Metabolic syndrome, Mediterranean diet, and polyphenols: Evidence and perspectives
Abstract
Metabolic syndrome (MetS) is defined as the co-occurrence of metabolic risk factors that includes insulin resistance, hyperinsulinemia, impaired glucose tolerance, type 2 diabetes mellitus, dyslipidemia, and visceral obesity. The clinical significance of MetS consists of identifying a subgroup of patients sharing a common physiopathological state predisposing to chronic diseases. Clinical and scientific studies pinpoint lifestyle modification as an effective strategy aiming to reduce several features accountable for the risk of MetS onset. Among the healthy dietary patterns, the Mediterranean diet (MedDiet) emerges in terms of beneficial properties associated with longevity. Current evidence highlights the protective effect exerted by MedDiet on the different components of MetS. Interestingly, the effect exerted by polyphenols contained within the representative MedDiet components (i.e., olive oil, red wine, and nuts) seems to be accountable for the beneficial properties associated to this dietary pattern. In this review, we aim to summarize the principal evidence regarding the effectiveness of MedDiet–polyphenols in preventing or delaying the physiopathological components accountable for MetS onset. These findings may provide useful insights concerning the health properties of MedDiet–polyphenols as well as the novel targets destined to a tailored approach to MetS.
1 INTRODUCTION
Metabolic syndrome (MetS) is a clinical definition encompassing a cluster of metabolic risk factors that predispose individuals to cardiovascular disease (CVD) and type 2 diabetes mellitus (T2DM). Epidemiologic evidence defines MetS as a highly prevalent worldwide disease. The increase of overweight and obese people globally and the impact this has had on health care systems, economies, and quality of life further support this evidence. Moreover, MetS allows the identification of high-risk subjects who are more likely to develop T2DM and CVD, and it facilitates epidemiologic and clinical studies leading to novel pharmacological strategies, lifestyle modifications, and preventive treatment approaches (Huang, 2009).
Although industrialized countries have a higher incidence of MetS, the widespread embrace of Western habits has provoked an increase of this physiopathology in other regions as well (e.g., Southeast Asia; Noleran, Carrick-Ranson, Stinear, Reading, & Dalleck, 2017; Rochlani, Pothineni, Kovelamudi, & Mehta, 2017). These findings have identified MetS as a pressing challenge for human health with profound implications for national health care systems. Thus, a better understanding of possible preventive strategies would seem to be extremely useful to improve human health in the future.
Clinical and scientific studies pinpoint lifestyle modification as an effective strategy to reduce several features accountable for the risk of MetS onset.
The Mediterranean diet (MedDiet) is a dietary pattern adhered to people living in the Mediterranean Sea basin. This diet is characterized by a high intake of vegetables, nuts, olive oil, and a moderate consumption of wine, along with rare consumption of red and processed meat, butter, and sugar drink (Guasch-Ferré, Merino, Sun, Fitó, & Salas-Salvadó, 2017).
Emerging evidence highlights the protective effect exerted by the MedDiet on the different components of MetS (Chiva-Blanch & Badimon, 2017). Particularly, the antioxidant and anti-inflammatory properties of MedDiet foods, such as olive oil, nuts, vegetables, and wine, have been documented. In turn, these beneficial properties seemed to be connected to the polyphenol content within these foods (Medina-Remon et al., 2017).
Identifying the principal polyphenols in MedDiet foods could provide interesting insights regarding the beneficial effect of this dietary pattern on MetS onset as well as the development of tailored strategies targeting some of the features accountable for this physiopathological state. Many studies have highlighted the effectiveness of these phytochemicals in supplementation strategies aiming to counteract some of the principal pathways responsible for the insurgence of chronic diseases, such as diabetes, CVD, and cancer (Dragan, Andrica, Serban, & Timar, 2015).
In this review, we aim to study the principal evidence linking the effectiveness of the MedDiet in preventing or delaying the physiopathological components accountable for MetS onset. In particular, we examine the principal polyphenols contained in typical MedDiet food, focusing our attention on their contribution in the regulation of mechanisms involved in key MetS features, such as insulin resistance (IR), endothelial dysfunctions, inflammatory response, oxidative stress, and dyslipidemia.
2 DEFINITIONS AND EPIDEMIOLOGY OF METS
MetS is characterized as the co-occurrence of metabolic risk factors that includes IR, hyperinsulinemia, impaired glucose tolerance, T2DM, dyslipidemia, and visceral obesity. Epidemiologic studies and scientific evidence show a relationship between MetS and the increased risk for CVD (Di Daniele et al., 2017).
The World Health Organization (WHO) in 1998 provided a unique definition for MetS, which takes into account the central physiopathological role played by IR (Alberti & Zimmet, 1998). IR was described as the primary requirement in the WHO definition (recognized as impaired fasting glucose, impaired glucose tolerance, or T2DM), and it must pair with at least two of the following criteria: (a) obesity (defined as waist/hip ratio >0.90—men; >0.85—women; or body mass index [BMI] > 30 kg/m2), (b) dyslipidemia (defined as triglyceride [TG] > 150 mg/dl or high-density lipoprotein cholestrol [HDL-C] <35 mg/dl—men; >39 mg/dl—women), (c) hypertension (i.e., >140/90 mmHg), and (d) microalbuminuria (defined as urinary albumin excretion of 20 μg/min or albumin-to-creatinine ratio of 30 mg/g; Huang, 2009). Although the WHO definition of MetS was the first to consider an interconnection among key metabolic features, the lack of routine performances of some measurements represented a limitation to its clinical application. In 1999, the European Group for the Study of Insulin Resistance (EGIR) revised the criteria used in the WHO definition. The EGIR proposed a model based on IR plus two of the following criteria: hypertension, dyslipidemia, and obesity; the latter measurement was simplified to waist circumference (Balkau & Charles, 1999).
Over the years, further revisions to the principles used to diagnose MetS were provided, aiming to introduce measurements and laboratory results commonly used in clinical management. The National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III) states that a MetS diagnosis can be made if three or more of the following criteria are met: (a) waist circumference (>40 in.—men; >35 in.—women), (b) blood pressure (>130/85 mmHg), (c) fasting TG (>150 mg/dl), (d) HDL-C level (<40 mg/dl—men; <50 mg/dl—women), and (e) hyperglycemia (fasting blood sugar >100 mg/dl; Di Daniele et al., 2017). The NCEP ATP III definition has been widely accepted and commonly used due to its simplicity and the prompt translatability into clinical and epidemiologic applications. Nevertheless, in 2005, the International Diabetes Federation (IDF) set up a novel criterion for MetS. This definition considered central obesity as the principal characteristic, which needed to pair with two of the four criteria elucidated in the NCEP ATP III definition (Zimmet, Magliano, Matsuzawa, Alberti, & Shaw, 2005). Obesity was considered as a central parameter rather than IR, given the difficulties in IR measurements in day-to-day clinical practice. It should be mentioned that obesity was calculated using ethnic-specific values, which takes into account the differences in body weight and waist circumference, thus recognizing the risk for T2DM and CVD across different populations (Zimmet et al., 2005).
Concerning the epidemiologic findings, the IDF estimated that MetS affected 25% of the population worldwide. Of that, the US population was the most highly affected, followed by Europeans. Southeast Asia has a lower incidence of MetS; however, this situation has shown a rapid increase toward Western levels (Nolan et al., 2017; Rochlani et al., 2017). Although the prevalence of MetS has marked regional differences and variations due to age, gender, and diagnosis criteria, the worldwide increase in overweight people (two billion estimated in 2030) has made MetS a pressing challenge for human health. Thus, improvement in human health in the near future requires a better understanding of the clinical significance MetS and an evaluation of possible preventive strategies.
3 COMMON PHYSIOPATHOLOGICAL PROCESSES AND CENTRAL FEATURES OF METS
Understanding the clinical significance of MetS and its related components is useful to recognize subjects exposed to the risk of T2DM and CVD. It is also useful to identify a subgroup of patients sharing this pathophysiologic state. Thus, given the promising implications for setting up tailored approaches, it is worthwhile to underline common biological processes (Rochlani et al., 2017). There are four main players involved in the initiation and progression of MetS: (a) IR, (b) metabolic inflexibility and mitochondrial dysfunction, (c) adiposopathy, and (d) inflammation (Figure 1). Examination of these four features is the simplest approach to describe the complexity and heterogeneity of MetS (Di Daniele et al., 2017; Huang, 2009).
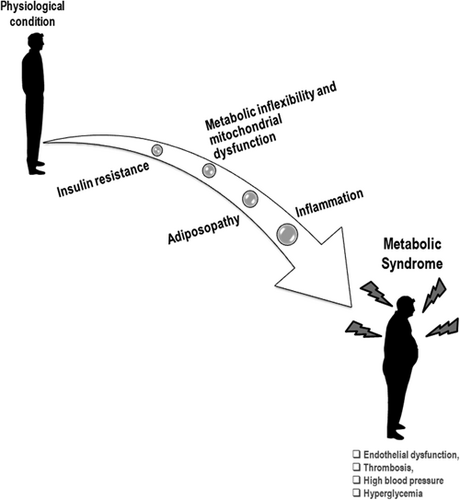
Representative illustration of the main players involved in initiation and progression of metabolic syndrome
3.1 IR
As previously discussed, IR and hyperinsulinemia (IR's surrogate marker), are useful predictors of T2DM and occur because of the decrease in insulin responsiveness in key metabolic tissues (i.e., skeletal muscle, fat, and liver; Di Daniele et al., 2017). IR evokes an abnormal response of adipose, muscle, and liver cells from hormone stimulation. Thus, IR exacerbates the deregulation of feedback mechanisms and exposes an organism to a pathological status. Researchers have described IR as promoting an increase in serum levels of free fatty acids (FFAs) due to impairment in the insulin-mediated inhibition of lipolysis occurring in adipose tissue (AT; Boden & Shulman, 2002). Circulating FFAs inhibit protein kinase in muscle cells (Hirabara et al., 2007), reducing their glucose uptake; on the other hand, FFAs promote gluconeogenesis and lipogenesis in the liver. The systemic consequence of these features lead to a hyperinsulinemic state to maintain the glycemic balance; accordingly, if this reparation fails, insulin secretion decreases (Rochlani et al., 2017). The IR-induced increase in glucose systemic levels affect endothelial cells first, given that vascular endothelium is the innermost layer in the vasculature cross-talking with the factors present in the bloodstream (Baur et al., 2006).
