A novel role of VEPH1 in regulating AoSMC phenotypic switching
Xiaofeng Shi and Caiming Xu contributed equally to this study.
Abstract
Abdominal aortic aneurysm (AAA) is a potentially lethal disease featured by focal dilatation in the aorta. The transition of vascular smooth muscle cells (SMCs) from a contractile/differentiated to a synthetic/dedifferentiated phenotype is considered to contribute to AAA formation and expansion. Our previous gene microarray data showed that Ventricular Zone Expressed PH Domain Containing 1 (VEPH1) expression increased in angiotensin II (Ang II)-infused aortic tissues. This study was thus performed to further explore the role of VEPH1. Herein, we first demonstrate that VEPH1 increases in the SMCs of Ang II-treated abdominal aortas. As in vivo, Ang II also upregulated VEPH1 expression in cultured hAoSMCs. The dedifferentiation of human aortic SMCs (hAoSMCs) was induced by a 24-hr stimulation of Ang II (1 μM)—the expression of contractile SMC markers, MYH11 and α-smooth muscle actin (α-SMA) decreased and that of synthetic markers, proliferating cell nuclear antigen and Vimentin increased. Inhibition of VEPH1 prevented Ang II-induced pathological dedifferentiation of hAoSMCs as indicated by the restored expression of MYH11 and α-SMA. In contrast, the forced overexpression of VEPH1 aggravated Ang II's effects. Furthermore, we demonstrated that VEPH1 and transforming growth factor-β1 (TGF-β1), a key regulator responsible for vascular SMC differentiation, negatively regulated each other's transcription. In contrast to VEPH1 silencing, its overexpression inhibited recombinant TGF-β1-induced increases in MYH11 and α-SMA and suppressed Smad3 phosphorylation and nuclear accumulation. Collectively, our study demonstrates that VEPH1 elevation promotes the synthetic phenotype switching of AoSMCs and suppressed the TGF-β1/Smad3 signaling pathway. Identification of VEPH1 as a pathogenic molecule for AAA formation provides novel insights into this disease.
1 INTRODUCTION
Abdominal aortic aneurysm (AAA) is a potentially lethal vascular disease involving a pathological dilation of the aortic wall (Sakalihasan, Limet, & Defawe, 2005). This disease is often asymptomatic until the fatal event of rupture (Sakalihasan et al., 2005). At present, endovascular or open surgical repair is the only treatment for AAA in clinics (Golledge, 2019; Lu, Rateri, Bruemmer, Cassis, & Daugherty, 2012). There is a lack of effective therapy for AAA due to poor understanding of the mechanisms underlying AAA pathophysiology.
Destruction of elastic media that involves smooth muscle cell (SMC) pathology is one of the main features of clinical AAA (Inoue & Node, 2009; Sakalihasan et al., 2005). SMCs retain a remarkable plasticity and form the walls of blood vessels (Frismantiene, Philippova, Erne, & Resink, 2018). Under a physiological condition, the differentiated medial vascular SMCs maintain quiescence and express contractile-associated proteins important for vessel contraction/dilation, such as the smooth muscle myosin heavy chain (MYH11) and α-smooth muscle actin (α-SMA; Frismantiene et al., 2018). However, SMCs can switch into a dedifferentiated phenotype in response to multiple environmental stimuli, and express synthetic molecules, such as proliferating cell nuclear antigen (PCNA) and Vimentin (Frismantiene et al., 2018; McDonald & Owens, 2007). Several earlier studies have revealed that promoting SMC differentiation is capable of limiting experimental AAA formation (Huang et al., 2018; Liang, Bai, et al., 2018). Such findings highlight the importance of identifying novel regulators of SMC plasticity.
