The effector cells and cellular mediators of immune system involved in cardiac inflammation and fibrosis after myocardial infarction
Yihai Liu and Jiamin Xu contributed equally to this study.
Abstract
The cardiac repair after myocardial infarction (MI) involves two phases, namely, inflammatory response and proliferative response. The former is an inflammatory reaction, evoked by different kinds of pro-inflammatory leukocytes and molecules stimulated by myocardial necrosis, while the latter is a repair process, predominated by a magnitude of anti-inflammatory cells and cytokines, as well as fibroblasts. Cardiac remodeling post-MI is dependent on the balance of individualized intensity of the post-MI inflammation and subsequent cardiac fibrosis. During the past 30 years, enormous studies have focused on investigating immune cells and mediators involved in cardiac inflammation and fibrosis, which are two interacting processes of post-MI cardiac repair. These results contribute to revealing the mechanism of adverse cardiac remodeling after MI and alleviating the impairment of cardiac function. In this study, we will broadly discuss the role of immune cell subpopulation and the involved cytokines and chemokines during cardiac repair post-MI, particular in cardiac inflammation and fibrosis.
Abbreviations
-
- CF
-
- cardiac fibrosis
-
- DAMP
-
- danger-associated molecular pattern
-
- MI
-
- myocardial infarction
-
- MSC
-
- mesenchymal stem cell
-
- PCI
-
- percutaneous coronary intervention
-
- PRR
-
- pattern recognition receptor
-
- ROS
-
- reactive oxidative species
1 BACKGROUND
Myocardial infarction (MI) is a leading cause of morbidity and mortality among people. When exposed to ischemic hypoxia, parenchymal and cardiac cells death programs were initiated (Li, Ren, Xia, Wei, & Xi, 2019). Meantime, the vascular endothelial cell integrity was impaired, being an access for leukocyte infiltration (Moccetti et al., 2018). Necrotic tissues release danger-associated molecular patterns (DAMPs), interacting with the cognate pattern recognition receptors (PRRs) expressed on circulating leukocytes to activate the immune response (Suetomi, Miyamoto, & Brown, 2019). A systemic inflammation response after MI included elevation of inflammatory cytokines, chemokines, and cell adhesion molecules as well as leukocytes (Fang, Moore, Dart, & Wang, 2015).
Cardiac repair after MI is a complex and fine-tuning series of events, including inflammatory phase (<4-day post-MI in murine) and reparative response (Bejerano, Etzion, Elyagon, Etzion, & Cohen, 2018). The former was initiated by tissue injury and necrosis, followed by sterile inflammation and infiltration of immune cells including neutrophils, monocyte/macrophages, dendritic cells, and lymphocytes (Swirski & Nahrendorf, 2018). And the latter was transited into inflammation resolution, characterized by myofibroblast proliferation, scar formation, and neovascularization (Frangogiannis, 2012). Inflammatory response facilitated cardiac repair, but excessive inflammation lead to adverse left ventricular remodeling (Westman et al., 2016).
A major strategy to treat MI was to perform percutaneous coronary intervention (PCI) for timely reperfusion; however, it is limited by the ischemic reperfusion injury because of abrupt reoxygenation, reactive oxidative species (ROS) generation, and activation of the complement pathway (Eltzschig & Eckle, 2011). The biological basis for cardiac repair after MI facilitates the interpretation of underlying mechanisms and the invention of novel therapeutic strategies.
2 EFFECTOR CELLS
2.1 Cells of the innate immune system in cardiac tissue inflammation and repair
2.1.1 Neutrophils
Neutrophils were the earliest leukocytes among the pro-inflammatory cells infiltrated in ischemic myocardium (Tourki & Halade, 2017). The activation of adhesive interaction between leukocytes and endothelial cells lead to the extravasation of neutrophils, which included selectins, integrins, and immunoglobulin superfamily molecules, such as VCAM-1 and ICAM-1 (Prabhu & Frangogiannis, 2016). Neutrophils were characterized by high plasticity potential, polarized to pro-inflammatory N1 by lipopolysaccharide and interferon-γ or anti-inflammatory N2 by interleukin-4 (Ma et al., 2016). Similarly, neutrophils underwent phenotypic changes over the MI time course (Daseke et al., 2019).
