Ganoderma lucidum polysaccharide inhibits UVB-induced melanogenesis by antagonizing cAMP/PKA and ROS/MAPK signaling pathways
Abstract
Ultraviolet (UV)-induced pigmentation is very common in clinical practice, but the current treatments are rarely effective, accompanied by some side effects. Ganoderma lucidum polysaccharide (GLP) is a natural antioxidant with no toxic side effects, which can antagonize UVB-induced fibroblast photo aging. The study aims to explore the role of GLP in inhibiting UVB-induced melanogenesis and its possible mechanism. The expression of melanogenesis genes such as microphthalmia-associated transcription factor (MITF), tyrosine (TYR), tyrosinase related protein 1 (TYRP1), tyrosinase related protein 2 (TYRP2), ras-related protein Rab-27A (Rab27A), and Myosin shows an upward trend after exposure of B16F10 and PIG1 cells to UVB irradiation, but GLP can downregulate the expression of genes related to UVB-induced melanogenesis. GLP can inhibit UVB-activated protein kinase A (PKA) and mitogen-activated protein kinase (MAPK) signaling pathways. Besides, GLP protects mitochondria from UVB damage and inhibits reactive oxygen species (ROS) production. Also, UVB-induced cyclic adenosine monophosphate (cAMP) can be inhibited. It has been found in the experiments of UVB-induced skin pigmentation in zebrafish that GLP is capable of inhibiting UVB-induced skin pigmentation. Meanwhile, it can greatly relieve erythema reaction in guinea pig skin caused by high-dosage UVB irradiation. In conclusion, this study shows that GLP can inhibit UVB-induced melanogenesis by antagonizing cAMP/PKA and ROS/MAPK signaling pathways and is a potential natural safe whitening sunscreen additive.
Abbreviations
-
- cAMP
-
- cyclic adenosine monophosphate
-
- CREB
-
- cAMP response element-binding protein
-
- GLP
-
- Ganoderma lucidum polysaccharide
-
- IgG
-
- immunoglobulin G
-
- MAPK
-
- mitogen-activated protein kinase
-
- MITF
-
- microphthalmia-associated transcription factor
-
- PKA
-
- protein kinase A
-
- PVDF
-
- polyvinylidene difluoride
-
- Rab27A
-
- ras-related protein Rab-27A
-
- ROS
-
- reactive oxygen species
-
- RT-PCR
-
- reverse transcription polymerase chain reaction
-
- TYR
-
- tyrosine
-
- TYRP1
-
- tyrosinase related protein 1
-
- TYRP2
-
- tyrosinase related protein 2
-
- UVB
-
- ultraviolet B
1 INTRODUCTION
Ultraviolet (UV)-induced facial pigmentation is very common in clinical practice (Cui et al., 2007; M. Kim, Han, Kim, Park, & Kang, 2016). Normal pigmentation can form skin color and protect the body from light damage, but hyperpigmentation often leads to chloasma, freckles, senile plaques, and other skin diseases (Matsui et al., 2015), seriously affecting patients’ appearance as well as their physical and mental wellbeing. Pigmentation mainly includes the synthesis and transport of melanin in melanocytes and the distribution and degradation of melanin in keratinocytes. Moreover, the synthesis and transport of melanin in melanocytes directly affect the process of pigmentation (Jeon et al., 2016). UV is the major external factor that causes facial pigmentation, whereas UVB is the main band that affects the synthesis and transport of melanin in melanocytes (Jian et al., 2014). At present, sunscreen, inhibition of tyrosinase viability, chemical peeling, laser, and so forth are methods typically used to prevent and treat UV-induced pigmentation, but they are rarely effective, costly, easy to relapse and accompanied by quite a few side effects (Dynoodt et al., 2013; Videira, Moura, & Magina, 2013). Therefore, it is of great significance for the development of whitening products to explore new methods for the prevention and treatment of UV-induced facial pigmentation.
In recent years, it has been found that traditional Chinese herbal medicine extracts are highly effective in inhibiting skin pigmentation, with characteristics of high efficiency, low cost and low side effects, embracing broad application prospects and huge social and economic benefits (Chen et al., 2015; Ko et al., 2014). Ganoderma lucidum is a long-standing traditional Chinese medicine used in China. It is capable of regulating immunity (Hsu et al., 2008) and delaying the aging process (Sudheesh, Ajith, Ramnath, & Janardhanan, 2010). Ganoderma lucidum polysaccharide (GLP) is one of the main active ingredients in G. lucidum and is active in antioxidation (Tie et al., 2012), regulation of immunity (Chan, Cheung, Law, Lau, & Chan, 2008), regulation of intestinal flora (Chang et al., 2015), antitumor (Li et al., 2015), and other pharmacological activities. Our preliminary study has also confirmed that GLP is effective in antagonizing UVB-induced fibroblast photo aging (Zeng et al., 2017). Recent studies have found that ganodermanondiol, another active ingredient in G. lucidum, is a potential whitening agent, which can inhibit melanin synthesis through the inhabitation of tyrosinase viability (J.W. Kim et al., 2016). However, it remains uncertain whether GLP is capable of antagonizing UV-induced pigmentation.