The transforming growth factor-β1 (TGF-β1) signaling pathway plays a pivotal role in vascular SMC differentiation (Zhang et al., 2019). Habashi et al. (2006) found that the TGF-β pathway is activated in a Marfan mouse model, and that the anti-thoracic aortic aneurysm/dissection (TAA/D) effects of angiotensin (Ang) II type I receptor blockers are associated with the deactivation of TGF-β signaling. In contrast, Huang et al. (2018) proved that vascular SMCs lacking TGF-β signals switched from the contractile to the synthetic phenotype. They also demonstrated that mice with impaired TGF-β signals had exacerbated TAA/D (Huang et al., 2018). These previous studies reveal that the TGF-β signaling pathway is dysregulated and involved in the formation of arterial aneurysms, however, its exact role is in debate.
Ventricular Zone Expressed PH Domain Containing 1 (VEPH1) was initially identified to express in the developing central nervous system of vertebrates by Muto et al., 2004). Later, Shathasivam et al. (2015) demonstrated that VEPH1 interacted with TGF-β receptor type-1 (TβRI) to impair the signaling transduction of the TGF-β pathway in cancer cells (Kollara, Shathasivam, Park, Ringuette, & Brown, 2019). Our group previously profiled the RNA transcript expression in abdominal aortic tissues of Ang II-infused apolipoprotein E-deficient (ApoE−/−) mice. Interestingly, the microarray results showed a >2-fold upregulation of VEPH1 in Ang II-infused aortas compared with the control (data not shown). There is a need to investigate the role of VEPH1 in AAA development because of its capability to orchestrate TGF-β signaling transduction.
In the present study, Ang II- or saline-infused mouse aortas were collected to analyze the expression patterns of VEPH1. In vitro, gain- and loss-of-function of VEPH1 experiments were performed to explore its role in the phenotypic switching of human aortic SMCs (hAoSMCs). Our work demonstrated that Ang II-induced elevation of VEPH1 contributed to SMC pathological dedifferentiation.
2 MATERIALS AND METHODS
2.1 Ethic statement
Experiments involving animals conformed to the Guide for the Care and Use of Laboratory Animals (National Institutes of Health). Procedures employed were ethically reviewed and approved by the Ethics Committee of Dalian Medical University.
2.2 Preparation of mouse aortic tissues
AAA was established in atherosclerosis-prone mice as described before (Daugherty, Manning, & Cassis, 2000). Fifteen-week-old male ApoE−/− mice (C57BL/6J background) were divided into two groups: AAA group, the mice were infused with Ang II (1 μg·kg−1·min−1 for 28 d; GL BioChem., Shanghai, China) via Alzet Osmotic minipumps (Model 2004; Alzet, Germany) that were implanted subcutaneously; sham-operated group, the mice were given normal saline (Dubang Pharmaceutical Co., Ltd., Dehui, China) through the minipumps. Before the surgical procedure, the mice were anesthetized via 2–3% isoflurane.
The formation of the arterial aneurysm was determined by recording the aortic diameters with a GE volusonE8 ultrasound system. On Day 28 post the Ang II infusion, the mice were killed to collect their aortas. After dissecting from fat and connective tissues, some of the aortic tissues were snap-frozen, and some were embedded into paraffin post fixation with 4% paraformaldehyde.
2.3 Elastin van Gieson (EVG) staining
Aortic tissue slices of 5-μm thickness were permeabilized with xylene and hydrated by incubating in gradient alcohols. After soaking in distilled water for 2 min, the slices were stained with EVG reagent for 15 min.
2.4 Immunofluorescent (IF) staining
For IF staining of aortic tissues, the antigens were retrieved by incubating the tissue slices in boiled citric acid for 10 min. For cell IF staining, the cells were mounted on glass coverslips, fixed in 4% paraformaldehyde for 15 min, and then treated with 0.1% TritonX 100 for half an hour. Tissue slices or cells were blocked with goat serum for 15 min. Primary antibodies used here included anti-VEPH1 polyclonal antibody (1:200 dilution; Novus Biologicals, Littleton, CO), anti-Vimentin monoclonal antibody (1:200 dilution; Santa (Shanghai), Shanghai, China), and anti-α-SMA monoclonal antibody (1: 200 dilution; Santa (Shanghai)). Samples were incubated with one or two of these primary antibodies at 4°C overnight, and then with the appropriate secondary antibodies labeled with Cy3 (red fluorescence) or FITC (green fluorescence). Images were photographed using a fluorescent microscope.
