RETRACTED: MicroRNA-29a-3p regulates abdominal aortic aneurysm development and progression via direct interaction with PTEN
Abstract
Various research studies have been conducted in deducing the role of microRNAs (miRNAs) in the pathogenesis and physiological processes of various systematic diseases. This study aims at demonstration of the important role played by miR-29a-3p, through association with phosphatase and tensin homolog (PTEN), in the regulation of abdominal aortic aneurysm development and progression. Quantitative real-time polymerase chain reaction (RT-qPCR) examined miRNA-19a-3p and PMEPA1 expression in multiplied vascular smooth muscle cells (VSMCs). Cell transfection upregulated or downregulated the genes and cell counting kit-8 assay determined cellular viability. RT-qPCR detected cellular proliferation and cell death using the cell proliferation and apoptosis biomarkers Ki87 and proliferating cell nuclear antigen, caspase-8 and caspase-3, respectively. Furthermore, luciferase assay analyzed the luciferase activity and western blot analysis determined miRNA-19a-3p and PMEPA1 protein expression in proliferation and apoptosis biomarkers. TargetScan 4.2 online software (www.targetscan.org) was used to perform the bioinformatics analysis so as to forecast the putative targets of miR-29a-3p and PTEN. The results inferred that there was an increased expression of miRNA-29a-3p found in AAA-mimic cells with increased cellular viability and significant pathological apoptosis. Further, when the expression of miRNA-29a-3p was downregulated, it reduced the cell viability of AAA cells. On the basis of the gene interplays, it can be understood that the PTEN was directly targeted by miRNA-29a-3p so as to regulate the AAA progression. Thus, PTEN was found to strengthen the proliferation effect of miRNA-29a-3p in AAA cells. The current study thus shed more insights about the molecular mechanistic roles of miRNA-29a-3p and PTEN, opening doors for novel therapeutic approach to AAA.
1 INTRODUCTION
Abdominal aortic aneurysm (AAA or triple A) is often asymptomatic except the feeling of pain at the time of rupture and is a localized enlargement that occurs in the abdominal aorta (Lee, Martin, & Mohiuddin, 2006; Lumsden, Lin, Bush, & Chen, 2006), which ends fatally (Patel et al., 2020). Close to 2–8% of the men above 65 years, with family history have the chance of being affected with and the prevalence among females is one-fourth as higher than males (Wilson, 2006). With high mortality rates, the patients with AAA rupture often (up to 65–75%) die before admission in hospital, while 90% meet fatality before they undergo surgical procedure (Frønsdal, Sæterdal, Harboe, Klemp, & Fure, 2014; Starodubtsev, 2017). Few additional risk factors are inclusive of high blood pressure, smoking, heart and blood vessel diseases (Wilson, 2006). As mentioned earlier, the family history, that is, genetic conditions also play a vital role in high-risk patients and the relevant conditions are termed as Marfan syndrome and Ehlers-Danlos syndrome (Davis, Rateri, & Daugherty, 2015; Hinchliffe et al., 2001). In vascular medicine, still the diagnosis and treatment procedures are highly challenging for AAA. Surgical procedure is recommended for the male patients if the diameter of AAA is above 5.5 cm whereas in females, the threshold is >5.0 cm (Davis et al., 2015; Golledge, Norman, Murphy, & Dalman, 2017; Sakalihasan, Limet, & Defawe, 2005).
