the order level. Bb M-16V enhanced the abundances of seven families, including Corynebacteriaceae, Bifdobacteriaceae, Coriobacteriaceae, Porphyromonadaceae, Christensenellaceae, Eubacteriaceae, and Erysipelotrichaceae, while it inhibited Peptostreptococcaceae.
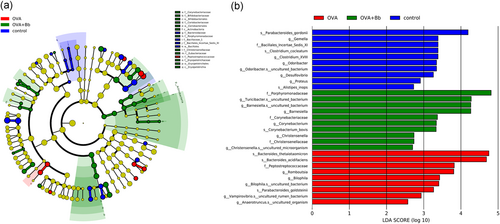
Besides, the comparison of LDA is exhibited (Figure 6b). Compared with the baseline composition of the microbiota in the control group, OVA sensitization had upregulated seven bacteria proportion, including, Bacteroides, Peptostreptococcaceae, Romboutsia, Bilophila, Parabacteroides, Vampirovibrio, and Anaerotruncus, but bacteria like Parabacteroides, Gemella, Bacillales, Clostridium, Odoribacter, Desulfovibrio, Proteus, and Alistipes were significantly downregulated. Simultaneously, Bb M-16V intervention had increased seven bacteria proportion, including Porphyromonadaceae, Turicibacter, Barnesiella, Corynebacteriaceae, Corynebacterium, Christensenella, and Christensenellaceae, reducing the abundance of bacteria increased by OVA.
3.6 The oral administration of Bb M-16V regulated the functional gene composition of gut microbiota
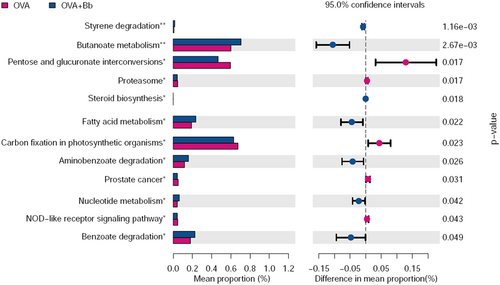
To explore the impact of Bb M-16V intervention on physiological function, we used PICRUSt to analyze and predict the functional gene composition in the metabolic pathways. The result showed that Bb M-16V significantly regulated the 12 subfunctional gene composition of gut microbiota (Figure 7). Before Bb M-16V treatment, OVA sensitization upregulated the functional genes, including Pentose and glucuronate interconversions, Proteasome, Carbon fixation in photosynthetic organisms, Prostate cancer, and NOD−like receptor signaling pathway, whereas after Bb M-16V treatment, six other functional genes, for instance, Styrene degradation, Butanoate metabolism, Fatty acid metabolism, Aminobenzoate degradation, and Benzoate degradation. The results indicated that Bb M-16V treatment significantly influenced the functional gene composition of gut microbiota.
3.7 The gut microbiota taxa in the feces associated with immune factors and immunoglobulin in serum
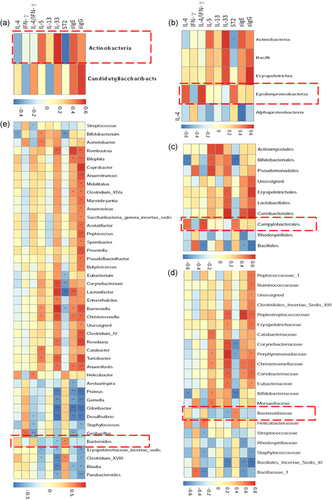
Next sparse canonical correlation analysis was used to identify nine immunological variables and gut microbiota taxa that associated with each other. The gut microbiota with the highest positive and negative loading indicated that most closely related to immunological variables. The results displayed that the concentration of Epsilonproteobacteria and Campylobacterales were positively correlated with the IL-4/IFN-γ (Figure 8a-e), indicating that these bacteria can induce Th2-mediated allergic reaction. On the other hand, Bacteroidaceae and Bacteroides were negatively correlated with IL-33 and positively correlated with ST2, while Actinobacteria was opposite to these bacteria, which corresponded with the analysis results of immune factors and gut microbiota (Figures 3d, 4, and 5).
4 DISCUSSION
In the current study, different doses of OVA were used to induce FA, corresponding Bb M-16V treatment group was also assigned. After the determination, we set up a mouse model of FA by 20 µg OVA as a tool for recognizing mechanisms, describing the vital immune components of the stage of effect of FA response, as well as revealing the composition changes of gut microbiota during the development of sensitization. Several crucial aspects of the impact of immune imbalance and dysbacteriosis in FA have emerged from our current study. The important function of Bb M-16V in maintaining the balance between the host intestinal immune system and microbiota composition was also demonstrated in this study.