2.5 In vitro cell culture
hAoSMCs, human aortic endothelial cells (hAoECs), and THP1 monocytes were obtained from Z.q.x.z Cell Research (Shanghai, China). They were allowed to propagate in specific SMC culture medium (ScienCell, San Diego, CA), EC medium (ScienCell), and RPMI-1640 (Sigma-Aldrich, St. Louis, MO) containing 10% fetal bovine serum (Sangon, Shanghai, China), respectively. THP-1 cells were induced to differentiate into macrophage-like cells based on previously described methods (Daigneault, Preston, Marriott, Whyte, & Dockrell, 2010).
2.6 Lentivirus infection
The CDS fragment and two exclusive short hairpin RNA (shRNAs) of homo VEPH1 gene (NM_024621) were inserted in Pljm-1 and Psico lentivirus shuttle vector, respectively. The corresponding lentivirus particles (108 TU/ml) were generated in 293 T cells, and used to infect hAoSMCs at a Multiplicity Of infection of 10:1.
2.7 RNA quantification
The messenger RNA (mRNA) expression levels of targeted genes were analyzed with real-time quantitative polymerase chain reaction (RT-qPCR). In short, total RNAs were reversely transcribed into complementary DNA via a Taq PCR Master Mix kit (BioTeke Bio., Beijing, China). The primers used in RT-qPCR analysis are listed in Table 1. The mRNA expression levels were determined with SYBR Green assays (Sigma-Aldrich) as per the manufacturer's standard protocols. The relative expression levels were shown as values.
| Genes | Sequences 5′–3′ |
|---|---|
| homo MYH11 F | CAGGATAGGGCAGAGCAA |
| homo MYH11 R | GCCAAGTAGCCACGACAC |
| homo α-SMA F | GGGGTGATGGTGGGAATG |
| homo α-SMA R | GCAGGGTGGGATGCTCTT |
| homo PCNA F | TCAAGAAGGTGTTGGAGGCA |
| homo PCNA R | TCGCAGCGGTAGGTGTC |
| homo Vimentin F | CGCCAGATGCGTGAAAT |
| homo Vimentin R | CGAAGGTGACGAGCCATT |
| homo TGF-β1 F | CGAAATCTATGACAAGTTCAAGCA |
| homo TGF-β1 R | GCTGAGGTATCGCCAGGAAT |
| homo VEPH1 F | AGGCAGTAGTTGAAATCTGTATC |
| homo VEPH1 R | CCAAAGGGTCTCAGGTTATGT |
| mus VEPH1 F | AGGCGGTGGTTGAAATCTGT |
| mus VEPH1 R | GGTCCGGTTGTAATTCTGTAAG |
- Abbreviations: F, forwards; R, reversed; qPCR, quantitative polymerase chain reaction.
2.8 Western blot
Protein fractions were extracted from mouse aortas or cells via cell lysis buffer supplemented with phenylmethylsulfonyl fluoride (Beyotime, Shanghai, China), and separated via sodium dodecyl sulfate polyacrylamide gel electrophoresis. Then proteins were transferred onto polyvinylidene fluoride membranes (Millipore, Billerica, MA) and blocked in 5% (M/V) skim milk for 1 hr. Anti-VEPH1 (1:1,000 dilution), anti-MYH11 (1:500 dilution; ABclonal, Wuhan, China), anti-α-SMA (1:500 dilution), anti-PCNA (1:1,000 dilution; ABclonal), anti-vimentin (1:200 dilution; Santa (Shanghai)), anti-Smad3 (1:1,000 dilution; ProteinTech, Rosemont, IL) and anti-phosphorylated Smad3 (1:1,000 dilution; Cell Signaling Technology, Danvers, MA) antibodies were used to probe the corresponding proteins at 4°C overnight. Then, immunoglobulin G conjugated to horseradish peroxidase were used to incubate the membranes for an additional 45 min. Protein bands were probed with BeyoECL Plus (Beyotime), and their intensities were analyzed with Gel Pro-analyzer software. β-Actin was used as an endogenous control.