In cardiovascular pathology, microRNAs (miRNAs) play a crucial role as regulators. Being ~20-nucleotide, single-stranded RNA molecules that target messenger RNA (mRNA), these miRNAs can possibly act as the targets for the suppression on AAA expansion. According to Kumar, Boon, Maegdefessel, Dimmeler, and Jo (2019), studies have underscored the association of miRNAs with AAA, based on the recent developments in miRNA microarray profiling research. There is still a lack of knowledge about their role in AAA disease and their therapeutic potential to prevent or arrest the development of aneurysm (Joviliano, Ribeiro, & Tenorio, 2017; Kumar et al., 2019). Various research studies have been conducted in deducing the role of miRNAs in the pathogenesis and physiological processes of cardiovascular diseases (Chan, Cheuk, & Cheng, 2017; Tian et al., 2018). However, special attention has been given to few miRNAs, for instance miR-29. miR-29a is a member of miR-29 family and has an association with fibrosis and formation of aneurysm (Boon & Dimmeler, 2011; Kriegel, Liu, Fang, Ding, & Liang, 2012). This miRNA is expressed aberrantly in different tumors and also in myocardial infarcted regions (Van Rooij et al., 2008). Maurer et al. (2010) mentioned that this miRNA creates an impact in various pathological processes inclusive of tumor growth and apoptosis. The upregulation of this miRNA has an association with notable reduction in the number of extracellular matrix proteins (Milewicz, 2012). It has also been identified to modulate the gene expression at the time of development and aging of aorta and at the time of development of aortic aneurysms (Lu, Rateri, Bruemmer, Cassis, & Daugherty, 2012). According to a study, the miR-29 promotes pathological hypertrophy as well as overall cardiac insufficiency of cardiac myocytes instead of fibrotic heart disease (Maegdefessel, Dalman, Tsao, 2014). These research investigations infer that miR-29 has a role in the development of cardiovascular diseases, though this role is highly questioned in cardiac function or atherosclerosis (Kriegel et al., 2012). In mice model, the potential of miR-29 silencing in treating aneurysm was proved when the locked-nucleic-acid (LNA)-modified antimiR silencing of miR-29 decreased the aorta dilation induced by angiotensin II. There is an association that exists between miR-29a-3p and cardiovascular diseases. But there are no studies available to deduce the role played by miR-29 vascular endothelial dysfunction and relevant mechanisms (De Rosa, Curcio, & Indolfi, 2014; McManus & Freedman, 2015).
Being a liquid and protein phosphatase, the phosphatase and tensin homolog (PTEN) acts as a crucial tumor suppressor protein in vascular integrity. The relevant modifications in PTEN signaling function as a significant initiating determinant that can drive pathological vascular remodeling (Milella et al., 2015). In this study, the authors experimented about the regulatory effects of miR-29a-3p and whether its putative gene targets, which create an impact on proliferation, apoptosis, and inflammation, are able to generate a novel therapeutic approach to counteract the AAA disease. This study aims at demonstration of the important role played by miR-29a-3p, through association of PTEN, in the regulation of abdominal aortic aneurysm development and progression.
2 MATERIALS AND METHODS
2.1 Cell treatment and transfection
The institutional ethical board approved the current study protocol. Primary aortic endothelial cells; normal, human (HAEC; ATCC® PCS-100-011) were propagated using the Dulbecco's modified Eagle's medium medium with streptomycin (100 µg/ml), l-glutamine (2 mM), and fetal bovine serum (7–10%) in line with the guidelines put forth by the manufacturer (Thermo Fisher Scientific, Inc.). In 37°C, the cell lines were cultured and incubated with 5% CO2 till it gets saturated or up to 48 hr, which ever was earlier.
2.2 Preparation of transfected and control cells
The authors procured the miRNA-29a-3p, PTEN mimics, and the relevant negative control mimics from Guangzhou Fulengen Co., Ltd. With the help of Lipofectamine 3000 (Beyotime, Shanghai, China), the cells (primary aortic endothelial cells) were transfected with miRNA-29a-3p and PTEN mimics or inhibitors that are inclusive of negative control mimics, in line with the instructions from the manufacturer. After 48 hr, the transfection efficiency was measured for 24 hr. Following this, the cells (1 × 105 cell per well) were propagated in six-well dishes and preserved by 120 nM angiotensin II (Beyotime) for 18 hr at 37°C, before preserving in 100 nM of lipopolysaccharide (Thermo Fisher Scientific, Inc.) for 4 hr at 37°C.
2.3 Quantitative reverse-transcription polymerase chain reaction (RT-PCR) assay
After the saturation, that is, formation of monolayer, the cells were harvested and the procedure to isolate total RNA was performed using TRIzol reagent according to the manufacturer's instructions (Thermo Fisher Scientific, Shanghai, China). The BeyoRT™ cDNA First Chain Synthesis Kit was used to perform the reverse-transcription for every specimen according to the guidelines from the manufacturer. Quantitative RT-PCR assays were performed using SYBR Green QPCR Mix (Thermo Fisher Scientific, China). The associated mRNA qRT-PCR detection kit was utilized to determine the miRNA-29a-3p and PTEN gene expression quantities for angiotensin II treatment, respectively, using an Applied Biosystems Vii7 RT-qPCR instrument (ABI, Vernon, CA). The primer sequences were synthesized by Genelily BioTech Co., Ltd. (Shanghai, China). After this, both U6 and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were normalized and the method was followed. The above-mentioned experiments were performed three times.