Physiologically, insulin-binding-mediated signaling primarily results in the activation of phosphoinositide 3-kinase (PI3K), its downstream target protein kinase B (also known as Akt, i.e., PI3K–Akt pathway), and the mitogen-activated protein kinase (MAPK) pathway (Muniyappa & Sowers, 2013). During IR, the balance between these two pathways is lost; particularly, the PI3K–Akt pathway is inhibited with a resulting deregulation of the downstream metabolic effects of insulin. Deregulation of the PI3K–Akt pathway causes a reduction in endothelial nitric oxide (NO) production in vascular cells. Insulin-induced NO in physiological conditions is responsible for the increase in blood flow, which is useful to enhance glucose uptake in skeletal muscle (J. A. Kim, Montagnani, Koh, & Quon, 2006). On the other hand, the MAPK pathway is unaffected by IR, thus resulting in endothelin 1 secretion as well as the expression of factors such as vascular cell adhesion molecules (VCAM) and E-selectin, which contribute to leukocyte–endothelial interactions and mitogen stimulation for vascular smooth muscle cells (SMC). Overall, this IR-mediated unbalance may lead to vascular abnormalities predisposing to atherosclerosis.
Other CVD-predisposing features, which are directly associable with IR onset, are the increase in serum viscosity, orientation toward a prothrombotic state, and increase of proinflammatory cytokines from AT (Juhan-Vague et al., 2003).
IR is strictly associated with atherogenic dyslipidemia as IR contributes to FFA spillover from the liver to the bloodstream. This leads to an increase in TG synthesis along with an augment in the production of apolipoprotein B (apoB) containing very low-density lipoprotein (VLDL). These events are responsible for the key features accountable for atherogenic dyslipidemia (i.e., high plasma TGs), low LDL, and high HDL.
IR mediates the increase in the VLDL level by altering its PI3K-induced clearance. Physiologically, insulin contributes to apoB degradation by acting on the PI3K-dependent pathway, thus impairing VLDL production and circulation. Moreover, insulin regulates the activity of lipoprotein lipase, whose major role consists in VLDL degradation. In sum, IR mediates the increase in TG-rich VLDL, which leads to an increase in LDL and a concomitant decrease in HDL, thus influencing the development of atherosclerosis (Manjunath, Rawal, Irani, & Madhu, 2013; Rochlani et al., 2017).
3.2 Metabolic inflexibility and mitochondrial dysfunction
Metabolic flexibility is the physiological process by which an organism adapts fuel oxidation (mainly fat and carbohydrates) in response to changes in nutrient availability. During fasting, healthy subjects predominantly meet energetic demand by lipid oxidation and fatty acids (FA) uptake. Under insulin-stimulated conditions (i.e., after meals), the organism orchestrates a shift toward glucose by activation of uptake, oxidative, and storage pathways along with the consequent suppression of lipid oxidation (Smith, Soeters, Wüst, & Houtkooper, 2018; Valentino et al., 2017). In MetS, an altered insulin-mediated substrate switching compromises this dynamic plasticity. During fasting, IR patients show a reduced ability of skeletal muscle cells to switch toward FA oxidation (FAO) with respect to healthy counterparts. Indeed, in response to lipid overload, skeletal muscles of patients with MetS present higher glycemic levels accompanied by lower FA uptake than those belonging to healthy subjects (Smith et al., 2018). This evidence supports the idea that an impaired FAO preludes IR and contributes to exposing subjects to MetS and its related complications.
Given the role played by mitochondria in orchestrating FAO, an impaired functionality of these organelles is also associated with IR. Abnormalities in mitochondria morphology, numbers, and functionality have been described in skeletal muscle cells of IR patients. At the molecular level, the expression profile of key genes regulating the oxidative machinery resulted in impairment, such as the peroxisome proliferator-activated receptor-γ coactivator 1-α (PGC-1α) and its downstream targets (Hesselink, Schrauwen-Hinderling, & Schrauwen, 2016). Unhealthy mitochondria jeopardize FA β-oxidation, causing a lipid overload in adipocytes with a relative increase in stress signals and proinflammatory mediators. Generally, this evidence indicates that mitochondrial dysfunction is crucial in causing IR and predisposes organisms to the risk factors of MetS (e.g., hypertension and T2DM; Mabalirajan & Ghosh, 2013).
3.3 Adiposopathy
AT has emerged as an active organ showing endocrine and immune features and playing a role in the development of MetS. In particular, white adipose tissue (WAT) is the “endocrinal” AT component, given its essential activity in the early stages of life for development and its proinflammatory properties. Nevertheless, starting from a young age, the adipokines (i.e., biologically active molecules secreted from WAT, especially from visceral adipose tissue [VAT]), are associated with cardiometabolic complications (Villarroya, Cereijo, & Villarroya, 2013). Although VAT accounts for only 12–20% of total body fat, it is strictly associated with MetS and CVD given the adipokines it secretes (Klein et al., 2007). Among them, VAT-released leptin controls energy homeostasis, and it has been known to have a proinflammatory activity by stimulating the Th1 pathway. On the other hand, adiponectin is an adipokine showing anti-inflammatory and antiatherogenic properties that contribute to a decrease in vascular reactivity, SMC proliferation, and improvement in the stability of atheromasic plaques (Pischon et al., 2004). Researchers have shown that an increase in VAT correlates with an increase in leptin levels along with concomitant decrease in adiponectin levels, thus exposing organisms to CVD risk (Rochlani et al., 2017).
An increase in AT mass is also associated with an augmented production of angiotensin II (Ang II), a key player in the renin–angiotensin system (RAS), which is known to strictly regulate blood pressure and fluid balance (Vaneckova et al., 2014). In addition, Ang II, as a potent activator of nicotinamide adenine dinucleotide phosphate (NAD(P)H) oxidase, contributes to the production of reactive oxide species (ROS) and leads to the increase of other sources of oxidative stress (Hitomi, Kiyomoto, & Nishiyama, 2007). Oxidative stress plays an important role in the pathogenesis of MetS by triggering or exacerbating many biochemical processes, including low-density lipoprotein (LDL) oxidation, expression of the redox sensitive nuclear factor κ light-chain enhancer of activated B cells (NF-κB), and platelet aggregation. Together, these processes contribute to signal cascades leading to dyslipidemia, diabetes, and CVD (Ando & Fujita, 2009).
Adipocyte hypertrophy is another feature induced by a hypercaloric state and is highly responsible for the activation of a paracrine signaling mechanism, which leads to the recruitment of fat cells to cope with the need for increased energy storage and to maintain AT physiologic functions (Bays et al., 2008). Consequently, the increase in fat storage predisposes to intracellular hypoxia that, in turn, mediates the release of FFAs into the bloodstream. This induces fat deposition in nonadipose sites, such as liver, muscle, pancreas, kidney, and blood vessels, causing lipotoxicity (Di Daniele et al., 2017). Lipotoxicity manifested at the muscular level induces IR. In the pancreas, it has a pejorative effect on insulin secretion. Overall, lipotoxicity contributes to the exacerbation of MetS features.
In general, these anatomic and functional alterations are accountable for the induction of “adiposopathy,” which in turn contributes to the worsening of MetS and increase in CVD risk factors.
3.4 Inflammation
The inflammation accompanying MetS has a particular presentation, and it is described as a “low-grade” chronic state that researchers and clinicians commonly name “meta-inflammation.” As previously described, obesity-induced oxidative stress and IR activate a signaling cascade having a proinflammatory effect. Given its susceptibility to lipolysis, VAT is also accountable for the release of many proinflammatory adipokines and cytokines, such as tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), IL-8, production of plasminogen activator inhibitor-1 (PAI-1), and C-reactive protein (CRP; Monteiro & Azevedo, 2010). It has been documented that IL-6 production by adipocytes increases with the rise of body fat and IR. Of note, this cytokine exerts its paracrine action by stimulating the production of CRP, the most commonly used marker to assess systemic inflammation. Experimental findings show a direct relationship between elevated levels of CRP and development of MetS, diabetes, and CVD (Tangvarasittichai, Pingmuanglaew, & Tangvarasittichai, 2016).
Besides the proinflammatory role played by adipocytes, adipose macrophages also participate in cytokines release. TNF-α, secreted by macrophages, presents in AT mass, and this increase in cytokine levels has been demonstrated to strictly depend upon AT status. In particular, TNF-α induces lipolysis by inactivating the insulin receptor in the AT. This increases FFAs while reducing adiponectin release (Monteiro & Azevedo, 2010). Researchers have demonstrated that hypertrophic AT expresses high concentrations of molecules, such as the macrophage chemoattractive protein (MCP-1) and the macrophage inhibitory factor (MIF-1), which represent chemoattractant stimuli for macrophages and peripheral lymphocytes, thus perpetuating the tissue immune invasion process (Leon-Pedroza et al., 2015). Once infiltrated into AT, macrophages change their anti-inflammatory profile (M2 macrophage) to a proinflammatory profile (M1 macrophage). This change induces aerobic glycolysis and is associated with inflammation and IR (Bluher, 2013). Additionally, studies on mouse models of obesity have shown that a lipotoxic milieu triggers a signaling cascade leading to immune cell modifications implicated in AT dysfunction, such as B and T lymphocytes, neutrophils, eosinophils, mast cells, and NK cells. This could contribute to promoting a vicious cycle of immune-metabolic degradation (Smith et al., 2018).