2.9 Enzyme-linked immunosorbent assay (ELISA)
The protein contents of matrix metalloproteinase-2 (MMP-2) and MMP9 were detected with ELISA kits (USCN Kit Inc., Wuhan, China) according to the manufacture's protocols.
2.10 Statistics
Data in this study were analyzed with GraphPad Prism software version 8.0 (GraphPad software, San Diego, CA). Values were presented as mean ± standard deviation. Student's t test was utilized to analyze data between two groups, while one-way or two-way analysis of variance followed by Tukey's multiple comparison test was used to compare data among multiple groups. A p < .05 was considered statistically significant.
3 RESULTS
3.1 Expression of VEPH1 in the AoSMCs of AAA mice
To establish experimental AAA, ApoE−/− mice were infused with Ang II (1 μg·kg−1·min−1) for a successive 28 d. Ultrasound images showed that ApoE−/− mice treated with Ang II displayed an enlarged aortic diameter (Figure 1a). Further EVG staining showed significant elastin degradation in the abdominal aortas of AAA mice (Figure 1b).
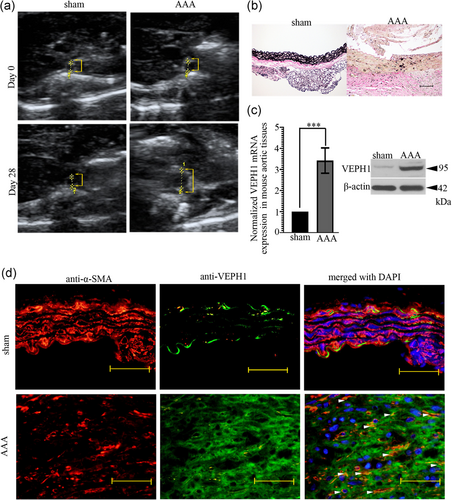
To gain an initial insight into the role of VEPH1 in AAA, we analyzed its endogenous expression in mouse abdominal aortic tissues via western blot and IF staining. VEPH1 mRNA and protein expression levels increased markedly in the Ang II-infused aortic tissues (Figure 1c). The aorta consists of varied types of cells, including SMCs. Thus, IF double staining using anti-α-SMA (a contractile marker for SMCs) and anti-VEPH1 antibodies was performed to analyze whether VEPH1 was expressed in the AoSMCs. Consistent with western blot data, IF images also showed that VEPH1 expression was hardly detected in control aortas, but increased significantly post Ang II administration (Figure 1d). We found that α-SMA+ cells in Ang II-infused aortas were less than that in normal aortas, but α-SMA+VEHP1+ cells are more in number (Figure 1d, white arrowheads). These pieces of data indicated that VEHP1 expression increased in AoSMCs after AAA induction.
3.2 VEPH1 is sensitive to Ang II stimulation in vitro
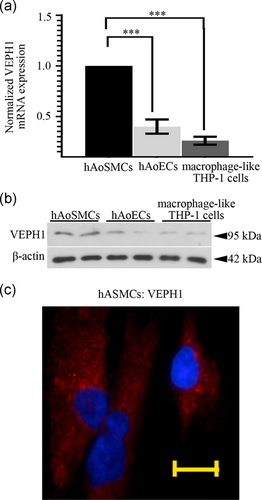
As VEPH1 expressed in mouse AoSMCs in vivo, we next performed RT-qPCR and immunoblotting analyses to determine its expression in primary hAoSMCs, hAoECs, and human THP-1 macrophage-like cells. The results showed that VEPH1 expression was detectable in the three types of cells with a relatively higher level in hAoSMCs (Figure 2a-c). As shown in the IF staining assay, VEPH1 mainly expressed in the cytoplasm of hAoSMCs (Figure 2c).