Infiltrating neutrophils could amplify granulopoiesis by releasing proteolytic enzymes for clearance of necrosis cells and matrix debris due to myelopoiesis triggered by MI (Sreejit et al., 2020). Besides, a study reported that neutrophil depletion could reduce infarct size by over 40% in in vivo models of perfused MI (Romson et al., 1983). Anti-RANKL antibody reduced infarct size and attenuated dysfunction via abrogating RNAKL-induced mouse neutrophils degranulation and migration (Carbone et al., 2016). Interestingly, neutrophil peak was established as an independent predictor of MRI-defined infarct size (Husser et al., 2011).
In reparative response, neutrophils helped in wound healing by secreting cytokines and growth factors. Apoptotic neutrophils released transforming growth factor-β (TGF-β) to activate anti-inflammatory programs in macrophages, resulting in the resolution of inflammation and fibrosis (Soehnlein & Lindbom, 2010). Another mechanism proposed was that the dying neutrophils released lactoferrin and annexin A1 to inhibit neutrophil recruitment, preventing the exacerbation of inflammation (Bournazou et al., 2009).
The neutrophils selectively degranulate over the MI time course, shifting from strengthening phagocytosis to contributing to scar formation mediated by secreting matrix metalloproteinase and stimulating extracellular matrix organization, respectively (Daseke et al., 2019).
2.1.2 Monocytes
After the inflammatory pathways were activated, mononuclear cells infiltrated necrotic myocardium tissues from the peripheral blood. Some mediators, such as TGF-β, complement, ROS and chemokines were involved in the regulation of monocyte infiltration, among which the most important was the MCP-1/CCR2 axis (Dewald et al., 2005). MI was also related to bone marrow activation and spleen monocytopoiesis, providing many monocytes for battle. A clinical study demonstrated that circulating CD16− monocytes expanded first (peak on Day 2.6), followed by CD16+ monocytes (peak on Day 4.8) in patients after MI. It suggested that monocytes may exert effects on the cardiac repair by performing different roles (Tsujioka et al., 2009; Figure 1; Nahrendorf, Pittet, & Swirski, 2010).
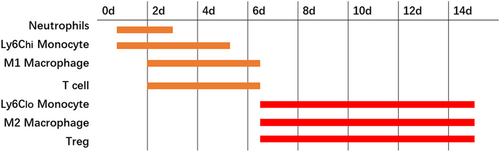
In the inflammatory phase, Ly6Chi monocytes dominated in the injured myocardium via chemokine receptor type 2, as a scavenger to fulfill their phagocytic and inflammatory function (Nahrendorf et al., 2007). Monocyte response has been associated with severe myocardial injury and poor cardiac function after MI (van der Laan et al., 2012).
However, in reparative stage, Ly6Clo monocytes accumulated preferentially to promote granulation tissue formation via CX3C chemokine receptor 1 (Nahrendorf et al., 2007). A study found that bone marrow-derived monocytes and macrophages produced myeloid-derived growth factor to repair the heart after MI (Korf-Klingebiel et al., 2015). Another study showed that classical (CD14(++)CD62L(+)) monocytes were associated with impaired LV function, large infarct size, and presence of microvascular obstruction, whereas nonclassical (CD14(+)CD62L(−)) monocytes were the opposite (van der Laan et al., 2012).
Generally, circulating derived monocytes play a pro-inflammatory role after infiltrating cardiac tissues. However, some subgroups based on flow cytometry labeling provide a protective role, which deserve further investigation using proteinomics or single-cell sequencing technology.
2.1.3 Macrophages
After induction of growth factors, including M-CSF and GM-CSF, monocytes matured and differentiated into macrophages. In the inflammatory phase, the macrophage was characterized by M1 macrophages, expressing augmented proteinase and tumor necrosis factor (TNF; Ma, Mouton, & Lindsey, 2018). Macrophage secretion of Ccl12 prevented initiation of reparative response and inhibited fibroblast conversion to myofibroblasts. Sustained chronic inflammation lead to adverse remodeling (DeLeon-Pennell et al., 2017).