Finally, the ability of lipids to interact with cell surface receptors, such as Toll-like receptors, emerges as further evidence about the triggering of the signal cascade response to inflammatory process activation. Nevertheless, it has to be pointed out that the combination of these factors, rather than a single molecule or pathway, underlines lipotoxicity-mediated inflammation (Hotamisligil & Erbay, 2008).
On the whole, these findings contribute to a recognition of the close link between meta-inflammation and enhancement of MetS causative features.
4 EMERGING FEATURES FOR METS: CALORIC RESTRICTION (CR) AND SIRTUINS
CR is emerging as a useful strategy accountable for weight loss in humans. Several studies have shown its effectiveness in regulating plasma levels of TGs, maintaining blood pressure, and modulating the levels of AT-derived hormones, such as adiponectin and leptin (Albiero, Avogaro, & Fadini, 2015).
At the molecular level, CR seems to elicit the deacetylation of many proteins, which act as nutrient sensors triggering the principal events accountable for the switch toward a new metabolic milieu. Among the proteins deacetylases, sirtuins emerge as the key metabolic sensors.
The mammalian sirtuin family consists of seven members (Sirt1–7) having different cellular localization and exerting various biological functions. Among them, Sirt1, Sirt3, Sirt4, and Sirt6 have been demonstrated to play an important role in metabolic control. Sirt1, the best-characterized sirtuin, shows a regulatory effect on chromatin remodeling and gene expression, thereby controlling metabolic homeostasis (Li, 2013). Interestingly, the majority of identified Sirt1 target proteins are involved in energy metabolism, such as enzymes ocentf glycolysis, glucose oxidation, the tricarboxylic acid (TCA) cycle, the electron transport chain, and FA β-oxidation. Sirt1-induced modifications occur in response to altered nutrient status, such as following high-fat feeding or CR. Indeed, Sirt1 counteracts IR by modulating insulin signaling and glycolytic pathways in obesity, diabetes, and heart failure.
Recent studies show that CR or changes in nutrition also affect the mitochondrial acetylome (Pougovkina et al., 2014). Among the three mitochondrial sirtuins (i.e., Sirt3, Sirt4, and Sirt5), Sirt3 is the major mitochondrial protein deacetylase (Ng & Tang, 2013; Torrens-Mas, Hernández-López, Oliver, Roca, & Sastre-Serra, 2018). Interestingly, mice lacking the homologous proteins Sirt4 and Sirt5 showed no such increased protein acetylation, suggesting that these proteins targeted low-abundance or a limited subset of acetylated proteins or, alternatively, targeted other lysine modifications (Pougovkina et al., 2014).
Proteomic analysis of mitochondrial lysine acetylation identified over 1,300 acetylated peptides and 1,047 proteins in metabolic pathways, including FA metabolism, glycolysis, and the TCA cycle (Lombard et al., 2007). Moreover, researchers have reported that a situation of over- and undernutrition alters mitochondrial protein acetylation (Hebert et al., 2013), creating abnormal metabolic states with high levels of acyl-CoAs (Giordano et al., 2005; Mucerino et al., 2017). Additionally, in chronic overfeeding or in a variety of clinical settings, including obesity and diabetes (Ukropcova et al., 2007), mitochondria are left in a state of indecision characterized by persistent oxidation of all three major fuels (glucose, FAs, and amino acids). Indeed, several studies showed that obese and/or diabetic humans fail to shift from FA to glucose oxidation during the transition from fasting to feeding (Smith et al., 2018).
5 CURRENT APPROACHES FOR METS
As previously discussed, the recent interest in MetS has increased due to cardiovascular risk involvement. According to recent evidence, MetS patients show a double risk of developing CVD compared to healthy subjects and a mortality rate of 1.5 times that of healthy subjects (Huang, 2009).
Global strategies commonly used for management and treatment of MetS mainly focus on a combination of lifestyle changes and pharmacological intervention (Amiot, Riva, & Vinet, 2016).
Babio et al. (2014) demonstrated that adherence to healthy habits, consisting of increased physical activity, dietary modifications, or weight loss, counteracts MetS and its components. Perez-Martinez et al. (2017) reported that weight loss induced by the synergistic action of CR approach (500/1,000 calories/day) and an increase in physical activity was effective in preventing MetS or treating the condition in overweight or obese subjects. Additionally, others have shown that dietary modifications reflecting a lower intake of saturated fats, cholesterol, sodium, and simple sugars are effective in controlling dyslipidemia, hyperglycemia, and hypertension (Rochlani et al., 2017).
Concerning pharmacology, the heterogeneous background of MetS still represents the principal issue in designing a unique pharmacologic treatment effective for preventing or delaying MetS. The common strategies consist in using a single drug or combination thereof that target one or more MetS components. These drugs include antiobesity drugs, thiazolidinediones, metformin, statins, fibrates, RAS blockers, glucagon-like peptide-1 agonists, sodium glucose transporter-2 inhibitors, and some antiplatelet agents such as cilostazol. The most effective drug-based interventions concern hypertension and atherogenic dyslipidemia, while pharmacological management of T2DM and obesity—the major components of MetS—continues to be unsatisfactory (van Zwieten, 2006).
According to these findings, scientists and clinicians concur in identifying lifestyle modifications as the first-line intervention for treatment of MetS (Lim & Eckel, 2014). This is further proved by the increase in programs based on lifestyle modifications and their relative follow-up data showing that modest long-term weight loss is associated with a reduction in MetS prevalence (Dalle Grave et al., 2010).
6 HEALTHY HABITS: MEDDIET AND METS
As discussed before, MetS complexity is principally due to its multifactorial condition and to the cross-action of genetic and environmental factors. However, clinical and epidemiological findings evidence a low relationship between hereditary factors and MetS; genetic predisposition is accountable for only 10% of MetS cases (Weiss, Bremer, & Lustig, 2013). Otherwise, environmental factors seem to be the most acknowledged causes responsible for MetS onset. Of note, dietary habits and physical inactivity are recognized as the principal risk factors. Unhealthy dietary patterns emerge as the more relevant factors exposing people to MetS (Martinez-Gonzalez & Martin-Calvo, 2013).
Given this situation, growing interest has emerged regarding the beneficial effects of fruit- and vegetable-based diets, such as the prevention of obesity, diabetes, and CVD (Amiot et al., 2016). In this context, the MedDiet has catalyzed the attention of clinicians and researchers who are attracted by its widely acknowledged healthful properties, which are associated with longevity.
6.1 MedDiet definition and characteristics
The traditional MedDiet was first described by Keys in the 1960s. The MedDiet referred to the dietary pattern of people living in the Mediterranean Sea basin, that is, Greece, southern Italy, and southern Europe. Although there are some differences in eating habits among these Mediterranean countries, the common features characterizing the MedDiet are (a) daily consumption of nonrefined cereals and products (e.g., whole grain bread, whole grain pasta, and brown rice), fresh fruits, vegetables, nuts, low-fat dairy products, and olive oil as the principal source of lipids; (b) moderate intake of wine (especially red wine); (c) regular physical activity. Moreover, adherence to the MedDiet also includes a moderate consumption of fish, poultry, potatoes, eggs, and sweets, and a monthly consumption of red meat (Gouveri & Diamantopoulos, 2015). Besides these characteristics, nutritional scientists identify plant-derived foods (e.g., nuts, olives, and fruits) as having beneficial impacts on human health given their high content of bioactive compounds, monosaturated and polyunsaturated FAs (PUFA), and polyphenols (Amiot et al., 2016). Contrary to common thinking, the MedDiet is not a low-fat diet; lipid sources consist mainly in foods rich in unsaturated FAs and antioxidants (e.g., olive oil, fish, and nuts), which are associated with a reduced risk of CVD. The optimal mix of vitamins, phytochemicals, and antioxidants, which are present in grains, fruits, and vegetables, also have beneficial effects, such as preserving the glycemic index, reducing TG levels, and lowering LDL oxidation and inflammation (Abete, Goyenechea, Zulet, & Martínez, 2011).
6.2 MedDiet and MetS: Epidemiologic studies
The results of several cross-sectional and perspective studies highlight the protective effect exerted by MedDiet adherence on the different components of MetS (Chiva-Blanch & Badimon, 2017). Here, we reported the most compelling epidemiological and clinical evidence suggesting a positive correlation between MedDiet pattern and MetS prevention or treatment.
In the “ATTICA Study,” Panagiotakos and colleagues described the protective effect of MedDiet adherence on MetS for 3,042 Greek subjects without CVD and diabetes. Their analysis showed a 20% decrease in the risk of having MetS for participants following the MedDiet irrespective of age, gender, physical activity status, lipids, and blood pressure levels. Particularly, Panagiotakos et al. (2004) described a relationship between the MedDiet and an increase in antioxidant capacity along with a low oxidized LDL-cholesterol concentrations. Again, a cohort study carried out at Seguimiento Universidad de Navarra after a 6-year follow-up revealed that subjects strictly adhering to the MedDiet showed a lower cumulative risk of experiencing MetS (Tortosa et al., 2007). These studies are further supported by data from a cross-sectional study that involved 808 elderly Spanish participants adhering to the MedDiet with a high cardiovascular risk. Subjects following the MedDiet showed a 56% lower risk for developing MetS (Babio, Bulló, & Salas-Salvadó, 2009).