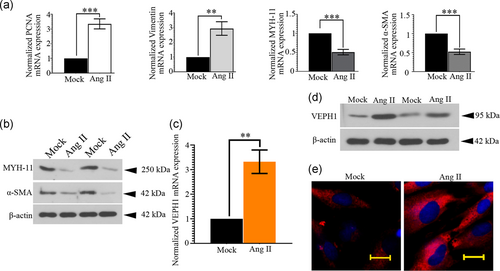
To better understand VEPH1's role in AAA formation, hAoSMCs were treated with 1 μM Ang II for 24 hr. By analyzing the expression of contractile (MYH11 and α-SMA) and synthetic (PCNA and Vimentin) SMC markers via RT-qPCR and/or western blot, we first confirmed that a 24-hr stimulation of Ang II promoted hAoSMC phenotypic switching. The hAoSMCs altered from a differentiated phenotype (contractile) to a dedifferentiated (synthetic) phenotype—the expression of MYH11 and α-SMA decreased, while that of PCNA and Vimentin increased in response to Ang II (Figure 3a,b). Interestingly, we noted that Ang II increased VEPH1 mRNA expression threefold in hAoSMCs (Figure 3c). The elevation of VEPH1 induced by Ang II was further validated with immunoblotting analyses (Figure 3d,e). These pieces of data suggested that VEPH1 upregulation occurred concurrently with the synthetic transition of hAoSMCs.
3.3 VEPH1 regulates the phenotypic switching of hAoSMCs
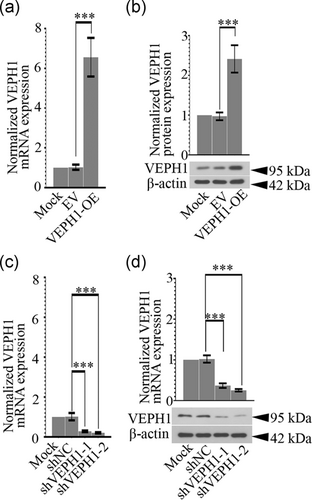
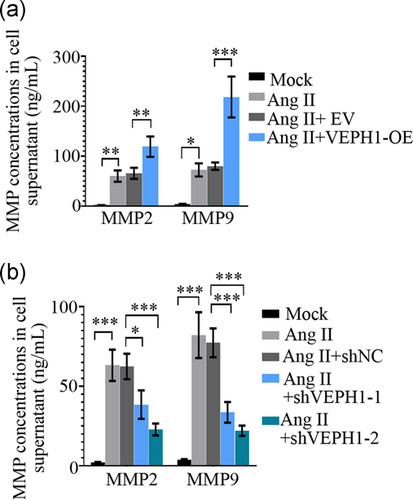
To evaluate how VEPH1 elevation regulates the phenotypic switching of hAoSMCs, its expression was upregulated or downregulated with specific lentiviruses. Cells harvested at 72 hr postinfection of VEPH1-OE, VEPH1 shRNA, or control lentiviruses were subjected to RT-qPCR (Figures 4a and 4c) and western blot analysis (Figures 4b and 4d). The data validated that the lentiviruses successfully altered VEPH1 expression in hAoSMCs. We noted that VEPH1 overexpression further augmented Ang II-induced alteration in contractile (MYH11 and α-SMA) and synthetic (PCNA and vimentin) SMC markers, while its downregulation induced the opposite alteration (Figure 5a-f). Furthermore, hAoSMCs exposed to Ang II produced more matrix metalloproteinase-2 (MMP2) and MMP9, indicating these cells switched into functional synthetic SMCs (Figure 6). VEPH1 silencing suppressed MMP generation provoked by Ang II (Figure 6). These pieces of data revealed a novel role of VEPH1 in regulating contractile-synthetic phenotypic switching of hAoSMCs.