2.4 Western blot analysis assay
The authors performed the western blot assay and the proteins were quantified for cell cycle as well as the apoptosis-related biomarkers such as Ki67, proliferating cell nuclear antigen (PCNA), caspase-3, and caspase-8. After exposing the HAEC to angiotensin II for different periods, the proteins were extracted, rinsed twice using cold phosphate-buffered saline, and then made to undergo lysis to test load up buffer. The load up buffer expression was 1.5% sodium dodecyl sulfate (SDS), 10% glycerol, 5 mM β-mercaptoethanol, bromophenol blue and 75 mM Tris (pH 7.0). Using 12% polyacrylamide gel electrophoresis (SDS-PAGE) gel, the whole cell lysates were separated and the proteins were moved onto a polyvinylidene fluoride membrane. In addition to the above, the coatings were also cultured and probed with antibodies, that is, Ki67, PCNA, caspase-3, and caspase-8, under 4°C overnight. After establishing the immunoblots, it was observed using ECL Western blot medium (Thermo Fisher Scientific). The internal controls were U6 and GAPDH. Each group analysis was repeated three times. In this study, the image J detection system was used to find out the band concentration.
2.5 Cell viability and proliferation assays by cell counting kit-8 (CCK-8) assay
With the help of CCK-8, both cell viability as well as its proliferation were determined. This is because the kit mentioned above enables simple assays that deploy WST-8 (2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium, monosodium salt), which, in turn, produce a water-soluble formazan dye upon bioreduction in the presence of an electron carrier, 1-methoxy PMS. In 96-well multiplying plates, the transfected cells of 4 × 103 density per well were planted between 0 and 48 hr. The cellular viability was measured using CCK (#C0037; Beyotime) standard protocol as per the manufacturer's specifications. The microplate detector apparatus (Molecular Devices, Sunnyvale) quantified the wavelength absorbance at 450 nm.
2.6 Bioinformatics analysis
In this study, TargetScan 4.2 online software (www.targetscan.org) was used to perform the bioinformatics analysis so as to forecast the putative targets of miR-29a-3p and PTEN. The software forecasts the miRNA-29a-3p biological targets by conducting a search for the availability of conserved sites, which align with the seed region of each miRNA. A total of 79 conserved mammalian miRNAs were used by TargetScan, from Rfam. In every transcript, the sites that possess high as well as low probability of targeting by miRNAs were displayed. This probability was determined after the inclusion of complete algorithms and parameters (site type, context++ score, context++ score percentile, weighted context++ score, conserved branch length, and PCT) for each miRNA candidate. After considering the aggregate PCT, cumulative weighted context++ score or only with conserved sites, the probability was ranked.
2.7 Luciferase reporter assay
The target sequence PTEN with the wild type (WT) or mutant type (MT) and miRNA-29a-3p connecting positions were produced and replicated into a pGL3 Dual-luciferase target vector (Promega) to create WT and MT PTEN plasmids. These WT or MT PTEN plasmids were cotransfected into treated human umbilical vein endothelial cells along with NC mimics or miRNA-29a-3p mimic (Sigma-Aldrich; Merck KGaA) using Lipofectamine 6000 following the manufacturer's instructions. Luciferase activity was measured using the Dual-Glo luciferase report assay system (Promega, Madison) after 48 hr using the manufacturer's instructions. The authors developed the target sequence PTEN with WT or MT as well as the miRNA-29a-3p connecting positions. This was then replicated into a pGL3 Dual-luciferase target vector (Promega) to yield WT and MT PTEN plasmids. Along with these WT or MT PTEN plasmids, the coinfection was performed for human umbilical vein endothelial cells with NC mimics or miRNA-29a-3p mimic (Sigma-Aldrich; Merck KGaA) with the help of Lipofectamine 6000 according to the instructions put forth by the manufacturer. The authors deployed Dual-Glo luciferase report assay system to quantify the luciferase activity (Promega) after 48 hr, in line with the guidelines from the manufacturer.