Intriguing results about the effectiveness of the MedDiet in preventing MetS are those obtained in studies conducted on a non-Mediterranean population. A 7-year follow-up study conducted on 1,918 participants of the Framingham Heart Study Offspring Cohort in the United States revealed that adherence to MedDiet patterns reduced the incidence of MetS. In particular, MedDiet subjects showed a reduction in MetS features, such as abdominal obesity and IR, and an improvement of lipid profiles (Rumawas, Meigs, Dwyer, McKeown, & Jacques, 2009).
The effectiveness of the MedDiet as an additional therapeutic strategy for subjects suffering MetS has been also tested. Esposito and colleagues provided the results of a 2-year cohort study consisting of 180 MetS patients divided into two groups: (a) MedDiet subjects, and (b) cardiac-prudent low-fat diet (i.e., fat intake <30%) subjects. At the end of the follow-up, the MedDiet subjects showed a 48% decrease in MetS features and an improved endothelial function. A reduction in body weight, IR, and inflammatory markers (i.e., IL-6, IL-8, and CRP) were documented (K. Esposito et al., 2004).
Finally, the paradox concerning the beneficial effects of the high-fat traditional MedDiet was assessed. To this purpose, within the PREvenction con DIetaMEDiterrànea (PREDIMED) study, 1,244 volunteers at high risk for CVD were categorized into two groups: (a) high-fat MedDiet (i.e., MedDiet supplemented with virgin olive oil or nuts) adherents, and (b) low-fat diet adherents. A 1-year follow-up revealed that the odds ratios for the reversion of MedDiet subjects to MetS, compared with the low-fat diet subjects, were 1.3 for the high-fat MedDiet supplemented with virgin olive oil and 1.7 for the high-fat MedDiet supplemented with nuts (Salas-Salvado et al., 2008). Of note, high-fat MedDiet subjects showed a decrease in blood pressure levels along with an increase in total polyphenol urine levels and NO in plasma. These results suggest that among other compounds, olive oil and nut polyphenols could play a pivotal role in exerting MedDiet beneficial effects (Chiva-Blanch & Badimon, 2017).
7 THE MEDDIET–POLYPHENOLS AND METS
Emerging evidence connects some of the main benefits of the MedDiet discussed above to the antioxidant and anti-inflammatory effects exerted by its principal components, such as extra-virgin olive oil (EVOO), nuts, vegetables, and wine (Barone et al., 2018). In turn, these beneficial properties seem to be related to the effect of polyphenols contained in these foods (Medina-Remon, Estruch, Tresserra-Rimbau, Vallverdu-Queralt, & Lamuela-Raventos, 2013). Many studies have demonstrated that the anti-inflammatory effect observed following EVOO consumption seem to be attributed to its polyphenolic content (Abe et al., 2011). Again, the favorable impact of olive oil polyphenols (OOPs) on CVD has been described. Clinical studies have disclosed the valuable results of olive oil consumption on endothelial function and inflammation markers with the antioxidant and anti-inflammatory properties of its polyphenols (Lopez-Miranda et al., 2010). Polyphenols in red wine have also been demonstrated to be accountable for modifications in lipoprotein profiles and redox mechanisms, which in turn exert a cytoprotective action (Di Daniele et al., 2017).
Accordingly, in the following paragraph, we review the effectiveness of polyphenols contained in MedDiet characteristic components, such as olive oil, red wine, and nuts, in counteracting the principal features predisposing or enhancing MetS onset.
7.1 Polyphenols: General features and characteristics
Phenolic compounds, also referred to as polyphenols, are a heterogeneous group of molecules representing the most abundant secondary metabolites from plants used in dietary patterns, especially the MedDiet. More than 8,000 different polyphenols have been described so far, each one showing property and bioavailability differences. A huge body of literature highlights their beneficial effects on human health, despite some nutritionists who do not consider them as essential micronutrients (Squillaro et al., 2018).
Physiologically, these molecules are produced to cope with environmental stressors prejudicing plant integrity, such as ultraviolet lights, free radicals, and uncommon temperatures. Concerning vegetable products in the Mediterranean basin, olives and grapes are very sensitive to stressors, and researchers have demonstrated that they enhance polyphenol production (Servili et al., 2004). Phenolic compounds share similar characteristics, that is, molecules with an aromatic ring structure and two or more hydroxyl groups showing high antioxidant capacity in vitro. They are categorized into five groups according to their chemical structure: phenolic acids, flavonoids, stilbenes, lignans, and others (Table 1).
| Polyphenol class | Basic chemical structure | Representative molecules | Principal sources | Mechanisms of action | |
|---|---|---|---|---|---|
| Polyphenols | Phenolic acids | 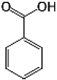 |
Ellagic acid; chlorogenic acid | Walnuts (Mora-Cubillos et al., 2015); coffee (van Dijk et al., 2009) | (↓) Inflammation and oxidative stress; lipid oxidation; endothelial dysfunction (S. R. Kim et al., 2014; Sanchez-Gonzalez et al., 2017; Suzuki et al., 2007; Winand & Schneider, 2014) |
| Flavonoids | 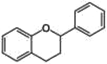 |
Apigenin; luteolin Quercetin; myrucetin Catechin; epicatechin Anthocyanins Cyanidin; delphinidin; malvidin |
Olive oil (Guasch-Ferre et al., 2017) Red wine (2000 et al., 2000) Red wine (Gronbaek et al., 2000) Blueberry (Chiva-Blanch & Badimon, 2017) Blueberry (Chiva-Blanch & Badimon, 2017) |
(↓) Inflammation and oxidative stress; lipid oxidation; intracellular lipid accumulation; endothelial dysfunction; insulin resistance (Basu et al., 2010; Camargo et al., 2010; Kwon et al., 2015; Medina-Remon et al., 2017; Ungvari et al., 2010; Wallerath et al., 2003) (↑) Glucose control (Camargo et al., 2010) |
|
| Stilbenes | 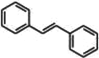 |
Resveratrol | Red wine (Gronbaek et al., 2000) | (↓) Inflammation; fatty storage and body weight; lipid oxidation; endothelial dysfunction (Fuhrman & Aviram, 2001; Mendez-del Villar et al., 2014; Ungvari et al., 2010; Wallerath et al., 2003) | |
| (↑) Mitochondrial biogenesis and functionality; Sirt1 induction (Allard et al., 2009; Lagouge et al., 2006) | |||||
| Lignans |  |
1-Acetoxypinoresinol, pinoresinol; 1-hydroxypinoresinol | Olive oil (Guasch-Ferre et al., 2017) | (↓) Inflammation; intracellular lipid accumulation; lipid oxidation (Camargo et al., 2010; Medina-Remon et al., 2017) | |
| Others | 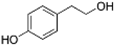 |
Oleuropein; hydroxytyrosol | Olive oil (Guasch-Ferre et al., 2017) | (↓) Inflammation; lipid oxidation; intracellular lipid accumulation; endothelial dysfunction; insulin resistance (Cao et al., 2014; Kwon et al., 2015; Medina-Remon et al., 2017; Rietjens et al., 2007; Takeda et al., 2014) | |
| (↑) Glucose control; insulin resistance; mitochondrial biogenesis and functionality; Sirt1 induction (Camargo et al., 2010; Kwon et al., 2015; Mendez-del Villar et al., 2014; Peyrol et al., 2017) |
- Note. The table shows the principal polyphenols contained within the representative MedDiet components (e.g., olive oil, red wine, and nuts). Polyphenols have been grouped according to the common classification.
- (↓): decrease; (↑): increase; MedDiet: Mediterranean diet.
Phenolic acids are widely present in foods, especially in acidic fruits and are further divided into two subgroups according to their chemical structure: benzoic acid-derivatives and cinnamic acid-derivatives. The latter group, to which p-coumaric, caffeic, ferulic, and sinapic acids belong, is the most commonly distributed within edible plants with respect to hydroxybenzoic acids, whose most representative molecule is ellagic acid (EA), which is very abundant in walnuts (Pandey & Rizvi, 2009).
Flavonoids are the most common polyphenols in human diets and, consequently, the most studied ones. Despite their abundance (more than 4,000 varieties have been described), they share a common chemical structure consisting of two aromatic rings that are bound together by three carbon atoms to form an oxygenated heterocycle (Manach, Scalbert, Morand, Rémésy, & Jiménez, 2004). According to the type of heterocycle, they are further divided into six subclasses: flavonols, flavones, flavanones, flavanols, anthocyanins, and isoflavones. In plants, these phytochemicals are responsible for the color and aroma of flowers and fruits, thus attracting pollinators and mediating fruit dispersion and development of seeds (Panche, Diwan, & Chandra, 2016).
Stilbenes are a class of polyphenols found in low amounts in human dietary patterns. Chemically, they consist of two phenyl rings connected by two carbon methyl bridges. Plants synthesize these molecules to counteract fungal infection or injuries. The most studied stilbene is resveratrol (3,4′,5-trihydroxystilbene), which is a phytochemical primarily found in grapes (Shamim et al., 2012). The body of literature describes the tremendous beneficial potential of this molecule on human health given its anticancer, antiaging, anti-inflammatory, and antioxidant properties (Gregorio, De Souza, de Morais Nascimento, Pereria, & Fernandes-Santos, 2016; Riccitiello et al., 2018).
Lignans are fiber-associated polyphenols found in many common foods as well as olive oil. Molecules in this group of phytochemicals share a 2,3-dibenzylbutane structure, formed by the dimerization of two cinnamic acid residues. Lignans have been considered as phytoestrogens given that they are metabolized into enterodiol and enterolactone in the mammalian gut (Pandey & Rizvi, 2009).
