Narrative review of the effects of antidiabetic drugs on albuminuria
Abstract
Diabetes mellitus is the most prevalent metabolic disorder worldwide. Glycemic control is the main focus of antidiabetic therapy. However, there are data suggesting that some antidiabetic drugs may have intrinsic beneficial renal effects and protect against the development and progression of albuminuria, thus minimizing the risk of diabetic nephropathy. These pharmacological agents can suppress upstream molecular pathways involved in the pathophysiology of diabetes-induced renal dysfunction such as oxidative stress, inflammatory responses, and apoptosis. In this narrative review, the pathophysiology of albuminuria in patients with diabetic nephropathy is discussed. Furthermore, the renoprotective effects of antidiabetic drugs, focusing on albuminuria, are reviewed.
1 INTRODUCTION
Diabetes mellitus (DM) is a global metabolic disorder of epidemic proportions with an increasing prevalence due to unhealthy lifestyle habits, thus mirroring the obesity pandemic (Semenkovich, Brown, Svrakic, & Lustman, 2015; Zimmet, Alberti, Magliano, & Bennett, 2016). This chronic disease results in an increase in morbidity and mortality through the development of macro- and microvascular complications, including diabetic nephropathy (DN; Al-Saeed et al., 2016; Russell & Cooper, 2015). DN is the most common cause of end-stage renal disease (ESRD) and is characterized by the histological changes of glomerulosclerosis, mesangial cell expansion, tubulointerstitial fibrosis, glomerular hypertrophy, podocyte loss, tubular-cell hypertrophy, and glomerular basement membrane thickening (Arora & Singh, 2013; Bhattacharjee, Barma, Konwar, Dewanjee, & Manna, 2016). These modifications are clinically presented as albuminuria (proteinuria), from the early stage of microalbuminuria progressing to overt proteinuria (De Zeeuw et al., 2004; Xu et al., 2015), leading to chronic kidney disease (CKD; Yaribeygi, Mohammadi, & Sahebkar, 2018g; Yaribeygi, Mohammadi, Rezaee, & Sahebkar, 2018e).
Although the exact pathophysiology of DN remains unclear, it involves inflammation, oxidative stress, and apoptosis, all of which are associated with poor glycemic control (Yaribeygi, Katsiki, Behnam, Iranpanah, & Sahebkar, 2018b; Yaribeygi, Mohammadi, & Sahebkar, 2018f). Renal hemodynamic changes and activation of the renin–angiotensin–aldosterone system (RAAS) also contribute to DN pathogenesis (Lin, Chang, Yang, Wu, & Chu, 2018).
Proteinuria has been linked to renal and cardiovascular (CV) outcomes both in patients with DN and with a nondiabetic renal disease, thus representing a valuable surrogate marker in clinical trials (Cohen-Bucay & Viswanathan, 2012; Cravedi & Remuzzi, 2013). Statins have been previously reported in meta-analyses to significantly decrease albuminuria in patients with DN (Qin, Dong, Fang, & Lu, 2017; Shen et al., 2016) and with nondiabetic CKD (Nikolic et al., 2013; Su et al., 2016). Similarly, angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs) exerted greater renal benefits, including protection from albuminuria development and progression, compared with placebo or other antihypertensive drugs (mainly calcium channel blockers) in patients with type 2 DM (Persson, Lindhardt, Rossing, & Parving, 2016; Vejakama et al., 2012). In this context, and according to the current guidelines of the American Diabetes Association, in the presence of albuminuria, ACE inhibitors/ARBs should be prescribed to minimize the risk of renal disease progression (Association, 2018). The same guidelines recommend that statin use may be considered in the presence of CV risk factors, including albuminuria (Association, 2018). Based on the above data, statins and ACE inhibitors/ARBs represent valuable therapeutic agents in patients with DN (Katsiki, Athyros, Karagiannis, & Mikhailidis, 2014; Umanath & Lewis, 2018).
Diabetic macro- and microvascular complications, including DN, are mainly developed via molecular pathways induced by chronic uncontrolled hyperglycemia (Asmat, Abad, & Ismail, 2016; Yaribeygi & Mohammadi, 2017; Zimmet et al., 2016). Antidiabetic drugs are used to achieve normoglycemia, thus reducing the risk of diabetic complications (Chaudhury et al., 2017; Hampp, Borders-Hemphill, Moeny, & Wysowski, 2014; Figure 1). However, apart from glucose-lowering, antidiabetic agents may also exert different metabolic and pharmacodynamic properties, thus forming their indications, side effects, and contraindications (Upadhyay et al., 2017). In this narrative review, the pathophysiology of albuminuria in patients with DN is discussed. Furthermore, we comment on the beneficial or harmful effects of antidiabetic drugs on albuminuria.