2.8 Statistical analysis
Separate trials were conducted three times and the experimental data is showcased here as average and standard error. In this study, GraphPad Prism 5 software as well as SPSS 18.0 version was used to analyze the study data. The authors applied Student's t test and analysis of variance. p < .05 is considered to be significant.
3 RESULTS
3.1 miRNA-29a-3p was upregulated in AAA-mimic cells and promoted cellular viability
As per the qRT-PCR assay results, there were relatively higher expressions of miRNA-29a-3p in angiotensin II-treated HAEC with time (0–48 hr) in comparison with the untreated cultured HAEC for AAA (Figure 1a, *p < .05). But the peak expression level was attained after 48 hr with the increase being gradually exponential at every time. From the outcomes of CCK-8 assay, it can be inferred that there was a dramatic increase in the cell viability among the angiotensin II-treated HAEC for AAA with time (0–48 hr) in comparison with untreated cultured HAEC (Figure 1b, *p < .05). But, as earlier, the peak proliferation could be observed after 48 hr with the increase being gradually exponential at every time. The proliferation biomarkers such as Ki67 and PCNA were examined using RT-PCR to confirm the cellular proliferation. There was a significant increase noted in the cellular proliferation with time (0–48 hr) in Ki67 biomarker examination, among angiotensin II-treated HAEC in comparison with untreated HAEC (Figure 1c, *p < .05). Even though the increase improved with time the peak expression level was achieved only at 48 hr. In case of PCNA biomarker, alike earlier, there was a significant increase in the proliferation time (0–48 hr) among angiotensin II-treated HAEC compared with the untreated HAEC (Figure 1c, *p < .05).
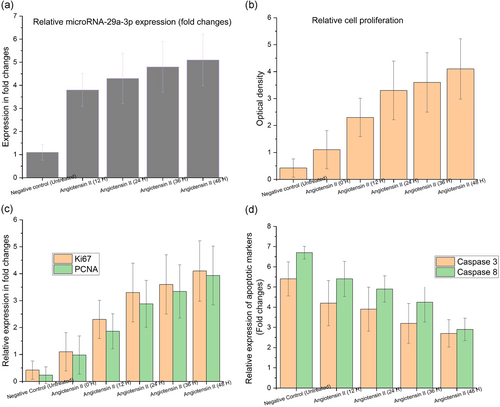
The peak expression level was attained after 48 hr with increase observed with time. Both caspase-8 and caspase-3 activity assays were conducted to confirm apoptosis. In comparison with the untreated HAEC (Figure 1d, *p < .05), there was a notable decrease in the apoptosis rate among angiotensin II-treated HAEC for AAA in caspase-8 results. But the decrease was gradually improving with time while the least expression was observed after 48 hr. In case of caspase-3 results, the cell apoptosis in angiotensin II-treated HAEC for AAA got notably reduced with time (0–48 hr) in comparison with untreated HAEC (Figure 1d, *p < .05). The least expression level was observed after 48 hr while the decrease improved with time. The next experiments made use of angiotensin II-treated HAEC. The general implications from these results are that the expression level of miRNA-29a-3p was elevated in AAA-mimic cells, which improved cellular viability with significant pathological apoptosis.
3.2 miRNA-29a-3p knockdown suppresses cell viability of AAA cells
CCK-8 assay was used to determine the impact of miRNA-29a-3p upon the cellular viability of angiotensin II-treated HAEC. First, transfection of angiotensin II-treated HAEC was performed using control mimics or miRNA-29a-3p-inhibitor during different time intervals in the range of 12–48 hr. After this, the knockdown efficiency of miRNA-29a-3p was evaluated using RT-qPCR. From the results, it can be inferred that there was a dramatic knockdown in the expression levels of miRNA-29a-3p for miR-29a-3p-inhibitor when contrasted with control mimics in all the varied times (12–48 hr) (Figure 2a, p < .05). In addition to the above, CCK-8 assay was used to assess the impact on cellular viability by miRNA-29a-3p among the angiotensin II-treated HAEC. The observations were carried out at different time intervals from 12 to 48 hr. From the results achieved at 12 hr, it can be inferred that the cell viability was low in the silenced miRNA-29a-3p group compared with control mimics (Figure 2a, p < .05). So, one more observation was performed after the completion of 24 hr. Those results showed a drastic reduction in the cell viability in silenced miRNA-29a-3p group compared with control mimics (Figure 2b, *p < .05). The cell viability was found to be the least after 48 hr in miRNA-29a-3p-inhibitor when compared with negative control group (Figure 2b, **p < .05). These results infer that the miRNA-29a-3p's knocked down expression inhibited cellular viability of AAA cells.