Other groups encompassing the molecules are not included in the previous four classes. An example is tyrosol and its derivates. Chemically, tyrosols are characterized by a phenethyl alcohol moiety that carries a hydroxyl group at the fourth position of the benzene group. Oleuropein (OL) and hydroxytyrosol (HT), the principal polyphenols in olive oil, are tyrosol-derived compounds (Gregorio et al., 2016).
7.2 The MedDiet food polyphenols: How do they effect MetS components?
7.2.1 OOPs
Olive oil is the most representative component of the MedDiet as well as its distinctive feature with respect to other dietary patterns. The composition of olive oil is unique and consists of two fractions. The saponifiable fraction is the most abundant (almost 98% of the total weight) and it consists mainly of TGs. The unsaponifiable fraction is extremely important given its high content in polyphenols, although it is the lesser component of olive oil (2% of total weight; T. Esposito et al., 2017; Peyrol, Riva, & Amiot, 2017). Despite the fact that the phenolic content of olive oil strictly depends on a mix of endogenous and exogenous factors (such as olive variety, agricultural environment, olive maturation stage, and processing techniques), the most representative components in EVOO are OL, HT, flavonols (such as apigenin and luteolin) and lignans (1-acetoxypinoresinol, pinoresinol, and 1-hydroxypinoresinol; Table 1; Guasch-Ferre et al., 2017). OL is the most abundant phenol compound in olives, and during fruit maturation it is hydrolyzed into different products; among them, HT is the most abundant phytochemical in EVOO.
The mean consumption of VOO in the MedDiet is almost 30–50 g/day, which accounts for a 200 μg polyphenol intake (Peyrol et al., 2017). Of them, HT is the principal phenolic compound adsorbed in the gut (Reboredo-Rodriguez et al., 2017).
Clinical evidence shows that the health benefits of OOPs can be attributed to their regulatory effects on genes and pathways involved in oxidative stress, atherosclerosis, inflammation, and mitochondrial function. Camargo and colleagues assayed by transcriptome analysis the gene expression profile in the postprandial state of volunteers adhering to an acute intake of a VOO-based breakfast supplemented with high and low polyphenol content, respectively. Their data showed that, in peripheral blood mononuclear cells of the former group, polyphenols induced an increase of messenger RNAs (mRNAs) belonging to genes involved in processes regulating obesity, dyslipidemia, and T2DM, such as NF-κB, cytokines, 5′-AMP-activated protein kinase (AMPK), or the activator protein-1 complex (Camargo et al., 2010). This evidence pairs with other data demonstrating the role of OOPs in lowering the inflammatory response of peripheral blood mononuclear cells and in decreasing the expression profiles of atherosclerosis-related genes (Konstantinidou et al., 2010).
The positive role played by OOPs in counteracting some features associable to the initiation and progression of MetS has been demonstrated in several in vitro and in vivo studies.
Researchers have demonstrated that an increase in body weight is a key factor predisposing to MetS. Several studies have shown that olive oil OL and HT counteract obesity by reducing intracellular TG accumulation and downregulating the expression profiles of adipogenesis-related genes, such as PGC-1α, lipoprotein lipase, acetyl CoA carboxylase-1, and carnitine palmitoyltransferase-1 (CPT-1; Cao et al., 2014; Hao et al., 2010). Other evidence has described the effectiveness of OOPs in regulating the expression profiles of factors involved in adipocyte proliferation, thus lowering fat tissue accumulation. Overall, these data suggest that the influence of polyphenol on adipocytes has a beneficial role in reducing risk of obesity (Peyrol et al., 2017).
Mitochondrial dysfunctions are strictly associated with obesity and metabolic inflexibility, leading to MetS. Evidence from an animal model of high-fat diet-induced obesity demonstrated the protective role of HT, administrated for 17 weeks, in stabilizing the mitochondrial complex-subunit expression profile and enhancing the quality of the organelles (Peyrol et al., 2017). Another study highlighted HT effectiveness in enhancing the expression of key genes regulating mitochondrial biogenesis and functionality, such as PGC-1α and CPT-1 (Hao et al., 2010). This evidence corroborates the emerging idea suggesting an OOPs-induced amelioration in mitochondrial oxidative metabolism and how this may counteract the events leading to metabolic inflexibility (Peyrol et al., 2017).
Moreover, OOPs seem to be effective in enhancing glucose tolerance and decreasing the events related to IR. The beneficial effects of dietary supplementation with OOPs or HT in reducing glucose plasma levels and decreasing insulin secretion have been tested in animal models (Hsu, Wu, Huang, & Yen, 2009). OL and luteolin administration were effective in improving glucose control in mouse and rabbit models of diabetes (Kwon, Jung, Park, Yun, & Choi, 2015). The possible mechanistic explanation behind the antihyperglycemic effect exerted by OOPs could consist in its mediated suppression of protein involved in carbohydrates transported toward the intestine (Saibandith, Spencer, Rowland, & Commane, 2017).
Another feature accounting for lowering intracellular defense and predisposing to MetS complications is the pro-oxidative state. In this context, the antioxidant properties of OOPs have been widely acknowledged in both animals and humans. Among OOPs, HT and OL are the most studied given that their chemical structure endowed them with a potent antioxidant ability. Their orthodiphenolic structure confers to these compounds a hydrogen-donating capacity able to scavenge free radicals. Interestingly, it has been hypothesized that HT seems to be involved in scavenging aqueous peroxyl radicals near the membrane surface, whereas OL exerts this action on the lipid peroxyl radicals within the membrane (Saija, Tomaino, Lo Cascio, Rapisarda, & Dederen, 1998). In a clinical study concerning healthy males, Covas et al. (2006) revealed an inverse correlation between an increase in the phenolic content of olive oil and a decrease in oxidative stress markers, such as hydroxyl-FAs and products of DNA-oxidative damage. Alirezaei and colleagues showed an increase in levels of antioxidant enzymes, such as glutathione peroxidase and catalase, in rat supplemented with OL. This led to an enhanced protection against ROS-induced lipid peroxidation. Indeed, the concentration of thiobarbituric acid reactive substances (an indicator of lipid peroxidation) strongly decreased in rat supplemented with OL (Alirezaei, Dezfoulian, Sookhtehzari, Asadian, & Khoshdel, 2014).
OOPs have also been demonstrated to reduce LDL oxidation, which is a deleterious mechanism accountable for alterations in lipoprotein conformation and predisposing to MetS. Moreover, HT and OL interfere with NF-κB activation, which is mainly due to ROS overproduction. This led researchers to hypothesize a direct ability of these phenolic compounds to reduce ROS levels. Studies showing a decrease in ROS levels in human endothelial cells following HT and OL treatment further corroborate this speculation (Carluccio, Calabriso, Scoditti, Massaro, & De Caterina, 2015).
Another physiopathological feature of MetS is meta-inflammation. Even in this case, OOPs, and especially HT, have been described as exerting anti-inflammatory action. Studies on rodent models have demonstrated the ability of HT to downregulate TNF-α, IL-6 cyclooxygenase-2 (COX-2) in liver, increase the expression of the anti-inflammatory cytokine IL-10, and reduce inducible NO synthase (Takeda et al., 2014). Zhang and colleagues demonstrated the inhibitory effect of HT on proinflammatory cytokine expression in immune cells. In particular, their data reported an HT-induced reduction in the TNF-α level as both protein and mRNA. This is an important finding, given the role played by this cytokine in macrophage activation, which, in turn, contributes to enhancing an inflammatory response (Zhang, Cao, & Zhong, 2009). Moreover, OL and HT have been demonstrated to counteract the TNF-α-induced proinflammatory effect on human adipocytes by stimulating adiponectin expression. This has profound implications, given that the exposure of adipocytes to TNF-α trigger immune cell infiltration (mainly monocytes and macrophages), which causes adipose dysfunctions linked to MetS (Scoditti et al., 2015).
The ability of OOPs to counteract inflammation also accounts for their protective action from atherosclerosis and endothelial dysfunctions. In an in vitro model of early atherogenesis, OOPs (mainly HT and OL) demonstrated their ability to modulate endothelial activation by the regulation of VCAM-1 and E-selectin expression (Carluccio et al., 2003). These events counteract the signaling cascade leading to monocyte binding to vascular endothelium, which is causative of atherogenic plaque formation. Experimental evidence has also demonstrated that OL and HT influence NO production (Medina-Remon et al., 2017). These data are in line with the results obtained by Rietjens, Bast, de Vente, and Haenen (2007), which demonstrated that HT induces an increase in the endothelial NO synthase phosphorylation (P-eNOS)/eNOS ratio, thus contributing to enhanced endothelium-dependent relaxation.
It has now emerged that OOPs are able to induce Sirt1 expression. In particular, HT has been described as activating Sirt1 signaling, which is in turn responsible for the transcriptional activation of antistress target genes, such as glutathione-S-transferase, γ-glutamyl cysteine synthetase, and NAD(P)H. This effect seems to be regulated through the interaction between Sirt1 and nuclear redox factor 2 (NRF2) signaling, which exert a protective effect against oxidative stress (Mendez-del Villar, González-Ortiz, Martínez-Abundis, Pérez-Rubio, & Lizárraga-Valdez, 2014). In addition, OL has been demonstrated to induce the expression of Sirt1 with a concomitant decrease of poly(ADP-ribose) polymerase-1 (PARP1). Functional associations between PARP1 and Sirt1 through NAD+ cofactor availability have been described. Thus, changes in NAD+ intracellular levels and/or PARP1 activity could influence Sirt1 activity to cope with a pro-oxidative milieu (Chung et al., 2010). These findings fuel speculations that activation of Sirt1 by OOPs may be beneficial for controlling oxidative stress and metabolism (Bayram et al., 2012).