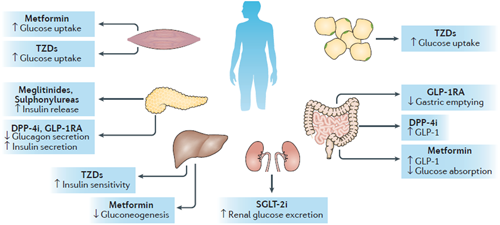
Target organs and action mechanism of antidiabetic drugs. The mechanism for metformin action remains uncertain: metformin might target the liver to reduce gluconeogenesis and skeletal muscles to enhance peripheral glucose utilization, with a possible role in the gut to increase levels of glucagon-like peptide 1 (GLP-1). Sulfonylureas and meglitinides increase insulin secretion in the pancreas. Thiazolidinediones (TZDs) act as insulin sensitizers in skeletal muscle, adipose tissue, and the liver. GLP-1 receptor (GLP-1R) agonists (GLP-1RA) target the pancreas to increase insulin secretion and reduce glucagon production, as well as act in the gut to reduce gastric emptying. Dipeptidyl peptidase 4 (DPP-4) inhibitors (DPP-4i) increase endogenous incretin levels by blocking the action of DPP-4. Sodium–glucose cotransporter 2 (SGLT-2) inhibitors (SGLT-2i) reduce renal glucose reabsorption. Reproduced with permission from (Zhou, Pedersen, Dawed, & Pearson, 2016). License code: 4415781322197. T2D, type 2 diabetes [Color figure can be viewed at wileyonlinelibrary.com]
2 PHYSIOLOGICAL IMPORTANCE OF ALBUMIN
The amount of renal albumin filtration is reported to be between 1 and 50 µg/ml of urine in dogs (Maack, 1992), about 400 mg/24 hr in humans (Mogensen & Sølling, 1977) and about 2.5–25 mg/24 hr in rats (Tencer, Frick, Öquist, Alm, & Rippe, 1998; Thelle, Christensen, Vorum, & Birn, 2006). The majority of albumin is subsequently reabsorbed in the proximal tubule (Jarad & Miner, 2009). The tubular albumin absorption capacity in proximal tubules of human is estimated at about 3.7 ng/min per mm tubule length, so when albumin levels in the filtrate exceeds this threshold, albuminuria occurs (Farquhar, 2006; Garsen, Rops, Rabelink, Berden, & van der Vlag, 2013; Jarad & Miner, 2009; Myers, Winetz, Chui, & Michaels, 1982; Park & Maack, 1984). In the normal physiological state, the amount of albumin excreted in the urine is 2.2 mg/24 hr in rats (Fuhrman et al., 2017; Russo et al., 2009). Albumin renal absorption involves the glomerular filtration barrier (GFB) that has a negative charge, thus affecting the amino acids/proteins penetration across the multilayer filtration membrane (Sancho-Martínez et al., 2015). This process maintains circulating albumin levels to preserve the plasma oncotic pressure needed for blood volume as well as for the transport of a variety of molecules and ions such as Ca2+, Mg2+, hormones, fatty acids, and hydrophobic vitamins (Birn & Christensen, 2006; Roche, Rondeau, Singh, Tarnus, & Bourdon, 2008).
Microalbuminuria is a clinical feature of renal insufficiency and it is considered as an independent risk factor for nephropathy as well as CV disease (Fuhrman et al., 2017; Yaribeygi & Mohammadi, 2017; Yaribeygi, Mohammadi, Rezaee, & Sahebkar, 2018d). The glomerular capillary membrane, also called GFB, inhibits the passage of proteins into Bowman’s capsule (Birn & Christensen, 2006). This membrane consists of three active layers consisting of fenestrated endothelial cells, the basement membrane and podocytes (PDs; Birn & Christensen, 2006). Evidence confirms that the contribution of all three layers is important for normal macromolecular filtration in renal glomeruli (Jarad & Miner, 2009).
2.1 Fenestrated endothelial cells
Due to the presence of overt fenestrations (50–100 nm size; ∼20% of surface area), the glomerular endothelium was initially regarded as the site of selectivity in the GFB (Jarad & Miner, 2009). Its coating glycocalyx layer is composed principally of proteoglycans and sialoproteins and extends to around 200 nm into the capillary lumen; glycocalyx “plugs” appear to cover these fenestrae (Menon, Chuang, & He, 2012). Disruption of this glycocalyx layer (by hyaluronidase and adriamycin) was shown to induce proteinuria (Menon et al., 2012). There is a further layer of proteins, known as the endothelial surface layer, into this glycocalyx which is in equilibrium with circulating plasma proteins that are too large to form any meaningful barrier (Reitsma, Slaaf, Vink, Van Zandvoort, & Oude Egbrink, 2007; Weinbaum, Tarbell, & Damiano, 2007). This layer may indirectly affect glomerular albumin filtration by modifying PDs behavior either physiologically, through soluble mediators, or pathologically, through an increased exposure of PDs to plasma components due to endothelial dysfunction (Satchell, 2013).