However, in reparative stage, M1 macrophages switched their phenotype to M2-like macrophage with enhanced expression of anti-inflammatory, profibrotic, and angiogenic factors. Macrophage depletion produced adverse effect on infarct size, left ventricular remodeling, and thrombus formation, which could be attenuated with macrophage restoration. Furthermore, it was concluded that the favorable effect was mediated by the increased percentage of M2 macrophage (Ben-Mordechai et al., 2013). The macrophages expressing the myeloid–epithelial–reproductive tyrosine kinase may contribute to transition to the reparative phase by efferocytosis of apoptotic cardiomyocytes (Wan et al., 2013).
Macrophages exhibited significant heterogeneity because embryonic macrophage subpopulation may promote a regenerative response, while monocyte-derived macrophages may induce inflammation to inhibit cardiac regeneration (Frangogiannis, 2015). Macrophage was also responsible for angiogenesis for mesenchymal stem cell (MSC) transplantation, which significantly increased local recruitment of macrophages to facilitate cardiac repair (M. Wang et al., 2015). After cocultured with MSCs, new macrophages defined as IL-10hi, IL-6hi, and TNF-αlo immunophenotype were generated, which were potential in cardiac repair (Kim & Hematti, 2009).
Circulating blood-derived macrophages play an unfavorable role in post-MI cardiac repair, while tissue-resident macrophages may promote inflammation resolution and fibrosis. However, more defined subpopulations need to be identified except for recognized M1 and M2 macrophages.
2.2 Cells of the adaptive immune system in cardiac tissue inflammation and repair
2.2.1 Lymphocytes
A large number of experiments confirmed that lymphocytes played an important role in orchestrating inflammatory response. Different immune cell subpopulation may participate in different process in cardiac repair (Ong et al., 2018).
T cells are generally divided into CD4+ helper T cells, CD8+ cytotoxic T cells, and regulatory T cells (Treg). Under inflammatory condition, cytokine induction and adhesive interaction recruited effector T cells to release pro-inflammatory mediators in the infarct zones. The depletion of CD4+ T cells is characterized by increased inflammation and fibrosis (Horckmans et al., 2017). During the early phase of MI, Navarro et al. reported an increased numbers of CD8 cells and a dysregulated CD4/CD8 ratio (Syrjala, Surcel, & Ilonen, 1991). In the late phase, regulatory T cells with suppressive properties were predominated, secreting IF-10 and TGF-β to take charge of modulation of inflammatory resolution (Bansal et al., 2019). A recent study showed that CD4+ Foxp3+ regulatory T cells were essential for favorable wound healing, scar formation, and inflammation resolution after MI, by modulating macrophage differentiation toward an M2-like phenotype (Weirather et al., 2014). It has been observed that adoptive transfer of Tregs and expansion of Tregs by IL-2 may help in repairing the infarcted heart (Sharir et al., 2014; Zeng et al., 2016).
2.2.2 Other components of the adaptive immunity
In addition to T lymphocytes, B cells also play an important role in activation of the inflammatory cascade by promoting mobilization of pro-inflammatory Ly6Chi monocytes (Zouggari et al., 2013).
Following MI, B lymphocytes interacted with monocytes and accelerated the process of injury (Zouggari et al., 2013). Mature B cells selectively released CCL7, a predictor of high post-MI mortality rate. While B lymphocytes depletion can suppress the inflammatory response (Yan et al., 2013; Zouggari et al., 2013). NK cells are generally downregulated following infarction and is related to impaired wound healing (Joshi et al., 2015). The activation of NK cells has been confirmed to reduce adverse remodeling by modulating the anti-inflammatory cytokines expression (Homma et al., 2013; Sobirin et al., 2012).