In sum, the evidence reported in this paragraph highlights the role played by OOPs in counteracting the principal mechanisms leading to the initiation and progression of some MetS features (Figure 2). In particular, results from in vitro and in vivo studies show the beneficial effect of these compounds in ameliorating IR, glucose and lipid metabolism, lipid oxidation, inflammation, and mitochondrial biogenesis and functionality. Despite the promising evidence, long-term studies have to be carried out to set up a valid supplementation protocol, establishing the best dose of polyphenol, and identifying the ideal food matrix. This may provide useful findings to increase the beneficial effects of MedDiet also by developing suitable functional foods.
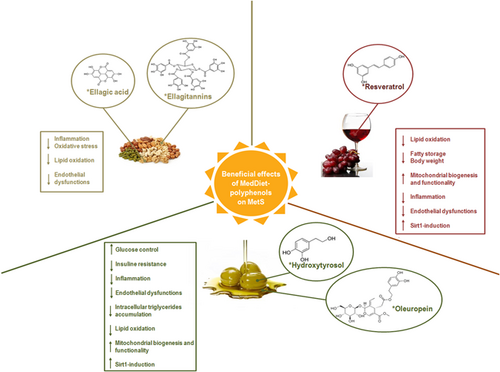
Schematic representation of the principal mechanisms responsible for metabolic syndrome (MetS) onset and/or progression regulated by Mediterranean diet (MedDiet)–polyphenols. *More representative and/or abundant polyphenol. ↑: increase; ↓: decrease [Color figure can be viewed at wileyonlinelibrary.com]
7.2.2 Red wine polyphenols (RWPs)
A moderate and regular wine consumption is another typical component of the MedDiet. Wine is a beverage obtained by the yeast fermentation of grape juice. This process is extremely important, given that it is accountable for the chemical modifications of many phytochemicals initially constituting fresh fruits. Currently, over 500 compounds have been described in wine, of which the major bioactive components are ethanol and polyphenols (Liu, Wang, Lam, & Xu, 2008). Polyphenols in red wine are highly concentrated, widely presented, and arise from the skin and seeds of grapes extracted during fermentation. The total amount of polyphenols in a glass of red wine is almost 200 mg with respect to the 30 mg in the same quantity of white wine. As described for olive oil, the polyphenol composition in wine varies according to endogenous and exogenous factors, such as type of grape, soil characteristics, environmental variations, and biological effects (fungi, insecticides, and fertilizers; Liu et al., 2008). Nevertheless, the principal classes of RWPs include flavonols (quercetin and myrucetin), flavanols (catechin and epicatechin), and anthocyanin and stilbenes (resveratrol; Gronbaek et al., 2000).
Clinical observations demonstrate that a moderate intake of red wine is associated with a reduction in risk for CVD, an improvement in the lipid profile, a decrease in the level of ox-LD, and protection against oxidative and inflammatory damage by strengthening the antioxidant defense system (Di Renzo et al., 2014).
Recently, polyphenolic compounds in red wine have been considered as the major candidates responsible for the beneficial properties associated with wine consumption. Growing evidence from in vitro and in vivo studies evidence the effectiveness of RWPs in protecting against MetS development.
Among RWPs, resveratrol is the most abundant polyphenol in red wine as well as the most studied. Resveratrol is produced by grapes in response to stress stimuli, such as fungal infection, injury, or ultraviolet exposure. Stressor-mediated signaling triggers the activity of stilbene synthase whose final product is resveratrol (Table 1; Liu et al., 2008). Chemically, this molecule exists in two isoforms: cis–trans isomers. Their distribution in red wine has been demonstrated to depend upon the fermentation process. Indeed, before grape juice fermentation, cis-resveratrol is the major isoform, but once this process finishes, the end product contains a large amount of trans-resveratrol, which is the most studied isoform responsible for the resveratrol-related pharmacological properties (Soleas, Yan, & Goldberg, 2001).
Besides the acknowledged effect of this molecule on several chronic diseases (e.g., cancer, myocardial infraction, and brain disorders), evidence supports its role protecting against development of some MetS features (Opie & Lecour, 2007).
Resveratrol has been demonstrated to activate the Sirt1 gene in many species, ranging from mammalians to insects, which has contributed to speculations that the protective effect exerted by CR against MetS development could be mimicked by this phytochemical (Allard, Perez, Zou, & de Cabo, 2009). Using a high-fat diet mice model, researchers demonstrated that resveratrol administration is effective in counteracting the effects induced by caloric excess and helps prevent MetS development (Lagouge et al., 2006). Also, the positive action of this polyphenol on body weight, fat mass, and BMI seemed to be attributed to the regulatory effect exerted on the peroxisome proliferator-activated receptor-γ (PPARγ) and its downstream genes mediating fat storage. This is also accountable for counteracting high-fat-diet-induced IR, which is an important feature in predisposing to MetS and its related diseases (Mendez-del Villar et al., 2014). AMPK is also a target of resveratrol, which enhances its activity by inducing phosphorylation of the α catalytic subunit. Recent evidence has associated this resveratrol-dependent AMPK activation with an increase of insulin sensitivity and metabolic profiles in rodents (Sun et al., 2007). Zhu, Wu, Qiu, Yuan, and Li (2017) also evidenced the effect of resveratrol in glucose lowering by AMPK activation; APMK was demonstrated to regulate energy balance by influencing insulin sensitivity and increasing glucose uptake.
The beneficial activity of resveratrol on mitochondria has also been described. In particular, this phytochemical promotes a Sirt1-induced activation of PGC-1α, thus contributing to mitochondrial biogenesis and enhancing oxidative phosphorylation. These favorable effects have been demonstrated in liver and skeletal muscle of mice fed with a high-caloric diet (Lagouge et al., 2006). Wang and colleagues demonstrated upregulation of Sirt1 levels in mouse fed with high-fat diet and supplemented with resveratrol. Their data supported the Sirt1-mediated activation of PGC-1α leading to an improved biogenesis, size, and density of mitochondria without inducing hepatic damages. Moreover, resveratrol treatment induced the upregulation of other genes such as nuclear respiratory factor 1 and transketolase, which regulated mitochondrial biogenesis as well as mitochondrial function (Wang et al., 2014). The protective role exerted by resveratrol on the biogenesis, function, and oxidative capacity of mitochondria is an important feature limiting their dysfunction, which is responsible for predisposition toward MetS. Resveratrol has also been demonstrated to exert an anti-inflammatory action by lowering the levels of NF-κB in macrophages and thus exerting a protective effect on murine endothelium. This regulatory effect on NF-κB has been attributed to resveratrol-mediated Sirt1 induction (Ungvari et al., 2010).
Besides resveratrol, the antioxidant properties and cardiovascular protective effects of RWPs have also been described. The administration of red wine, or catechin and quercetin, in a mice model of atherosclerosis showed a reduction in ox-LDL with a concomitant attenuation in atherosclerosis progression. Of note, quercetin seems to exert a major role in inhibition of LDL oxidation with respect to catechin, although they both share the effect of limiting macrophage uptake of oxo-LDL and in suppressing foam cells (Fuhrman & Aviram, 2001). RWPs have also been shown to promote endothelial-dependent vasodilatation by acting on NO enhancement and release. This is due to two principal causes: (a) the RWPs-induced eNOS enhancement through its phosphorylation, which seems to be mediated by the redox sensitive PI3K–Akt pathway (Ndiaye, Chataigneau, Chataigneau, & Schini-Kerth, 2004), and (b) the RWPs-mediated induction of eNOS expression levels through transcriptional activation (Wallerath, Poleo, Li, & Förstermann, 2003). This appears to be causative of the promising potential of RWPs on MetS, given evidence showing that eNOS-deficient mice are most likely to suffer from MetS and its related features, such as IR, dyslipidemia, and meta-inflammation (Liu et al., 2008).
Another key feature predisposing to MetS is the activation of monocyte and leukocytes adhesion to the endothelium. RWPs have been demonstrated to counteract this feature by downregulating the expression of pro-atherosclerotic and prothrombotic factors, such as VCAM-1, intercellular adhesion molecule 1 (ICAM1), and MCP-1 (Oak et al., 2003).
Overall, these findings support the effectiveness of RWPs, especially resveratrol, in playing a role protecting against the development of key features that predispose or enhance MetS onset, such as dyslipidemia, inflammation, and mitochondria biogenesis and functionality (Figure 2).
Despite resveratrol has been considered in innovative approaches for its beneficial potential, a better understanding of its efficacy and safety on MetS will contribute to the determination of recommendations for setting up translational diet therapy. In general, the further elucidation of the common mechanisms of action between MetS components and long-term RWPs supplementation will provide new insights into the impact of MedDiet on human health.
7.2.3 Nuts polyphenols
Nuts are another distinctive trait of the MedDiet, given their large consumption in this dietary pattern. The beneficial effects evoked by nuts have been documented. Clinical and epidemiological evidence has associated their intake with the low incidence of many MetS-related features, such as obesity, hyperglycemia, dyslipidemia, and inflammation (Mora-Cubillos et al., 2015). Despite the great amount of literature in this regard, the bioactive components in nuts as well as the healthy properties linked to the whole nut have been poorly characterized and studied. Recently, the identification of polyphenols as among the well-known micronutrients present in nuts (i.e., essential vitamins and minerals, mono- and PUFAs, and fiber) has provided interesting speculations about their beneficial potential on MetS (Ros, 2010).