2.2 Glomerular basement membrane (GBM)
This includes type-IV collagen (COL4) and laminin, along with sulfated proteoglycans (Menon et al., 2012; Miner, 2012). The glomerular basement membrane (GBM) consists of β2-laminin mainly, whereas tubular basement membranes have β1-laminin chains (Menon et al., 2012). COL4 structure is unique in the GBM since it is enriched in α-3, 4, and 5 chains, whereas most other basal lamina is constituted of α-1 and 2 chains (Menon et al., 2012). Overall, the GBM contains three layers that is, lamina rara externa, lamina densa, and lamina rara interna (Figure 2, Table 1; Farquhar, 1981, 2006; Menon et al., 2012; Reitsma et al., 2007).
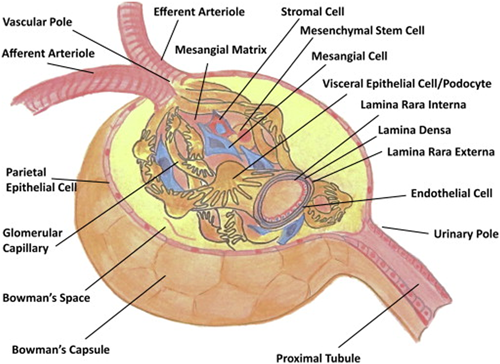
The structure and cellular components of the glomerulus. The cell types, glomerular components, and vascular structures are indicated. Reproduced with permission from (Barton & Sorokin, 2015). License code: 4415790561786 [Color figure can be viewed at wileyonlinelibrary.com]
| Antidiabetic agents | Reno-protective effects |
|---|---|
| Insulin | Re-stores podocytes function, normalized blood glucose, and suppresses subsequent inflammation and oxidative stress, keeps GFB integrity |
| SGLT2i | Reduces albuminuria secondary to lower blood glucose and subsequent oxidative stress |
| Biguanides | Suppresses oxidative damage and inflammatory responses, suppresses PDs apoptosis, improves endothelial dysfunction, metabolic control, blood pressure, lipid profile, and insulin sensitivity |
| Thiazolidinediones | Suppresses the loss of anionic charges of GFB, inhibits of hyperinsulinemia, hyperglycemia, high blood pressure, endothelial dysfunction, renal cells proliferation, and renal inflammation |
| Sulfonylureas | No evidence of a renoprotective effect |
| DPP-4 inhibitors | Reduces albuminuria via lowering blood glucose and blood pressure, suppresses endothelial-to-mesenchymal transition; oxidative stress and lipotoxicity and inhibit lipotoxicity-mediated inflammation, fibrosis, and apoptosis |
| GLP-1 receptor agonists | Same effect to DPP-4 inhibitors, declines albuminuria dependent to hypoglycemic effects and lowering blood pressure, also suppresses oxidative stress and lipotoxicity, and inhibit lipotoxicity-mediated inflammation, fibrosis, and apoptosis |
- Note. DPP-4: dipeptidyl peptidase-4; GFB: glomerular filtration barrier; GLP-1: glucagon-like peptide-1; PDs: podocytes.
It is established that any abnormality in the GBM can lead to varying degrees of albuminuria (Jarad & Miner, 2009). For example, laminin β2; lamb2 (laminin subunit beta-2) knockout mice have mild albuminuria without any significant changes in endothelial cells or PDs (Satchell & Tooke, 2008). The equivalent disorder in humans is Pierson syndrome with GBM malfunction and albuminuria (Witte, Kogan, & Goebel, 2006). Similarly, a COL4 disease known as Alport syndrome is closely associated with GBM disruption and albuminuria (Kashtan, 1999; Kashtan & Michael, 1996; Mochizuki et al., 1994). The GBM has some unique features, that is, it is leakier at the site of the endothelial cells and has an anionic charge which is mostly conferred by a lattice-like network formed by the sulfated glycosaminoglycan moieties of constituent the proteoglycans (Menon et al., 2012). During the early stages of DN, the progressive widening of the GBM may be accompanied by loss of the negative charges in the glomerular capillary wall, thus allowing albumin penetration (Menon et al., 2012).
2.3 Podocytes (PD)s
They are differentiated and highly specialized epithelial cells of mesenchymal origin that are located on the urinary side of the GFB (Menon et al., 2012; Mundel & Kriz, 1995). PDs are covered by an anionic layer of glycocalyx that is mainly composed of podocalyxin (Menon et al., 2012). These cells support the loop of glomerular capillaries from the visceral part of the Bowman’s space and interact at specific cell-to-cell junctions called slit diaphragms (SDs; Greka & Mundel, 2012; Menon et al., 2012; Pavenstadt, Kriz, & Kretzler, 2003). SDs have different types of proteins that is, zona occludens-1, catenin, and P-cadherin (Menon et al., 2012) and they are ladder-like structures with a thick central strand (consisting of multiple layers of nephrin strands) connecting to adjacent foot processes (FP; Martin & Jones, 2018).