In particular, it is well-known that sex hormones have a great influence on the immune system. Estrogen can increase antibody responses to infection by activating B cells, while androgens have the opposite effect (Cook, 2008; Fairweather, Frisancho-Kiss, & Rose, 2008; Fairweather, Petri, Coronado, & Cooper, 2012). Estrogen can increase inflammation either by driving T helper 1 (Th1) and Th17 responses or activating macrophages by increasing the regulatory arm of the adaptive immune response (Papenfuss et al., 2011; Straub, 2007). Therefore, the sex difference in post-MI cardiac repair is becoming a hot spot for future researchers.
3 MOLECULAR MEDIATORS OF THE IMMUNE SYSTEM IN CARDIAC INFLAMMATORY AND REPAIR PHASE
3.1 Cytokines
Cytokines, a group of polypeptide or glycoprotein factors, can bind to specific membrane-bound receptors to induce various physiological responses by acting in an autocrine or paracrine fashion (Noels, Weber, & Koenen, 2019). In cardiac repair post-MI, they were classified as pro- or anti-inflammatory proteins. The triggers of cytokines after MI included mechanical stress, ischemic induction, ROS stimulus, and self-amplification (Figure 2; Nian, Lee, Khaper, & Liu, 2004). Whether cytokines were stimulant or suppressant to heart was determined by the activated signaling pathways (Bartekova, Radosinska, Jelemensky, & Dhalla, 2018).
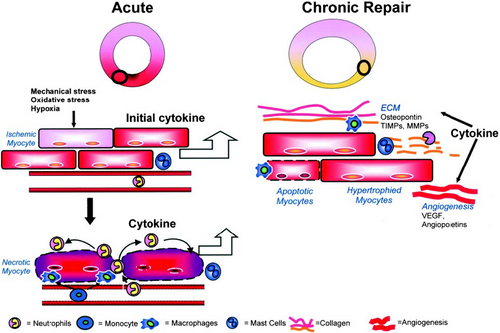
In the inflammatory phase, the pro-inflammatory cytokines such as IL-1, TNF, and IL-6 were upregulated post-MI (Sanchez-Alonso, Alcaraz-Serna, Sanchez-Madrid, & Alfranca, 2018). The cytokines and growth factors in hypoxic conditions contributed to the occurrence of intramyocardial hemorrhage, which, in turn, augmented inflammation response. IL-1, critical in stimulating inflammatory response, was involved in the pathogenesis of reverse cardiac remodeling. Both genetic and pharmacologic targeting of IL-1 has shown to protect the infarcted heart from adverse remodeling (Abbate et al., 2008; Bujak et al., 2008). In the reparative process, anti-inflammatory cytokines IL-10 and TGF-β were induced to inhibit pro-inflammatory cytokines and chemokine synthesis. TGF-β-induced myofibroblast transdifferentiation, stimulated synthesis of extracellular matrix proteins, and inhibited matrix degradation (Laiho, Saksela, Andreasen, & Keski-Oja, 1986). Besides, TGF-β may induce homing of bone marrow MSCs in the myocardial repair post-MI via regulating stromal cell-derived factor 1 (SDF-1)/chemokine (C-X-C Motif) receptor 4 (CXCR4) axis (S. J. Zhang, Song, He, & Yu, 2016). Chronically, sustained expressed cytokines caused myocyte phenotype transition and activation of matrix metalloproteinases, enhancing the reverse cardiac remodeling (Nian et al., 2004). The cellular signaling mechanisms underlying the cytokine-induced cardiostimulatory or cardiodepressant response included immediate effects, namely, sphingolipid, phospholipid, and cNOS-derived NO-dependent pathways and delayed effects, namely, β-AR uncoupling, iNOS-derived NO, and sphingomyelinase-dependent pathways (Prabhu, 2004).
Besides, cytokines may be responsible for the switch of pro- and anti-inflammatory pathways, consequently for the transition from inflammatory process to reparative process. For example, insulin-like growth factor-1 Ea may favor a reduction in inflammatory Ly6C+ monocytes and an increase in anti-inflammatory CD206 + macrophages by shifting the balance of innate immune cell population (Gallego-Colon et al., 2015).