Walnuts have a higher polyphenolic content than those of the nuts commonly featured in the MedDiet, such as almonds, hazelnuts, pistachios, and peanuts. The polyphenolic range in walnuts consists in 1,500/2,000 mg per 100 g and, interestingly, its extract shows an enhanced antioxidant potential (Vinson & Cai, 2012). The most abundant polyphenols in walnuts are ellagitannins (EL) that, upon consumption, are hydrolyzed to release EA, a compound belonging to the phenol acid class (Table 1). Despite the growing interest in EL and EA, a comprehensive understanding of their mechanisms of action has not been fully provided. This is due to the complexity associated with their metabolism, which depends upon many factors, such as dietary sources, absorption rate, and interaction with gut microbiota (Mora-Cubillos et al., 2015). Nevertheless, researchers have described the antioxidant and the anti-inflammatory properties of EL and EA in cellular and animal models (Sanchez-Gonzalez, Ciudad, Noé, & Izquierdo-Pulido, 2017), thus leading to the speculation that these properties contribute to the prevention and/or amelioration of MetS. As previously discussed, proinflammatory pathways and oxidative stress are strongly connected. In vitro evidence documented the positive role of EA in reducing the expression levels of TNF-α, IL-6 chemokine, and C–C-motif ligand-2. These genes have been acknowledged not only for their role in triggering inflammatory responses but also the crucial roles they play in the development of IR and cardiovascular complications once secreted by adipocytes and macrophages in fat deposits (Winand & Schneider, 2014). EA supplementation in New Zealand rabbits on a high-fat diet lowered lipid peroxidation and attenuated oxidative stress-induced dysfunctions (Yu, Chang, Wu, & Chiang, 2005). The ability of EA to counteract LDL oxidation during oxidative stress has been demonstrated in human plasma, in vitro, thus suggesting a promising potential of walnut polyphenols in regulating lipid profiles and supporting antiatherogenic activities (Sanchez-Gonzalez et al., 2017). Again, Papoutsi and colleagues showed that walnut extract and EA alone contributes to the downregulation of the expression levels of TNF-α, VCAM, and ICAM in endothelial cells. This evidence supports the anti-inflammatory potential in the polyphenolic content of walnuts to lower endothelial dysfunction (Papoutsi et al., 2008).
Although these findings contribute to expectancies about the beneficial role exerted by nut polyphenols in the prevention or amelioration of the physiopathological features of MetS (Figure 2), further clinical and epidemiological studies should be carried out. This may provide useful insights in determination whether single polyphenols or polyphenol-rich foods are the suitable candidate for the development of supplementation approaches.
7.2.4 Other MedDiet–polyphenols and phytochemicals
Besides the aforementioned MedDiet–polyphenol foods, other sources of polyphenols and phytochemical characterize MedDiet healthy properties.
Berries assumption is associated with anti-inflammatory and antioxidant potential due to the polyphenols contained in these fruits, such as anthocyanins, cyaniding, delphinidin, and malvidin (Table 1; Chiva-Blanch & Badimon, 2017). Particularly, blueberry consumption has been tested in clinical trials showing antihypertensive and antioxidant effects along with an amelioration of endothelial function and IR in MetS patients (Basu et al., 2010; Johnson et al., 2015). Experimental evidence obtained in animal models demonstrate that blueberry anthocyanins (BBAs) improve blood pressure. This effect is due to the increase of eNOS levels and the concomitant decrease of vasoconstriction evoked by NO-mediate pathways (Di Daniele et al., 2017). Data obtained in an acute mouse model of type 2 diabetes demonstrated hypoglycemic activity of BBAs. This effect is played by hydroxyl groups in the B ring of anthocyanins, which induced insulin secretion (Grace et al., 2009). DeFuria and colleagues showed that blueberries impacted on adipocyte physiology and inflammatory macrophages in AT. Their data led to hypothesize that this effect could be due to BBAs modulatory action on MAPK- and NF-κB stress signaling pathways, which are able to regulate cell fate and inflammatory genes (DeFuria et al., 2009).
In general, BBAs seem to improve vascular tone and cope oxidative stress, determining a protective effect against IR and inflammation-mediated complications.
Caffeinated beverage consumption are also associated to health benefits of MedDiet. An epidemiologic study conducted on an Italian cohort demonstrated the effect of coffee intake in lowering the risk of MetS onset. This was associated to the polyphenols contained in coffee (Grosso, Marventano, Galvano, Pajak, & Mistretta, 2014). Chlorogenic acid (CGA) is an ester of caffeic acid (Table 1); it is associated to various protective effects, such as suppression of body fat accumulation and glycemic regulation. Studies on animal models reveled that CGA consumption increased the sensitivity to insulin and slowed the glucose circulating levels following glucose load (Bassoli et al., 2008; van Dijk et al., 2009). This effect may be due to CGA regulatory effect on AMPK, a sensor and regulator of energy balance. Thus, CGA exerts its modulatory action on lipid and glucose regulation via AMPK (Ong, Hsu, & Tan, 2013). An antihypertensive action of CGA has been hypothesized. Suzuki et al. (2007) showed that CGA increased NO bioavailability, thus improving endothelial function with the concomitant reduction in blood pressure levels. Moreover, phenolic compounds of coffee seems to be also involved in inflammatory modulation. Even in this case CGA demonstrated a significant antioxidant effect that contribute to reduce the expression of proinflammatory cytokines. CGA regulates NF-κB activation via redox-related pathways, which lead to an immunomodulatory effect (S. R. Kim et al., 2014).
In general, these findings contribute to speculate the positive role of BBAs and CGA in controlling glucose regulatory processes and exerting an immunomodulatory action. These effects may contribute to counteract or delay MetS onset.
Although in this review we principally focus on polyphenols impact on MetS components, the bioactive effects of other phytochemical characterizing MedDiet are also worth mentioning.
Tomato is a MedDiet typical food, whose antioxidant properties have been already acknowledged. In particular, the beneficial effects of this vegetable are associated to lycopene, a carotene phytochemical, which activates antioxidative enzymes (e.g., superoxide dismutase and catalase) and shows anti-inflammatory and insulin-sensing properties (Di Daniele et al., 2017). This characteristics primarily lead to a mitigation of obese-related inflammation. In mouse models, lycopene supplementation resulted in a downregulation of proinflammatory cytokines (IL-6 and TNF-α), adipokines (resistin and leptin), and chemokines (MCP-1), along with upregulation of anti-inflammatory proteins (IL-10 and transforming growth factor-β; Fenni et al., 2017). These findings demonstrate the positive impact of lycopene on adipose inflammatory status. In particular reduction in IL-6, TNF-α, and MCP-1 lowers macrophage infiltration, which contribute to fuel meta-inflammation and its related compliances.
In addition, lycopene showed a regulatory activity on genes involved in FA β-oxidation, such as CPT-1 and peroxisome proliferator-activated receptor-α (PPARα; Zaghini et al., 2002). The latter is the master regulator of FAO in liver and the former is the rate limiting enzyme facilitating FA translocation in mitochondria. This evidence leads to hypothesize a positive action of lycopene in controlling mitochondria functionality and lipid metabolism. Moreover, antioxidant properties of carotenoids, as lycopene, result in counteracting lipid peroxidation, given their lipophilicity combined to free radical scavenging ability (Hadley, Clinton, & Schwartz, 2003).
Overall, this evidence suggests that lycopene is involved in reduction of lipotoxicity, meta-inflammation and inflexible hepatic FA metabolism, which are involved in MetS onset.
Epidemiologic and clinical studies support the positive role of omega-3 PUFAs (n-3 PUFAs) on MetS-related disease. The MedDiet sources of these compounds are fishes rich in eicosapentaenoic acid and docosahexaenoic acid (e.g., sardine), along with plants rich in α-linolenic acid (e.g., nuts; Harper & Jacobson, 2003). The n-3 PUFAs bioactivity has been analyzed in in vitro and in vivo models, which evidenced the favorable effects of these molecules on body weight reduction and metabolic profile improvement. By a mechanistic point of view, n-3 PUFAs induce the upregulation of transcriptional factors controlling adipogenesis (i.e., PPARα and PPARγ). This suggests that these molecules mediate healthy expansion of AT upon feeding and contribute to an healthy metabolic phenotype (Albracht-Schulte et al., 2018).
Anti-inflammatory activity is another feature associated to n-PUFAs administration, which is responsible for reduction of NF-κB transcriptional factor and its related proinflammatory cytokines, IL-1, IL-6, and TNF-α, usually elevated in obese subjects (Albracht-Schulte et al., 2018). This elicits an anti-inflammatory effect leading to a reduction in macrophage infiltration into AT, as demonstrated in mouse model supplemented with n-3 PUFAs. These data are also supported by clinical evidence demonstrating a decrease in M1 macrophage in AT of obese patients following n-3 PUFAs supplementation (4 g/day; Spencer et al., 2013). The shift from an anti-(M2) toward a proinflammatory (M1) phenotype predisposes to IR and meta-inflammation. Accordingly, Montserrat-de la Paz et al. (2017) showed that saturated FA induce monocyte toward M1 macrophage differentiation, contrary to PUFA, which trigger M2 phenotype. Besides the reduction of AT inflammation, omega-3 counteracts IR also by improving glycogen synthesis. Studies in muscle of mouse fed an high-fat diet evidenced the positive action of omega-3 in maintaining normal PI3K activity and GLUT4 expression levels, which lead to improve glucose uptake (Kuda et al., 2009).
Despite these favorable impacts on MetS components (especially IR and meta-inflammation), long-term data have to be produced to support the clinical relevance of n-3 PUFAs. However these findings and the underlying mechanisms provide encouraging expectative.