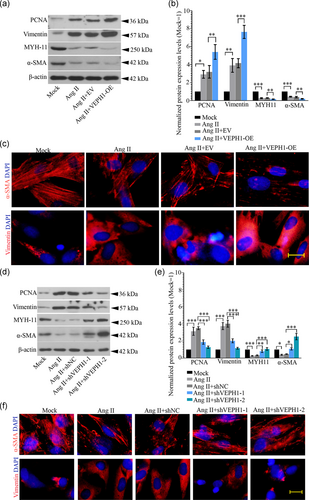
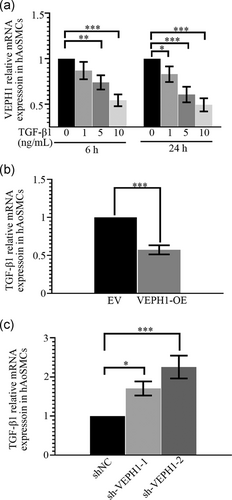
3.4 VEPH1 and TGF-β1 negatively regulate the transcription of each other in hAoSMCs
hAoSMCs were treated with multiple concentrations of recombinant TGF-β1 for 6 or 24 hr and then harvested to determine VEPH1 expression with RT-qPCR. As indicated in Figure 7a, TGF-β1 suppressed the transcription of VEPH1. Furthermore, VEPH1 was forced to overexpress or be knocked down in hAoSMCs, and the results illustrated that VEPH1 negatively regulated TGF-β1 mRNA expression (Figure 7b,c). The shVEPH1-2 with a better knockdown efficiency on endogenous VEPH1 induced a higher upregulation in TGF-β1 mRNA expression (Figure 7c). These pieces of data collectively suggested VEPH1 and TGF-β1 act as suppressors of each other.
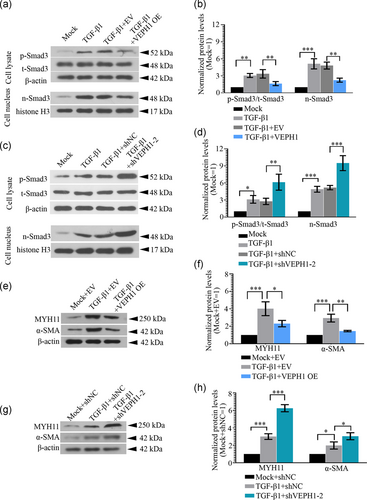
3.5 VEPH1 inhibits the contractile phenotypic transition of hAoSMCs by suppressing the TGF-β1/Smad3 signaling pathway
hAoSMCs were infected with VEPH1 overexpression or knockdown lentiviruses (shVEPH-2) for 72 hr, and then stimulated with 5 ng/ml TGF-β1 for 30 min. The protein levels of p-Smad3 and t-Smad3 were determined with western blot. VEPH1 elevation suppressed Smad3 signaling transduction activated by TGF-β1, while its silencing functioned in the opposite manner (Figure 8a-d). To induce hAoSMC differentiation, 5 ng/ml TGF-β1 was used to treat cells for 72 hr post a 48-hr lentivirus infection. Additional data revealed that the TGF-β1-induced increases in SMC markers, MYH11 and α-SMA, were further augmented by VEPH1 silencing, but weakened by VEPH1 overexpression (Figure 8e-h).
4 DISCUSSION
Imbalanced vascular SMC plasticity often leads to maladaptive phenotype alterations, which occurs almost in all SMC-driven vascular diseases (Frismantiene et al., 2018). Various environmental cues, including mechanical forces, growth factors, inflammatory mediators, modulate SMC polarization (Frismantiene et al., 2018). The activation of the renin–angiotensin system (RAS) is a major risk factor correlated to AAA pathogenesis (Daugherty et al., 2000; Peshkova, Schaefer, & Koltsova, 2016). Atherosclerosis-prone mice infused with low dose Ang II can develop pronounced AAA (Daugherty et al., 2000; Saraff, Babamusta, Cassis, & Daugherty, 2003). Therefore, although varied animal models have been developed to simulate the clinic AAA formation (Patelis et al., 2017), we decided to perform our work in the Ang II-infused AAA mouse model.