3.2 Chemokines
Chemokines is comprised of a group of chemotactic cytokines, regulating the trafficking of immune cells and participating in cardiac repair and remodeling. Based on the difference of the structure of polypeptide chains, chemokines are divided into CC, CXC, CX3C, and XC subfamilies. In general, the CC chemokines are potent chemoattractants for mononuclear cells, while CXC chemokines are mainly involved in chemotactic neutrophils (Noels et al., 2019). These chemokines form a complex network to endorse recruitment of leukocyte subpopulations to infarcted myocardium (Figure 3; Cavalera & Frangogiannis, 2014).
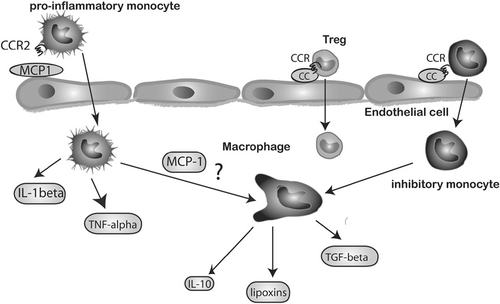
The CC chemokines MCP-1/CCL2 was obviously upregulated in the infarct myocardium. A study demonstrated that mice treated with a competitive MCP-1/CCL2 inhibitor exhibited reduced infarct size and monocyte infiltration after ischemic and reperfusion injury, and ameliorated post-MI remodeling (Liehn et al., 2010). A study demonstrated that loss of the chemokine receptor CCR5 was associated with accentuated inflammation and worse adverse remodeling, in part due to impaired recruitment of Tregs in the infarcted heart (Dobaczewski, Xia, Bujak, Gonzalez-Quesada, & Frangogiannis, 2010).
CXC chemokines were markedly upregulated in the infarct myocardium and promoted neutrophils infiltration. A protein that bound and neutralized CXC chemokines called evasin-3 could reduce infarct size via attenuating neutrophils recruitment (Montecucco et al., 2010).
Other chemokines were also important for cardiac repair post-MI. For example, SDF-1/CXCL with angiogenic property promoted cardiomyocyte survival and enhanced the regenerative capacity of progenitor cells (Frangogiannis, 2011).
4 ROLE OF THE IMMUNE SYSTEM IN CARDIAC FIBROSIS (CF) POST-MI
4.1 CF post-MI
CF after MI is a process of self-repair and inflammation of the myocardium. After 5 days of MI, monocytes/macrophages and endothelial cells cooperate with angiogenesis to promote blood supply to granulation tissue. Type I collagen is synthesized by myofibroblasts, which protect the heart muscle from rupture. After 2–3 weeks of MI, mononuclear/macrophage disappeared and granulation tissue matured to form a collagen cross-linked scar (Blankesteijn et al., 2001; Cleutjens, Blankesteijn, Daemen, & Smits, 1999; Ertl & Frantz, 2005).
It is currently believed that the occurrence of myocardial fibrosis is associated with the renin-angiotensin-aldosterone system (Meyer, Hodwin, Ramanujam, Engelhardt, & Sarikas, 2016; J. H. Wang et al., 2016), inflammatory response and oxidative stress (Detterich, 2017; Suthahar, Meijers, Sillje, & de Boer, 2017), various cytokines (Khalil et al., 2017; Souza et al., 2017; Steele et al., 2017; M. Zhang, Ai, Mei, Hu, & Zhang, 2017), gas signaling molecules, autophagy (Song et al., 2017), and microRNA (Yuan et al., 2017). In this study, we focused on the effects of inflammation on CF after MI, especially the interplay between immune cells or mediators and cardiac fibroblasts.
4.2 Crosstalk between cardiac fibroblasts and immune cells
The excessive activation of immune response to myocardial infarct may also make important contributions to local inflammation, and they play an important role in the activation of cardiac fibroblasts (Sattler, Fairchild, Watt, Rosenthal, & Harding, 2017). Myocardial fibroblasts account for 60–70% of the total number of heart cells. Changes in the biological properties and function of cardiac fibroblasts are helpful for the repair of damaged myocardial tissue and maintenance of cardiac function, but its long-term effects lead to a gradual increase in myocardial stiffness, decreased myocardial compliance, and ventricular systolic and diastolic dysfunction (Shyu, 2017). Studies have shown that after cardiac injury, inflammation persists through upregulation of cytokine release, leading to fibroblast proliferation and metalloproteinase activation (Frangogiannis, 2012). A study by Masanori Kawaguchi et al. has also revealed that cardiac fibroblast may mediate the inflammation process and the inflammasome can serve as a novel therapeutic target (Kawaguchi et al., 2011).