Another phytochemical with an high healthy potential is vitamin E, a lipid-soluble vitamin existing in two major subgroups: tocopherol (TF) and tocotrienol (T3). Vitamin E, particularly αTF, is found in vegetable oils, olives, and nuts; epidemiologic studies evidenced its antihypercholesterolemic, antiobesity, and antioxidant activity (Wong, Chin, Suhaimi, Ahmad, & Ima-Nirwana, 2017). Alcalá et al. (2015) demonstrated that αTF supplementation (150 mg/kg, twice a week) in obese mice fed with high-fat diet reduced oxidative stress and inflammation, thus improving insulin sensitivity and hypertriglyceridemia. Indeed, αTF has been demonstrated to reduce lipid peroxidase and downregulate TNF-α and CRP, suggesting a potential role in ameliorating redox status and inflammatory response in MetS patients (Devaraj, Leonard, Traber, & Jialal, 2008). Vitamin E mediates the reduction of the proinflammatory cytokine IL-6 expression levels in MetS patients exerting a beneficial effect in chronic inflammation and its related complications (Wong et al., 2017).
The beneficial role of vitamin E on dyslipidemia and vascular tone has been supposed. This phytochemical prevents oxidation of LDL-cholesterol by inducing NF-κB activation. Inhibition of LDL oxidation counteract vascular endothelial apoptosis exerting a protective action against impaired vasodilatation (Kuwabara, Nakade, Tamai, Tsuboyama-Kasaoka, & Tanaka, 2014).
In general, this evidence confirms the beneficial effect of MedDiet phytochemicals, such as lycopene, omega-3, and vitamin E, on MetS onset. These molecules mainly regulate the inflammatory pathways activated in MetS and control lipid metabolism ameliorating the physiology of AT.
8 METS AND DEPRESSION: MEDDIET ON MENTAL HEALTH
Emerging evidence indicates depression among the risk factors predisposing to MetS (Koponen, Jokelainen, Keinänen-Kiukaanniemi, Kumpusalo, & Vanhala, 2008).
Data obtained from cross-sectional and cohort studies demonstrated an high prevalence of MetS in patient with depressive disorders, evidencing a relationship between these conditions. Associations among depression and MetS features, such as central obesity, chronic inflammation, IR, and oxidative stress, have been described. These data pair with the evidence showing that conventional antidepressive treatments may interplay with various components of MetS (Mezuk, Eaton, Albrecht, & Golden, 2008; Pan et al., 2012). In particular, oxidative stress and inflammation emerge as the principal physiopathological features shared from both conditions. The role of oxidative stress in depressive disorder has demonstrated by the increase of lipid peroxidation in patients suffering depression. This is further accompanied by the activation of immune system and the release of proinflammatory cytokines, such as IL-6 and TNF-α (Scapagnini, Davinelli, Drago, De Lorenzo, & Oriani, 2012). Results from experimental in vivo animal models reveal that administration of IL-6 and TNF-α elicits depressive-like behaviors (Maes et al., 2012). Inflammatory cytokines, in turn, evoke the mitochondria-induced ROS production which is responsible for the increased levels of oxidative stress. An altered redox status in the central nervous system of patients suffering depression was observed (Scapagnini et al., 2012).
Dietary compounds with antioxidant activity capable of counteracting the effect of ROS emerge as a suitable strategy to support the conventional antidepressants therapies (Scapagnini et al., 2012). Epidemiological evidence show that healthy dietary patterns characterized by high content of fruit, vegetables, fish, olive oil and low content of animal foods associate with a decreased risk of depression. This evidence bestow upon MedDiet and its components intriguing expectations, especially for the high content of polyphenols contained within its foods. Results from in vivo and in vitro studies suggest that MedDiet–polyphenols play a role in brain health and in regulation of depression-linked aspects (Godos, Castellano, Ray, Grosso, & Galvano, 2018). OL and HT have been investigated for their neuroprotective activity against inflammation and oxidation. In particular, HT supplementation in rats evoked an increase in transcription factors, such as Forkhead box O1 (FoxO1) and FoxO3, involved in enhancement of mitochondrial function as well as in decrease of oxidative stress (Zheng et al., 2015). Analogously, the phenolic compounds in olive oil showed anti-inflammatory effects through the regulation of TNF-α, COX-2, and NF-κB (Davinelli et al., 2016).
Studies in rat models demonstrated the effectiveness of resveratrol in alleviating anxiety- and depression-like behavior. This effect was due to its antioxidant activity, which contributed to exert a neuroprotective action (Ge, Xu, Qin, Cheng, & Chen, 2016). Moreover, resveratrol administration in a depression-induced rat model revealed the anti-inflammatory potential of this molecule. In particular the antidepressant-like effects of resveratrol were accompanied by a reduction of proinflammatory cytokines, that is IL-1β and TNF-α, in rat hippocampus. Furthermore, resveratrol treatment attenuated the activation on NF-κB, demonstrating that inhibition of proinflammatory cytokines may depend by regulation of NF-κB signaling pathways (Ge et al., 2016).
In addition, resveratrol is a potent activator of Sirt1, whose reduction in hippocampus has been associated with increased depressive behavior (Abe-Higuchi et al., 2016). This lead to suppose other mechanistic activity of this polyphenol against depression.
On these premises, MetS and depression are important problems in the field of health; the identification of strategies targeting their common features may ameliorate well-being of people worldwide. In particular, MedDiet and the nutraceutical molecules contained within its food components, such as polyphenols, may represent a useful strategy to alleviate the incidence of depressive behaviors on MetS components and vice versa.
9 CONCLUSIONS AND PERSPECTIVE
As discussed in the present review, clinical and epidemiological studies have highlighted the beneficial effects of a close adherence to the MedDiet in preventing MetS and in delaying or counteracting its onset.
The substantial features characterizing the healthy properties of the MedDiet on MetS can be summarized as follows: (a) use of olive oil as the primary source of dietary fat, (b) habitual consumption of nuts, and (c) moderate intake of wine during meals. Evidence from in vitro and in vivo studies links the role of these components and the nutrients therein contained in ameliorating the etiology of components predisposing to MetS, such as dyslipidemia, hyperglycemia, meta-inflammation, and oxidative stress (Grosso, Mistretta et al., 2014). Among the well-known micronutrients present in olive oil, red wine, and nuts (e.g., essential vitamins and minerals, mono- and PUFAs, and fiber), polyphenols provide interesting speculations about their role, as a MedDiet cornerstone, in preventing the development and progression of MetS. The evidence discussed herein suggests that, in some cases, polyphenols are effective in enhancing the beneficial effect of MedDiet foods in which they are found. Molecules, like OL and HT (olive oil), resveratrol (red wine), and EL and EA (nuts), have been shown to regulate mechanisms responsible for the events leading to the initiation and maintenance of some of MetS features (Figure 2).
In addition to the described roles played by MedDiet–polyphenols in positively regulating MetS components, growing evidence has revealed their influence on Sirt1 as another key feature linking the MedDiet to longevity and good health.
As previously argued, resveratrol, OL, and HT have been demonstrated to exert regulatory action on Sirt1 by inducing its transcription, thereby triggering Sirt1-mediated events. This evidence pairs with the fact that Sirt1 activation mimics CR's beneficial effect in humans, thus increasing interest in the MedDiet as a source of healthy foods. MedDiet–polyphenols appear to be the safest way to benefit from the potential effects induced by a CR-mimetic strategy. Indeed, the failure of pharmacological molecules aiming to do this, given their severe side effects, open an intriguing scenario for the MedDiet and its principal phytochemicals.
Despite this promising evidence, clinical and epidemiological studies should produce further insights to address some issues concerning the effectiveness of MedDiet–polyphenols on MetS. More long-term randomized trials must be carried out to evaluate the protective effect of a higher intake of polyphenols on MetS. Additional epidemiological observations could provide precious insight to assess whether single polyphenols or a combination of them are more appropriate in eliciting a beneficial healthy effect (Chiva-Blanch & Badimon, 2017).
The lack of correspondence between the beneficial effects of polyphenols evidenced in in vitro and in vivo studies and the less satisfying data from clinical studies is also accountable for the difficulties in setting up a valid supplementation protocol, establishing the best dose of polyphenol, and identifying the ideal food matrix. This is also due to the bioavailability of polyphenols and their interactions with human gut microbiota. The greatest amount of polyphenols (90–95% of the total intake) accumulate in the large intestinal lumen where they are subjected to the enzymatic activity of the gut microbial community, thus modifying each other (Amiot et al., 2016). As described for EA, the variability of human microbiota might be accountable for the difference in polyphenol metabolization, thus influencing or varying their bioactive properties.
In conclusion, the intake of polyphenols may be responsible for the beneficial and protective effects of the MedDiet on MetS. However, further studies should be undertaken by researchers and clinicians to further understand the impact of MedDiet–polyphenols on good health. Such findings may also be effective for the development of polyphenol supplementation strategies aiming to maximize the health effects of the MedDiet. In particular, the CR-mimetic effect exerted by MedDiet–polyphenol-mediated induction of Sirt1 may represent a promising target for development of effective therapies for some MetS features, such as obesity and T2DM.
ACKNOWLEDGMENTS
This study was supported from PO FESR 2014-2020—Regione Campania, Asse 1—obiettivo specifico 1.2, Progetto “Sviluppo di nanotecnologie Orientate alla Rigenerazione e Ricostruzione tissutale, Implantologia e Sensoristica in Odontoiatria/oculistica (SORRISO)”—pdt1–000410; Project “Studio di fattibilità per la progettazione di sistemi di rilascio nanoparticellari a base di idrossidi doppi lamellari (layered double hyroxide—LDH) in grado di veicolare, in modo controllato e prolungato nel tempo, molecole biologicamente attive tipo Ca e/o F” (Prot. N. 0000836/2018) sponsored by Elleva Pharma S.r.l.; and PON I&C 2014-2020 FESR progetto “Micro/nanoformulati innovativi per la valorizzazione di molecole bioattive, utili per la salute e il benessere delle popolazione, ottenute da prodotti di scarto della filiera ittica (FOR.TUNA)” F/050347/03IX32.
CONFLICTS OF INTEREST
The authors declare that there are no conflicts of interest.