Nephrin is a transmembrane protein of the immunoglobulin superfamily found in the SDs (Martin & Jones, 2018). It is hypothesized that an interaction between a nephrin and another nephrin (homologous) or nephrin-1 (heterologous) from an adjacent PD is responsible for the development of the “pore” of the SDs (Farquhar, 2006; Martin & Jones, 2018; Menon et al., 2012). SDs represent the final barrier of the GFB against the filtration of macromolecules (Menon et al., 2012). In cases of nephrin-1 mutations, the slit pores become thinner, shorter and less organized, thus creating larger pores and channels which may result in albuminuria (Jarad & Miner, 2009). PDs are also the main site of production and release of angiotensin II (Ang II); Ang II enhances the generation of reactive oxygen species by PDs, thus promoting PD autophagy (Ferrell et al., 2013; Yadav et al., 2010). Moreover, any mechanical stress on PDs (e.g., by hyperfiltration) can further augment Ang II production (Campbell, Raij, & Mundel, 2011; Petermann et al., 2005). Overall, the activation of the RAAS may affect macrophage recruitment and inflammatory responses in PDs (Noronha, Fujihara, & Zatz, 2002; Shrivastava & Bhatia, 2010).
PDs can also release other active biochemical substances that are involved in the development of albuminuria (Abbate et al., 2002; Menon et al., 2012). For example, angiopoietin-like-4, a podocyte-secreted glycoprotein, is overexpressed in DN and is linked to albuminuria (Menon et al., 2012). DN is strongly related to endothelial dysfunction caused by vascular endothelial growth factor (VEGF) inhibition (Neufeld, Cohen, Gengrinovitch, & Poltorak, 1999). VEGF is secreted by PDs and is then localized to the cell membrane and FPs; in cases of PD injury, VEGF is secreted in lower amounts, resulting in endothelial dysfunction and albuminuria (Sugimoto et al., 2003; Zhu, Wu, Dahut, & Parikh, 2007).
3 GLOMERULAR PROTEIN FILTRATION: THE ROLE OF TUBULAR REABSORPTION
The specific structure of the GFB predominantly restricts the transport of large and negative-charged molecules, for example, albumin (Cravedi & Remuzzi, 2013). The size selectivity is mostly attributed to the fenestrated endothelial cells, the lamina densa layer of the GBM and the pores of the SDs, whereas the glycocalyx layer of the fenestrated endothelial cells, the lamina rara externa, and lamina rara interna of the GBM as well as the anionic glycocalyx layer of the PDs are responsible for the charge selectivity (Cravedi & Remuzzi, 2013; Menon et al., 2012; Reitsma et al., 2007).
Charge selectivity has been confirmed by studies using charged dextran molecules showing that those with a negative charge have limited transport through the GFB, whereas those that are positively charged or neutral molecules have a more free passage (Greka & Mundel, 2012; Jarad & Miner, 2009). Therefore, in a healthy “physiologic” glomerulus, the transport of albumin, being a large and anionic molecule, across the GFB is restricted (D’amico & Bazzi, 2003; Menon et al., 2012). The GFB inhibits the passage of molecules by a radius above ~45 A°, that is, albumin by 60 A° (D’amico & Bazzi, 2003; Ferrell et al., 2013). However, a small fraction of total plasma can pass across the GFB and appear in the Bowman’s space filtrate by a ’shunt pathway’ created by a small number of SDs pores (D’amico & Bazzi, 2003). This pathway is very limited in physiological states but largely extended in nephrotic patients (D’amico & Bazzi, 2003; Tencer et al., 1998).
In relation to the size selectivity theory in the filtration process, several studies have demonstrated that the glomerular filtration rate (GFR) is inversely associated to the radius and molecular weight of the filtrated molecules (Jarad & Miner, 2009). However, the precise location of the size selectivity, as well as the contributions of PDs, SDs, GBM, and other elements of the endothelium are not completely understood (Greka & Mundel, 2012; Jarad & Miner, 2009). Almost 94% of the filtered albumin is effectively absorbed by proximal tubules, having an absorption capacity of about 3.7 ng/min per mm of tubule length (Jarad & Miner, 2009). Any increase in protein filtrate concentration may exceed the tubular reabsorption capacity, thus resulting in albuminuria (Jarad & Miner, 2009).