The immune cells interacted with cardiac fibroblasts in a diverse way after MI, so that T cells can promote myocardial fibrosis under long-term stress conditions. Th1 has been found to directly stimulate fibroblasts to differentiate into myofibroblasts and promote CF remodeling (Nevers et al., 2017). In addition, some previous studies have demonstrated that adoptive transfer of Tregs, which generally have anti-inflammatory effects, reduces the infiltration of neutrophils, monocytes, and lymphocytes into the heart after MI and reduces myocardial fibrosis (Cao, Xu, & Xiong, 2013; Tang et al., 2012). Moreover, activated B lymphocytes produce cytokines, which directly lead to myocardial dysfunction by depressing contractility, inducing fibroblast differentiation into myofibroblasts, and inducing myocyte apoptosis (Lund, 2008; Nian et al., 2004). Several subpopulations of lymphocytes (Saxena et al., 2014) and mast cells (Schirone et al., 2017) also make important contributions to local inflammation and play an important role in the activation of cardiac fibroblasts.
At present, prevention and reversal of CF has become one of the important purposes of post-MI treatment (Bageghni et al., 2019). In view of the central role of immune inflammation in the pathogenesis of CF, researchers are now expecting to alleviate cardiac remodeling by anti-inflammation therapy.
5 CONCLUSIONS
Necrotic cells and injured extracellular matrix released DAMPs, binding to the PPRs of the immune cells to activate a cascade of inflammatory mediators (Timmers et al., 2012). It is well-known that inflammatory response exerted cytotoxic injury to the myocardium (Ong et al., 2018); in addition, leukocytes also secreted protease, cytokines and reactive oxygen species, which not only scavenged necrotic tissues, but also damaged surrounding viable cardiomyocytes. Even though many studies showed that anti-inflammatory therapy was effective for cardiac repair in large animal models, a lot of clinical trials proved unsatisfactory. A study reported that an increased inflammation promoted angiogenesis and myofibroblast formation, favoring cardiac repair, but the effect was not persistent (Shao et al., 2015). To sum up, the effect of inflammatory response remains controversial.
A short course of anti-inflammatory treatment with colchicine can lead to reduced infarct size in MI patients (Deftereos et al., 2015). Metoprolol, the β1-adrenergic-receptor antagonist, inhibited neutrophil migration mediated by neutrophil-platelet interaction, which reduced infarct size in acute MI patients (Garcia-Prieto et al., 2017). Broad-spectrum anti-inflammatory agents, such as nonsteroidal anti-inflammatory drug, may produce a short-term effect on inflammatory response, but it would cause catastrophic consequence in myocardial repair. Currently, there is no specific medical-targeted therapy to relieve myocardial fibrosis. So, it was meaningful to selectively target pro-inflammatory signals for the discovery of potential therapy, and improve intervention strategies. According to the change of signal pathways involved in the cardiac repair process, temporal consideration was a key determinant of therapeutic intervention after MI.
In summary, our study broadly discussed the role of immune cell subpopulation and the involved cytokines and chemokines during cardiac repair post-MI. On one hand, active suppression and resolution of inflammation facilitated transition to reparative process and reduced inflammatory damage; on the other hand, proper inhibition of the overactive fibrosis attenuated adverse remodeling in patients with MI. Moreover, future study should also be tailored to gender given the difference of innate and adaptive immune response between male and female (Hammes & Levin, 2011).
ACKNOWLEDGMENT
This study was supported by the Funds for Distinguished Young Scientists in Nanjing (JQX15002).
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Y. L. wrote the original draft; J. X. and M. W. refined the manuscript; L. K. and B. X. designed the study.