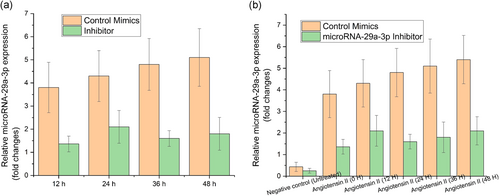
3.3 miRNA-29a-3p directly binds to 3′-untranslated region (3′-UTR) of PTEN to moderate PTEN expression and AAA progression
Bioinformatics analysis was conducted using TargetScan software to substantiate the assertion that miRNA-29a-3p has a direct interaction with PTEN in the regulation of AAA progression. Figure 3a shows that the results found matching pairing sequences among miRNA-29a-3p and 3′-UTR of PTEN. So the authors developed the luciferase reporter vector that possess WT or MT miR-29a-3p binding locations in PTEN so as to authenticate the binding. As a consequence, the transfection of control mimics or miR-29a-3p mimics to WT or MT was performed to confine the luciferase activity. From the results, it can be inferred that there was a dramatic increase in the luciferase activity of WT PTEN 3′-UTR for miR-29a-3p mimic transfected cells. However, this luciferase activity was found to be decreased in those cells that were infected with negative control (Figure 3a, p < .05). But there was no such impact observed in luciferase activity of MT- PTEN (Figure 3a, p < .05). RT-qPCR was used to examine the PTEN expression levels at different times such as 12–48 hr in HAEC. The results showcased a steep reduction in the expression of PTEN in angiotensin II-treated HAEC for AAA with time (0–48 hr) in comparison with untreated HAEC (Figure 3b, p < .05). But the decrease gradually went up with time while the least expression was observed after 48 hr. From these results, it can be inferred that PTEN was a direct target of miRNA-29a-3p in the regulation of AAA progression.
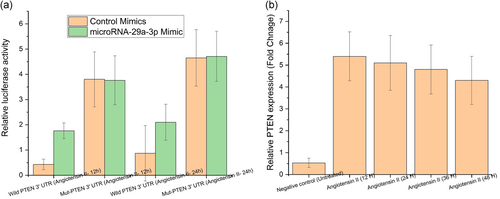
3.4 Downregulated PTEN strengthens the proliferation influence of miRNA-29a-3p in AAA cells
As discussed in the sections above, the miRNA-29a-3p manifestations after 48 hr were significantly higher in AAA angiotensin II-treated HAEC. Further after 48 hr, the PTEN expression was notably knocked down in AAA angiotensin II-treated HAEC. So the current section discusses about the angiotensin II-treated HAEC (48 hr) exposed model adopted for the study. Furthermore, CCK-8 assay was performed to explore the molecular mechanisms that underlie between PTEN and miR-29a-3p during cellular proliferation. At first, the angiotensin II-treated HAEC after 48 hr was transfected with miRNA-29a-3p-NC, miRNA-29a-3p mimic + si-control, or miRNA-29a-3p mimic + si-PTEN. The next step was RT-qPCR to assess the PTEN expression. The results showcased that the PTEN expression in miRNA-29a-3p mimic + si-PTEN group got decreased in comparison with miRNA-29a-3p-NC and miRNA-29a-3p mimic + si-control groups (Figure 4a, p < .05). Further, a significantly higher cellular viability was observed from the CCK-8 assay results among miRNA-29a-3p silenced (miRNA-29a-3p mimic + si-control) and control (miRNA-29a-3p-NC) groups. However, among overexpressed (miRNA-29a-3p mimic + si-PTEN) transfected