4 PATHOPHYSIOLOGY OF ALBUMINURIA
Albuminuria results from an imbalance between glomerular filtration and tubular reabsorption of proteins (Cravedi & Remuzzi, 2013). Tubular albumin reabsorption mainly occurs in the S1 and S2 (and to a lesser extent in S3) segments of the proximal tubules via ’endocytosis’ across the apical extremities of the epithelial cells (Cravedi & Remuzzi, 2013; D’amico & Bazzi, 2003). In these segments, the epithelial cells have extensive apical structures enabling them to effectively reabsorb more albumin from the tubular filtrate; thus, only a negligible amount of albumin remains in the excreted urine (Cravedi & Remuzzi, 2013). The proximal tubule reabsorbs about 71% of the filtrated albumin, the loop of Henle about 23% and the collecting duct about 3% (Tojo & Kinugasa, 2012).
The exact mechanism of albuminuria is not fully elucidated, but it is suggested that the ’shunt pathway’, observed only in a very limited proportion of nephrons under physiological conditions, is largely extended, resulting in a greater penetration of albumin across the GFB (Guasch, Deen, & Myers, 1993; Jarad & Miner, 2009). Such large shunt pathways are developed in the presence of damaged PDs, SDs, and glomeruli, as well as in tubular sclerosis, leading to further albumin filtration and less reabsorption (Guasch et al., 1993; Jarad & Miner, 2009). Furthermore, there is a detectable defect in charge selectivity in animal models of nephropathy, permitting negative molecules to be filtrated across the GFB (Deckert et al., 1993; Gall, Rossing, Kofoed-Enevoldsen, Nielsen, & Parving, 1994). In contrast, a decrease in anionic charges of the GBM via the removal of agrin (an anionic element in PDs) had no significant impact on the urinary albumin excretion (Harvey & Miner, 2008; Harvey et al., 2007). Therefore, it seems that all elements should function properly to create an effective barrier against filtration of macromolecules and negative elements (Jarad & Miner, 2009).
5 THE EFFECTS OF ANTIDIABETIC DRUGS ON ALBUMINURIA
5.1 Insulin
Exogenous insulin is essential for the treatment of type 1 DM and also commonly used to treat type 2 DM (Van den Berghe et al., 2006). Studies have reported that insulin therapy may reduce renal dysfunction and albuminuria independent of its glucose-lowering effect (Pilz et al., 2014). In this context, Pilz et al. reported that insulin resistance was associated with microalbuminuria and the onset of DN (Pilz et al., 2014). This finding was in accord with other studies reporting that insulin resistance was strongly related to different levels of albuminuria in patients with type 2 DM (Kim, Montagnani, Koh, & Quon, 2006; Stehouwer & Smulders, 2006; Welsh & Coward, 2010). Insulin signaling plays a key role in the function of PDs (Welsh & Coward, 2010). De Cosmo et al. in 2012 suggested that both insulin resistance and hyperinsulinemia are associated with endothelial and PDs dysfunction, thus resulting in albuminuria (De Cosmo, Menzaghi, Prudente, & Trischitta, 2012). Therefore, it seems that insulin affects GFB integrity, by preventing the loss of PDs and reducing albuminuria (El-Atat, Stas, McFarlane, & Sowers, 2004; Jauregui, Mintz, Mundel, & Fornoni, 2009). Studies in patients with type 1 and type 2 DM demonstrated a strong relationship between the insulin signaling pathway and microalbuminuria, thus suggesting that insulin is an effective therapeutic agent for the maintenance of normal endothelial permeability and GFB integrity (El-Atat et al., 2004; Hsu et al., 2011; Parvanova et al., 2006).
5.2 Sodium-glucose co-transporter 2 inhibitors (SGLT2i)
SGLT2i normalize plasma glucose levels by inhibiting glucose reuptake in renal proximal tubules, thus inducing glycosuria (Idris & Donnelly, 2009). Apart from their normoglycemic effects, SGLT2i have been shown to reduce albuminuria (Vallon et al., 2013). In this context, Vallon et al. demonstrated that empagliflozin, an SGLT2i, diminished albuminuria in streptozotocin-induced diabetic animals secondary to glucose-lowering (Vallon et al., 2013). Gallo et al. reported that empagliflozin reduced renal fibrosis and prevented DN in mice, although albuminuria was not improved (Gallo et al., 2016). However, Cherney et al. found that empagliflozin markedly reduced both micro- and macroalbuminuria in patients with type 2 DM independently of its metabolic or hemodynamic effects (Cherney et al., 2016). In the randomized placebo-controlled Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients–Removing Excess Glucose (EMPA-REG OUTCOME trial; Wanner et al., 2016), empagliflozin was found to significantly reduce the risk of incident or worsening nephropathy (defined as progression to macroalbuminuria) in patients with type 2 DM with established CV disease. There was a significant relative risk decrease of 38% in the progression from micro- to macroalbuminuria in the empagliflozin-treated patients compared with placebo (Wanner et al., 2016). However, no significant differences were observed in the rates of incident albuminuria between the two groups. In an exploratory analysis from the EMPA-REG OUTCOME trial, patients on empagliflozin were significantly more likely to experience improvements in albuminuria than those on placebo (Cherney et al., 2017).