The synthesis of melanin is a multistage enzymatic reaction process in which tyrosine (TYR), tyrosinase related protein 1 (TYRP1), and tyrosinase related protein 2 (TYRP2) are the key enzymes (Dynoodt et al., 2013). In addition, microphthalmia-associated transcription factor (MITF) can induce TYR expression (Kordass et al., 2016; Park, Kosmadaki, Yaar, & Gilchrest, 2009). Therefore, MITF, TYR, TYRP1, and TYRP2 are often used as markers for melanogenesis. After melanogenesis, intracellular melanin transport is formed within the melanosomes from the perinuclear region to the direction of dendritic stretch. During the process, the dynamic complex molecules formed by ras-related protein Rab-27A (Rab27A), Myosin, and other proteins participate in the process of melanosome transport (Jian et al., 2014; J.H. Kim et al., 2015). Consequently, Rab27A and Myosin are often used as markers for melanin transport. Existing research has shown that cyclic adenosine monophosphate/protein kinase A (cAMP/PKA) and mitogen-activated protein kinase (MAPK) are the main regulatory pathways of melanogenesis (Drira & Sakamoto, 2016; Ren et al., 2016). Activation of the cAMP/PKA signaling pathway in melanocytes after UVB irradiation can promote the expression of MITF, which promotes the expression of genes related to melanogenesis (Chiaverini et al., 2008; Park et al., 2009). Furthermore, after exposure to UV irradiation, melanocytes experience increased reactive oxygen species (ROS) content, which can stimulate the MAPK signaling pathway to promote melanogenesis (Peng, Lin, Wang, Shih, & Chou, 2014).
The study explored whether GLP could antagonize UVB-induced melanogenesis. It was confirmed by cell and animal experiments that GLP can antagonize UVB-induced pigmentation. Its mechanism is relevant to the fact that GLP can antagonize UVB-activated cAMP/PKA and ROS/MAPK signaling pathways. The findings of the study suggest that GLP is a potential, natural, and safe agent for whitening and sunscreening.
2 MATERIALS AND METHODS
2.1 Culture of cells
In the presence of 5% CO2 at 37°C, immortalized human melanocyte cell line PIG1 (a gift from Dr. Caroline Le Poole, Loyola University Chicago) was cultured in the 254 (#M-254-500; Gibco, CA) medium supplemented with 5% fetal bovine serum (Biological Industries, Israel), 1% Penicillin-Streptomycin (Gibco), and 1% human melanocyte growth supplement (HMGS) (#S0025; Gibco). Meanwhile, melanoma cells from kunming (KM) mice (B16F10 cells) were cultured in dulbecco's modified Eagle medium (DMEM) (Hyclone, UT) medium supplemented with 10% fetal bovine serum and 1% Penicillin-Streptomycin (Gibco).
2.2 Determination of cell viability
After exposure to UVB irradiation, GLP (purchased from Shanxi Ciyuan Biotech Co., Ltd., Xi'an, China), or Malto (Yuanye Biotech Co., Ltd., Shanghai, China) for 24 hr, and the viability of B16F10 cells was determined with MTT assay. Specific procedures involved in methyl thiazolyl tetrazolium (MTT) essay can be referred to in our previously published paper (Zeng et al., 2017).
2.3 Determination of melanin content
B16F10 cells (2.5 × 105 cells per 35 mm dish) were divided into four groups: control group, UVB group (20 mJ/cm2 × 3d), GLP group (40 ug/ml), UVB+GLP group (20 mJ/cm2 UVB×3d, 40 ug/ml GLP). The cells were digested, centrifuged, and then photographed. Thereafter, the cells were lysed with 100 μl 1 mol/L NaOH in a water bath at 70°C for 2 hr. Then, the absorbance of the cells was determined at 490 nm with a microplate reader (PerkinElmer EnVision xcite, UK).
2.4 Reverse transcription polymerase chain reaction experiments
Total RNA in B16F10 and PIG1 cells was extracted with TRizol reagent (Invitrogen, Carlsbad, CA), and RNA was transcribed reversely into complementary DNA with Toyobo RT kit (Toyobo Co., Ltd., Osaka, Japan) according to the manufacturer's instructions. The reverse transcription polymerase chain reaction (RT-PCR) reaction was performed on a real-time fluorescence quantitative PCR instrument (LightCycler 480II, Mannheim, Germany) using a KOD SYBR® qPCR kit (Toyobo Co., Ltd.). All messenger RNA expression levels were normalized by GAPDH. Primer sequences are shown in Supporting Information Table 1.