Our data showed that VEPH1 hardly expressed in control aortas, but expressed in aortic media post Ang II infusion in mice. It has been reported that VEPH1 is restricted to express in the eye and kidney of healthy adult mice (Muto et al., 2004), suggesting that the elevation of VEPH1 observed in Ang II-infused aortas may be a pathological consequence induced by RAS activation. In addition to inducing aortic dilatation and elastic fragment, Ang II also triggers synthetic SMC phenotype switching (Liang, Cheng, et al., 2018; Sachdeva et al., 2017). The synthetic SMCs can be characterized by osteopontin, MMP-9, and Vimentin, while the contractile SMCs express MYH11 and α-SMA (Branchetti et al., 2013; Salabei et al., 2013). We noted that although α-SMA+ cells in Ang II-infused aortas were less than that in normal aortas, the α-SMA+VEHP1+ cells are more in number. We next explore the role of VEPH1 in phenotypic switching of hAoSMCs in vitro.
Our data revealing that both the mRNA and protein expression levels of VEPH1 were upregulated by Ang II suggested VEPH1 to be a novel molecule sensitive to RAS activation. In vitro, in consistent with previous studies (Cui et al., 2016; Nagayama et al., 2015), we also demonstrated that Ang II successfully triggered the synthetic switching of hAoSMCs. To further explore how VEPH1 affected SMC phenotypic switching, its expression was forced to increase or decrease in hAoSMCs. In the presence of Ang II, VEPH1 silencing increased the expression of contractile SMC markers (MYH11 and α-SMA) and decreased that of synthetic SMC markers (PCNA and Vimentin). Two shRNAs were designed to knock VEPH1 down. Although both shVEPH1-1 and -2 significantly reduced VEPH1 expression, the knockdown effect of shVEPH1-2 was better. Interestingly, the effects of shVEPH1-2 on SMC markers were much stronger than that of shVEPH1-1. Such results suggest that VEPH1 expression is indeed correlated to SMC phenotypic switching.
Synthetic differentiation of vascular SMCs is accompanied by an enhanced release of pro-inflammatory and chemotactic cytokines (Scirocco et al., 2016). In response to Ang II infusion, our previous microarray data showed that pro-inflammatory cytokines interleukin-1β (IL-1β) and IL6 increased threefold (p < .05) and fivefold (p = .072), and chemotactic factors C-C Motif Chemokine Ligand-6 (CCL-6), CCL8, C-X-C Motif Chemokine Ligand (CXCL) 1 and CXCL16 increased over twofold (p < .05). Synthetic vascular SMCs also release more MMPs that are able to digest extracellular matrix proteins (Huang et al., 2018; Johnson, 2007). The present microarray data demonstrated that the expression of several MMPs, such as MMP2, MMP9, MMP3, MMP14 and MMP27 increased in Ang II-infused aortas as compared to that in normal aortas. In vitro, hAoSMCs exposed to Ang II released more MMP2 and MMP9 into the cell medium, which was suppressed when VEPH1 was silenced.
The pathogenic role for TGF-β is attributable in part to the detection of elevated TGF-β1 in human and experimental animal AAA tissues. The canonical TGF-β signaling cascade is initiated by TGF-β ligands that bind to type I and/or type II TGF-β receptors (TβRI and TβRII) to phosphorylate and induce the nuclear translocation of Smad2 and Smad3, thereby modulating the expression of target genes (Gadue, Huber, Paddison, & Keller, 2006; Weiss & Attisano, 2013). If the promiscuous activation of canonical TGF-β pathways contributes to aneurysmal pathology, then the blockade of Smads is expected to mitigate disease severity (Lindsay & Dietz, 2014). However, TGF-β neutralization or Smad4 deletion unexpectedly exacerbates aortic dilation (Chen et al., 2016; Holm et al., 2011; Huang et al., 2018). Loss of canonical TGF-β signaling may augment noncanonical signaling to pathogenic levels. Elevated TGF-β signaling is either proaneurysmal or antianeurysmal, depending on the context (Andelfinger, Loeys, & Dietz, 2016).