Heerspink et al. showed that dapagliflozin, another SGLT2i, exerted renoprotective effects in patients with type 2 DM by lowering albuminuria and reducing the risk of long-term renal complications (Heerspink, Johnsson, Gause-Nilsson, Cain, & Sjöström, 2016a). Dapagliflozin significantly decreased albuminuria in an animal model of DN (Abdel-Wahab et al., 2018) as well as in patients with type 2 DM with renal impairment (Fioretto, Stefansson, Johnsson, Cain, & Sjöström, 2016). In a pooled analysis of Phase 3 clinical trials, dapagliflozin was reported to reduce albuminuria (assessed by the urinary albumin-to-creatinine ratio; Petrykiv et al., 2017a). Furthermore, dapagliflozin further improved albuminuria in patients with type 2 DM on ACE inhibitors or ARBs (Heerspink et al., 2016a; Petrykiv, Laverman, de Zeeuw, & Heerspink, 2017b).
Canagliflozin, another SGLT2i, was reported to improve albuminuria and tubulointerstitial markers in patients with DN independently of its glucose-lowering effects (Heerspink et al., 2016b; Takashima et al., 2018). In the Canagliflozin Cardiovascular Assessment Study (CANVAS) program (Neal et al., 2017), canagliflozin tended to protect against the progression of albuminuria compared with placebo in patients with type 2 DM at high CV risk. Of note, an increased risk of amputation was observed in relation to canagliflozin therapy (Neal et al., 2017). In a prespecified exploratory analysis of the CANVAS program, canagliflozin was shown to significantly reduce albuminuria (Perkovic et al., 2018).
Overall, these data strongly suggest that SGLT2is have renoprotective effects with beneficial effects on inflammation, oxidative stress, blood pressure, uric acid, and renal hemodynamics, thus protecting kidney function and preventing DN development and progression (Heerspink, Kosiborod, Inzucchi, & Cherney, 2018; Katsiki, Athyros, & Mikhailidis, 2016, 2017; Mende, 2017; Yaribeygi, Butler, Atkin, Katsiki, & Sahebkar, 2018a, 2018c).
5.3 Metformin
Metformin is an effective hypoglycemic drug (Orecchioni et al., 2015). It has been suggested that metformin may also exert renoprotection through inhibition of oxidative stress-induced PDs apoptosis, thus maintaining their integrity and density (Nasri et al., 2013). Kim et al. demonstrated that metformin prevented PD injury in an experimental model of type 2 DM by improving the redox state in the glomeruli (Kim, Shon, Kim, & Kim, 2012). The authors suggested that metformin may act as an AMPK (adenosine monophosphate-activated protein kinase) activator that regulates lipid and glucose metabolism as well as an inhibitor of mitochondrial respiratory chain (complex I) that is suppressed in the diabetic kidney; metformin can restore its activation and so re-adjust the oxidative balance (Kim et al., 2012; Liu, Li, Zeng, Liu, & Wang, 2008). Furthermore, metformin significantly decreased nephrin levels, GBM thickness and the foot process fusion rate in diabetic rats (Zhai et al., 2017).
Metformin significantly reduced albuminuria in both patients with DM and diabetic rats (Abbasi et al., 2004; Amador-Licona, Guı́zar-Mendoza, Vargas, Sánchez-Camargo, & Zamora-Mata, 2000). In a randomized controlled trial, metformin was shown to markedly improve renal insufficiency and diminish albuminuria without any effects on GFR in patients with type 2 diabetes (Pan et al., 2016). Endothelial dysfunction is the main initiator of renal albumin excretion; long-term metformin treatment significantly improved endothelial dysfunction by suppressing oxidative damage and reducing inflammation via decreases in the levels of vWF (von Willebrand factor), sVCAM-1 (soluble vascular adhesion molecule-1), t-PA (tissue-type plasminogen activator), PAI-1 (plasminogen activator inhibitor-1), CRP (C-reactive protein), and sICAM-1 (soluble intercellular adhesion molecule-1; De Jager et al., 2014). It has also been suggested that metformin can reduce albuminuria via effects on glycemic control, blood pressure, lipid profile, and insulin sensitivity (Amador-Licona et al., 2000). In contrast, there are studies reporting no beneficial effects of metformin on renal insufficiency and albuminuria (Imano et al., 1998; Lachin et al., 2011; Schernthaner et al., 2004). Therefore, further research is required to elucidate the full range of metformin’s renal effects.