First, immune imbalances play an important role in the FA. Our observations demonstrate that an increase in the Th2-type cytokine IL-4 is accompanied by an upregulation of the IL-4/IFN-γ in the context of allergic disease and that IL-4 can be partially activated with the enhancement of functional IL-33/ST2 signaling, which may partly explain the correlation between the IL-33/ST2 signaling and the development of FA. Our data further suggest that the absence of IL-33/ST2 signaling may hinder the role of OVA, which is also a contribution of Bb M-16V. Some studies have presented similar conclusions in other antigen-specific mouse models (De Grove et al., 2018; Teufelberger et al., 2018).
Second, many studies have shown that the FA are accompanied by the dysbiosis in recent years (Fu, Fu, Wang, Xie, & Wang, 2019; Ly, Litonjua, Gold, & Celedon, 2011). We have identified the changes of dynamic dysbiotic in the intestinal flora of FA mice induced by OVA, evidenced by the failure of gut microbiota of mice with FA to protect against FA. Whereas, in the same model, the beneficial microbiota of mice treated with Bb M-16V has an obvious protective effect as compared to that of FA mice, indicating an imperative role of dysbiosis in FA. On the other hand, Bb M-16V treatment reconstructs the microbiota in terms of the increment of Actinobacteria as well as the decrement of five bacterial taxa, which is partially consistent with previous studies. The study found a significant decrease of Actinobacteria in the feces of the infant with FA (Ling et al., 2014). Nishijima et al. (2016) found that the Japanese exhibited a higher life expectancy, possibly related to the higher abundance of Actinobacteria. Whereas the proportion of Bacteroidaceae in the intestinal flora of infants with allergy was higher (Loo et al., 2018). The association between some bacterial communities and allergic diseases is still contentious. The study indicated that Bacteroidetes were significantly enriched in patients with FA (Berni Canani et al., 2018). Whereas a significantly higher rate was found in nonallergic infants compared to infants with FA (Diaz et al., 2018). Moreover, there is not enough data on whether the Epsilonproteobacteria and Campylobacterales affect allergic diseases, and further research is needed to clarify the mechanism. Although there is some debate about these mechanisms, these observations may raise some questions worthy of further exploration.
In addition, we applied LEfSe software to analyze the gut microbiota community in the FA and Bb M-16V-intervened group, attempting to explore the bacterial taxonomic markers related to Bb M-16V intervention, which have been proved to be a critically important way to transform the molecular and genomics data into the clinical applications. The data showed that Bacteroides could be used as a typical biomarker of the FA group, while that of the intervention group was Porphyromonadaceae. In other words, the most significant difference before and after the intervention was that the abundance of Bacteroides decreased while Porphyromonadaceae increased. These data might prove useful for the rapid identification of FA through clinical microbioassay. Meanwhile, the changes in microbial community structure are associated with the variation of functional gene abundance. Therefore, we used PICRUSt to predict functional gene abundance and metabolic pathways. The results indicated that the process of FA might be accompanied by the activation of proteasome and NOD-like receptor signaling pathway, which was prone to the release of inflammatory factors and disease outcomes such as prostate cancer. While Bb M-16V interventions might help degrade harmful compounds, promote fatty acids metabolism, and inhibit the production of inflammatory factors, reducing the risk of cancer.
Finally, we have attempted to further explore the correlation between immunological variables and gut microbiota taxa. The correlation analysis revealed the contribution of the immune response to maintaining the intestinal homeostasis. The results highlighted the association between the immune system and FA, with evidence that it is mediated in part by gut microbiota.
In summary, we speculate that the existence of an antibacterial Th2 response may aggravate the pathogenic immunoreaction to food and may play a key role in the initiation, persistence, and outcome of the disease. Raising awareness of FA from this animal model study may provide insights into the etiology of FA in humans and experimental confirmation of epidemiological findings, contributing to developing new strategies for preventing and treating FA.
ACKNOWLEDGMENTS
This work was funded by grants from the China Postdoctoral Science Foundation (Grant No. 2019M661567), China, and the Dipro Medical Research Foundation (MIHC/2019/02/Di-AMV/BL02), China. We also gratefully acknowledge the assistance of Genesky Biotechnologies, Inc., Shanghai, 201315 detecting intestinal flora.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHORS CONTRIBUTIONS
N. L. designed the study and drafted the manuscript. Y. Y. participated in the study design. X. C. conducted laboratory experiments and carried out the data collection. S. G. and Q. Z. participated in data processing. All the authors reviewed and approved the final version of the manuscript.
Open Research
DATA AVAILABILITY STATEMENT
All data included in this study are available upon reasonable request from the corresponding author.