groups (Figure 4a, p < .05), the cell viability got visibly decreased. The proliferation biomarkers authenticated the outcomes for both Ki67 and PCNA after conducting the RT-qPCR assay. The results inferred that there was a dramatic reduction in the proliferation among miRNA-29a-3p mimic + si-PTEN group when compared with the high proliferation rate in miRNA-29a-3p-NC and miR-29a-3p mimic + si-control groups for both Ki67 and PCNA (Figure 4b, p < .05). The apoptosis biomarkers authenticated the outcomes for both caspase-8 and caspase-3 after performing the RT-qPCR. The results inferred that there was a significantly increased apoptosis rate in miR-29a-3p mimic + si-PTEN group when compared with the reduced proliferation in miRNA-29a-3p-NC and miRNA-29a-3p mimic + si-control groups for both caspase-8 and caspase-3 (Figure 4c, p < .05). In addition, the protein quantities of PTEN were determined using western blot analysis. In case of proliferation biomarkers such as (Ki67 and PCNA), the PTEN expression was found to be drastically low in miR-29a-3p mimic + si-PTEN group compared with the peak proliferation in miRNA-29a-3p-NC and miRNA-29a-3p mimic + si-control groups for both Ki67 and PCNA (Figure 4e, p < .05). There was an instant increase in the PTEN protein expression in miRNA-29a-3p mimic + si-PTEN group, in case of apoptosis biomarkers (caspase-8 and caspase-3), and when these results were compared, there was less proliferation observed in miRNA-29a-3p-NC and miRNA-29a-3p mimic + si-control groups for both caspase-8 and caspase-3 (Figure 4c, p < .05). So, it can be generally understood that the PTEN strengthened the proliferation effect of miRNA-29a-3p in AAA cells.
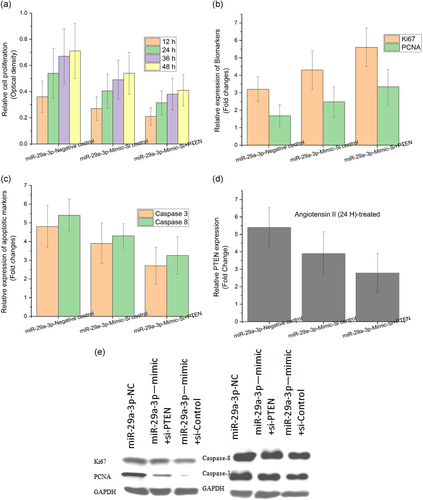
4 DISCUSSION
Globally, AAA is one of the leading causes of morbidity and mortality. Various clinical studies conducted across different populations infer that AAA is prevalent among 2.4–16.9% in males, while in females, it is in the range of 0.5–2.2%, above 65 years (Sidloff et al., 2014; Vasdekis et al., 2008). Acute rupture is the most severe form of clinical consequence, which often (close to 80%) leads to mortality. AAA patients, close to 60%, die due to cardiovascular diseases such as myocardial infarction or stroke, which infers that there exists a relationship between AAAs and atherosclerosis (Lederle et al., 2009; Moxon et al., 2010). It remains a big clinical challenge to decode the underlying mechanisms and appropriate medical intervention for reducing the mortality and morbidity of AAAs, though there were numerous efforts taken so far following traditional approaches. The discovery of a novel gene regulation method via miRNAs, their validation as markers and modulators of vascular remodeling in pathological conditions paved the way for new therapeutic pathways to develop innovative and insight-based therapies (Iyer, Rowbotham, Biros, Bingley, & Golledge, 2017; Wanhainen et al., 2017).