5.4 Thiazolidinediones
Thiazolidinediones (TZDs) are selective agonists of the peroxisome proliferator-activated receptors (PPARs), thus controlling the expression of proteins and enzymes involved in glucose and lipid metabolism (Yki-Järvinen, 2004). TZDs are insulin sensitizers that lower insulin resistance and enhance glucose absorption in peripheral tissues (Yki-Järvinen, 2004). There are studies supporting the ability of TZDs to decrease albuminuria in both diabetic animals and patients with DM (Sarafidis, Stafylas, Georgianos, Saratzis, & Lasaridis, 2010; Yamashita, Nagai, Takamura, & Nohara, 2002). In this context, Yamashita et al. demonstrated that pioglitazone reduced albuminuria in an animal model of type 1 DM via suppression of the loss of anionic charges of GFB without affecting blood pressure or glucose (Yamashita et al., 2002). Miyazaki et al. showed that rosiglitazone prevented DN development by reducing albuminuria and improving renal function in patients with type 2 DM (Miyazaki, Cersosimo, Triplitt, & DeFronzo, 2007); rosiglitazone ameliorated inflammation by inhibiting the expression of tumor necrosis factor alpha (TNF-α; Miyazaki et al., 2007). Pioglitazone also improved inflammatory markers in patients with DM after kidney transplantation (Kharazmkia et al., 2014) as well as ameliorated oxidative stress, renal fibrosis and preserved kidney function in diabetic rats (Gumieniczek, 2003; Toblli et al., 2009).
Overall, the available data suggest that TZDs can prevent renal dysfunction and attenuate albuminuria in patients with DM via inhibition of hyperinsulinemia, hyperglycemia, endothelial dysfunction, oxidative stress, and inflammation (Miyazaki et al., 2007; Yamashita et al., 2002; Zheng & Guan, 2007).
5.5 Sulfonylureas
Sulfonylureas lower glucose by stimulating insulin secretion from the beta pancreatic cells via the blockage of the ATP-sensitive K+ channels (KATP) (Sur1-Kir6.2) in beta cells; these channels are depolarized, causing voltage-gated Ca2+ channels to open and subsequently stimulating pro-insulin secretion (Sola et al., 2015). Sulfonylurea-induced hypoglycemia is a clinically important side effect that limits the use of these drugs in daily practice (Shorr, Ray, Daugherty, & Griffin, 1996).
There are studies reporting that sulfonylureas may reduce albuminuria in both patients with type 2 DM and diabetic rats (Biederman et al., 2005; Goldshtein et al., 2016; Hung et al., 2013). In an animal study, sulfonylureas reversed the increased albumin excretion and improved glomerulosclerosis (Biederman et al., 2005). Further research is needed to elucidate the mechanisms of a direct if any, the renoprotective effect of these drugs.
5.6 Dipeptidyl peptidase-4 (DPP-4) inhibitors
DPP-4 inhibitors prevent GLP-1 inactivation, thus increasing the levels of native glucagon-like peptide-1 (GLP-1), consequently leading to glucose-dependent insulin secretion and glucagon suppression (Ahren, 2007). DPP-4 inhibitors appear to exert renal benefits in patients with type 2 DM (Tanaka et al., 2016). In this context, these drugs were reported to protect from the development and progression of albuminuria via improvements in oxidative stress, inflammation, and endothelial dysfunction (Haluzík, Frolík, & Rychlík, 2013). DPP-4 inhibitors may also suppress lipotoxicity and inhibit lipotoxicity-mediated inflammation, fibrosis, and apoptosis in renal tubular cells (Tanaka et al., 2016) as well as prevent oxidative stress-induced tissue damage in the kidneys during chronic hyperglycemia (Kanasaki et al., 2014).
There are studies showing that DPP-4 inhibitors can reduce albuminuria (Haluzík, Frolík, & Rychlík, 2013; kim et al., 2016). This effect may be also attributed to blood pressure and glucose lowering (Hattori, 2011). Sitagliptin, linagliptin, vildagliptin, alogliptin, and saxagliptin have all been reported to decrease albuminuria in patients with type 2 DM (Cooper et al., 2015; Groop et al., 2013; Helal, Zaki, & Said, 2018; Mori, Okada, Arao, & Tanaka, 2014; Mosenzon et al., 2016; Sakata et al., 2013; Trevisan, 2017). Among DPP-4 inhibitors, linagliptin has the highest affinity for DPP-4 and the largest volume of distribution, thus allowing its penetration into kidney tissue and tight binding to resident DPP-4 (Kanasaki, 2018). Of note, DPP-4 is predominantly present in the kidneys, especially in the presence of DN (Kanasaki, 2018). Furthermore, linagliptin is the only DPP-4 inhibitor not principally excreted in the urine, thus not requiring dose adjustments in patients with renal impairment (Gallwitz, 2013). Linagliptin has been shown to improve glomerulosclerosis, tubulointerstitial fibrosis, and albuminuria via antioxidant and antifibrotic effects in animal diabetic models (Kanasaki, 2018; Spencer, Yang, Sullivan, Klein, & Stanton, 2018). The Efficacy, Safety, and Modification of Albuminuria in Type 2 Diabetes Subjects with Renal Disease with LINAgliptin (MARLINA-T2D) study evaluated linagliptin effects on hyperglycemia and renal function in patients with type 2 DM with albuminuria (Groop et al., 2017). In this trial, linagliptin decreased albuminuria compared with placebo but this effect was not significant. The results of the Cardiovascular and Renal Microvascular Outcome Study With Linagliptin in Patients With Type 2 Diabetes Mellitus (CARMELINA), when published, may further elucidate the impact of linagliptin on albuminuria (Rosenstock et al., 2018).