As activation of the TGF-β1 signaling pathway promotes the differentiation of SMCs by increasing the expression of SMC markers (Hao, Gabbiani, & Bochaton-Piallat, 2003), we next explored the role of canonical TGF-β/Smad3 signaling in VEPH1-regulated differentiation of hAoSMCs. In hAoSMCs, we found that VEPH1 and TGF-β1 negatively regulated each other's transcription. Furthermore, TGF-β1-induced elevation of MYH11 and α-SMA, and nuclear translocation of Smad3 in cultured hAoSMCs was suppressed by VEPH1 overexpression but augmented by its knockdown. This negative regulatory role of VEPH1 on TGF-β/Smad3 pathway in hAoSMCs was supported by earlier findings reported by Shathasivam's group in cancer cells (Kollara et al., 2019; Shathasivam et al., 2015; Shathasivam et al., 2017). These data together indicate that a potential circular regulatory mechanism between VEPH1 and TGF-β1.
Identification of molecules that contribute to AAA formation based on experimental animal models is a common approach adopted by many research groups, including us (Patelis et al., 2017). Nonetheless, lack of direct evidence demonstrating VEPH1's elevation in human AAA tissues is a limitation of our present study. Of note, a profile (GDS3903) from NCBI Gene Expression Omnibus (GEO) repository showed that the expression of VEPH1 tended to upregulate in the human intracranial arterial aneurysm samples as compared to the normal cerebral artery, suggesting that VEPH1 upregulation may be correlated to arterial aneurysm formation in humans. Moreover, our work revealed VEPH1 as a sensitive molecule to RAS activation not only in mouse aorta but also in human AoSMCs in vitro. Hence, there is a great possibility that VEPH1 increases in human AAA samples. Although GDS3903 data together with our current findings imply an involvement of elevated VEPH1 in arterial aneurysm development in clinics, there is still an urgent need to determine its expression directly in human AAA samples.
In addition to the synthetic switching of vascular SMCs, injured vascular endothelium and pathological infiltration of inflammatory cells, such as macrophages, are also intensively correlated to AAA pathogenesis (Brangsch et al., 2017). By labeling AoECs with anti-CD31 antibody and infiltrated macrophages with anti-CD68 antibody in mouse abdominal aortas, we also found abundant VEPH1 in AoECs but less in infiltrated macrophages in mouse AAA tissues (data not shown). It has been demonstrated that though in ovarian cancer xenografts VEPH1 overexpression inhibits vascularization by impairing the function of vascular endothelial cells (Shathasivam et al., 2017), in THP-1 macrophage-like cells, VEPH1 expression increased upon Ang II or ox-LDL stimulation (Figure s1). A hypothesis that the elevated VEPH1 contributes to vascular endothelium injury and macrophage activation during AAA formation is thus proposed, and will be validated by our group in the future.
In summary, our work demonstrates that the elevation of VEPH1 induced by Ang II promotes to the transition of AoSMCs from a contractile phenotype to a synthetic phenotype. Identification of VEPH1 as a pathogenic molecule for AAA formation provides novel insights into this disease.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
X. S., C. X., and L. L. designed the experiments, acquired and analyzed the data. X. S. drafted and C. X. revised the manuscript. Y. L., H. W., W. M., Y. T., and H. Y. acquired and helped to analyze the data. All authors gave final approval of the version to be published.
Open Research
DATA AVAILABILITY STATEMENT
All data are included in this manuscript.