The lentiviral hyperexpression of miR-21-induced cell proliferation decreases the apoptosis in aortic wall along with protective impact upon the progression of aneurysm. The overexpression of miR-21 significantly reduced the expression of PTEN protein (Maegdefessel, Azuma, Toh, Deng, et al., 2012; Maegdefessel, Azuma, Toh, Merk, et al., 2012). This results in the increased phosphorylation and activation of AKT, a major part in proproliferative and antiapoptotic pathways. When a locked nucleic acid–modified antagomir is systematically injected, targeting miR-21, it notably reduces the proproliferative impact of downregulated PTEN, resulting in significant increase in AAA size. The results were similar in mice model with AAA augmented by nicotine as well as in human aortic tissue samples from patients who undergo surgical repair of AAA (with more pronounced effects observed in smokers; Milewicz, 2012). Being novel gene manifestation controlling factor, the miRNAs have the ability to diminish the transformation activity of mRNA translating proteins. This occurs when the former gets attached with the targeted mRNA 3′-UTR and stimulate the controlling properties (Liu et al., 2010). The miRNAs do play an important role in apoptosis, cell differentiation, cell proliferation, and metabolism (Wanhainen et al., 2017). As a consequence, various investigations concluded that multiple miRNAs are closely related in the onset and progression of AAA. These relationships also control the external cell matrix degeneration, vascular soft muscle cellular growth and death, inflammation and angiogenesis. The miR-21 modulation in AAA disease is a common practice followed in treating cardiovascular disease as a protective physiological response. For instance, natriuretic peptides (e.g., brain natriuretic peptide) get released from left ventricle during high filling pressures. The sole aim of these peptides is to inhibit the volume overload and to ensure the cardiac function. Similar way, in arresting the AAA expansion and vascular disease progression, a significant role is played by the modulation of miR-29 expression (Milewicz, 2012; Slusarz & Pulakat, 2015). The development of AAA results in the increased expression of miR-29a-3p and decreased expression of PTEN. This finally results in pro-proliferative and antiapoptotic response of smooth muscle cells inside the vessel wall, most likely in an attempt to safeguard the aorta from further expansion and ultimate rupture. This was the case observed in both the murine AAA models and one human represented AAA samples. The miR-29 induced inhibition of PTEN/PI3K/AKT signaling pathway remains crucial in arresting the expansion of AAA. This is the mechanism that occurs when miR-21 was overexpressed through treatment with pre-21. In addition, when treated with anti-21, the pro-proliferative effects of downregulated PTEN got reduced. This occurs by preventing the upregulation of miR-21, resulting in the marked acceleration of AAA development and at times, rupture in nicotine-supplemented animals (Maegdefessel, Azuma, Toh, Deng, et al., 2012; Maegdefessel, Azuma, Toh, Merk, et al., 2012).
As far as aortic dilatation is concerned, the study identified that miR-21 got notably upregulated in both the established murine models of AAA disease: porcine pancreatic elastase infusion model in C57B/L6 mice and the Ang II-infusion in ApoE−/− mice. In the models mentioned above, vascular smooth muscle cell-specific miR-21 target genes modify the proliferation and apoptosis (Maegdefessel et al., 2013; Maegdefessel, Dalman, et al., 2014). The PTEN was found to be the only target gene, which got notably downregulated three times at the time of development and progression of aneurysm. Being a crucial tumor suppressor gene, PTEN a lipid and protein phosphatase functions as a significant negative regulator in PI3K pathway. When LNA modified antagomiR was systematically injected against miR-21, it reduced the proproliferative impact of downregulated PTEN resulting in a substantial upsurge in AAA expansion. In addition, there were significant protective effects observed upon the expansion of aneurysm when the aortic PTEN with pre-miR-21-loaded lentivirus was downregulated. This would have occurred by inducing the massive proliferation in the aortic wall in both murine models (Maegdefessel et al., 2013; Maegdefessel, Azuma, Toh, Deng, et al., 2012; Maegdefessel, Azuma, Toh, Merk, et al., 2012; Maegdefessel, Dalman, et al., 2014).
5 CONCLUSION
The current research article comprehensively discusses the molecular mechanism between miRNA-29a-3p and PTEN in the development and progression of AAA. This study fills the research gap existing in this area. Based on the gene interplays, it can be understood that the PTEN was directly targeted by miRNA-29a-3p so as to regulate the AAA progression. Thus, PTEN was found to strengthen the proliferation effect of miRNA-29a-3p in AAA cells. The current study thus throws more light on the molecular mechanistic roles of miRNA-29a-3p and PTEN, opening doors for novel therapeutic approach to AAA.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Y. Z., M. W., and J. H.: concepts, design, data analysis, statistical analysis, manuscript preparation, manuscript review, guarantor. P. X. and H. W. definition of intellectual content, literature search, experimental studies, data acquisition, manuscript editing.
ETHICS STATEMENT
Ethical approval was obtained from IEC of Sichuan Academy of Medical Sciences, Sichuan Provincial People's Hospital. Informed consent was obtained from the participants with the option to withdraw them from the study at any time.
Open Research
DATA AVAILABILITY STATEMENT
All data provided in this study, as well as the raw data can be obtained on request from the corresponding author.