5.7 Glucagon-like peptide-1 receptor agonists (GLP-1 RAs)
GLP-1 RAs are administered by subcutaneous injection, leading to supraphysiological levels of GLP-1 (Kalra et al., 2016). There are both daily (exenatide, liraglutide, and lixisenatide) and once-weekly (exenatide and dulaglutide) administered GLP-1 RAs currently available in the market (Lee & Lee, 2017).
GLP-1 RAs have been reported to exert renal benefits. The exact mechanisms involved in the process are not fully elucidated yet (Thomson & Vallon, 2018). Improvements in inflammation and oxidative stress may be involved in the process (Fujita et al., 2014; Muskiet, Smits, Morsink, & Diamant, 2014). Furthermore, it has been suggested that GLP-1 RAs may cause renal vasodilation and diuresis (Thomson & Vallon, 2018). These drugs can also reduce blood pressure and glucose; it is not known if this drug-induced blood pressure reduction is due to decreased sodium intake or enhanced renal sodium excretion (Thomson & Vallon, 2018). Of note, there are GLP-1 receptors and DDP-4 in the proximal tubule, presumably degrading filtered GLP-1 (Thomson & Vallon, 2018).
Exenatide, liraglutide, lixisenatide, and dulaglutide have been reported to reduce albuminuria in patients with type 2 DM or diabetic animal models (Cavusoglu et al., 2014; Tuttle et al., 2017; Zobel et al., 2017). In this context, in the Evaluation of Lixisenatide in Acute Coronary Syndrome (ELIXA) trial (Pfeffer et al., 2015) as well as in a post hoc analysis of this study (Muskiet et al., ), lixisenatide was shown to prevent the progression of albuminuria in patients with type 2 DM with a recent acute coronary event. Similarly, in the Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER) trial, fewer participants in the liraglutide group experienced the new-onset of persistent macroalbuminuria than in the placebo group among patients with type 2 DM at high CV risk (Mann et al., 2017). Even after 2 years of liraglutide therapy, albuminuria was significantly decreased, thus suppressing renal function deterioration in patients with type 2 DM with impaired kidney function (Hiramatsu, Ozeki, Ishikawa, & Furuta, 2017). Furthermore, the renoprotective effects of liraglutide were observed on top of multifactorial treatment, including inhibitors of the RAAS in patients with type 2 DM and albuminuria (von Scholten et al., 2017).
6 CONCLUSIONS
Based on the available data, insulin, metformin, pioglitazone, SGLT2i, DPP-4 inhibitors, and GLP-1 RAs may exert direct renoprotective effects through suppression of inflammatory responses, inhibition of free radical generation and oxidative injury and/or prevention of apoptotic pathways and cellular death. These effects are additional to the reduction of hyperglycemia, induced by all antidiabetic drugs, that is, indirectly associated with suppression of oxidative stress and inhibition of apoptosis. However, the renoprotective effects of antidiabetic drugs require further elucidation in large prospective clinical trials., the exact underlying molecular mechanisms should be also evaluated in future studies. Finally, owing to the recent trend toward identification and use of natural products and phytochemcials with antidiabetic activity (Asgary, Rafieian-Kopaei, Shamsi, Najafi, & Sahebkar, 2014; Panahi et al., 2018; Xu, Li, Dai, & Peng, 2018), it would be ideal to explore the potential impact of these compounds on renal function.
CONFLICTS OF INTEREST
NK has given talks, attended conferences and participated in trials sponsored by Amgen, Angelini, Astra Zeneca, Boehringer Ingelheim, Mylan, Novo Nordisk, Sanofi, Servier and Win Medica. The other authors declare that they have no conflicts of interest.
AUTHORS CONTRIBUTION
HY and AS did the initial research and draft that was reviewed and amended by SA and NK.
ETHICAL STATEMENT
The authors have no ethical conflicts to disclose.
FUNDING
No funding support was received for this study.