2.5 Determination of intracellular ROS content
After B16F10 cells were treated with UVB irradiation, GLP, or UVB+GLP for 24 hr, the ROS content in the cells was determined by ROS detection kit (KeyGen Biotech. Co., Nanjing, China) in accordance with the instructions. The cells were observed under a fluorescence microscope (Olympus IX71, JPN). Fluorescence intensity was measured with a multifunction microplate reader (PerkinElmer EnVision xcite).
2.6 Western blotting
Cell proteins were extracted with lysis buffer and centrifuged at 12,000 rpm for 10 min at 4°C. The protein concentration was determined by the bicinchoninic acid (BCA) protein assay, and then the proteins were boiled for 5 min before the experiment. Twenty micrograms of protein sample was applied to each well, separated by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (Well Biological Co., Ltd., Changsha, China) and transferred to a polyvinylidene difluoride (PVDF) membrane (Millipore). After 2 hr of blocking with 5% bovine serum albumin (BSA), the membranes were incubated overnight with antibodies to Myosin (#3402; CST), Rab27A (#69259; CST), MITF (#MABE78; Abcam), TYRP1 (#MABC592; Millipore), TYRP2 (NBP1-56058; Novusbio), TYR (ab180753; Abcam), cAMP response element-binding protein (CREB; #9197; CST), p-CREB (#9198; CST), p38 (#9926; CST), extracellular signal regulated kinase (ERK) (#9926; CST), c-Jun N-terminal kinase (JNK) (#9926; CST), P-p38 (#9910; CST), P-ERK (#9910; CST), P-JNK (#9910; CST) or GAPDH (ab181602; Abcam). The PVDF membrane was washed with phosphate buffered solution+Tween 20 (PBST) and then incubated with horseradish peroxidase (HRP)-labeled goat anti-rabbit immunoglobulin G (IgG) or anti-mouse IgG secondary antibody (CST) for 90 min at room temperature. The binding antibody was detected by electrochemiluminescence (ECL) chemiluminescence.
2.7 cAMP-GloTM assay
The cells were lysed using the cAMP-Glo™ Assay kit (Promega Corporation, Madison, WI) to release cAMP, followed by addition of test solution containing PKA and kinase substrate cAMP. The Kinase-Glo reagent was then added to terminate the PKA reaction, and ATP was detected by luciferase reaction. A multifunction microplate reader was used to determine cAMP concentration.
2.8 Immunofluorescence microscopy
B16F10 cells were grown on coverslips. After treatment with UVB, GLP or UVB+GLP, the cells were fixed with 4% paraformaldehyde for 30 min. Afterwards, they were incubated in PBS with 1% BSA for 20 min at 37°C. The cells were then incubated with the melanosome marker NKI-beteb antibody (NBP2-29407; Novusbio) for 45 min at 37°C, and the cells were rinsed three times with PBS and incubated with Alexa488-labeled goat anti-mouse IgG (#4408; CST) for 30 min. Finally, the nuclei were dyed blue with 4’,6-diamidino-2-phenylindole (DAPI). A laser confocal microscope (ZEISS LSM800, JPN) was used to take images.
2.9 Determination of mitochondrial membrane potential
JC-1 mitochondrial membrane potential was determined according to the instruction manual provided by the manufacturer (Beyotime, Shanghai, China). The cells were observed under a fluorescence microscope (Olympus IX71, JPN) and the fluorescence intensity was measured on a multifunction microplate reader.
2.10 In vivo experiments
On the first day after hatching, zebrafish in the normal control (NC) group were cultured with ordinary culture medium; zebrafish in the UVB group were exposed to 300 mJ/cm2 UVB irradiation every day; zebrafish in the GLP group were cultured with GLP with a concentration of 4 mg/ml; zebrafish in the UVB+GLP group were irradiated with 300 mJ/cm2 UVB every day, and at the same time cultured in GLP with a concentration of 4 mg/ml. The same treatment continued for 5 days. Changes in pigment content in zebrafish were observed. KM mice and guinea pig were epilated with a hair removal cream (Veet; HuBei, China) on the back and then divided into four groups. The mice in NC group were wet-applied with PBS on the back skin for 1 hr every day; the mice in UVB group were exposed with 300 mJ/cm2 UVB irradiation on the back three times a week; the mice in GLP group were wet-applied with 40 mg/ml GLP solution on the back for 1 hr every day; the mice in UVB+GLP group was irradiated with 300 mJ/cm2 UVB on the back three times a week, and, at the same time, wet-applied with 40 mg/ml GLP solution on the back for 1 hr every day. The experiment lasted for 8 weeks. Changes in the skin pigmentation of KM mice were observed by dermoscopy. After the KM mice were killed, the skin tissue on the back was taken, paraffin-embedded, sliced, and stained with silver stain. The guinea pig was divided into the same four groups. However, the UVB dosage was 500 mJ/cm2. Twenty four hours after the treatment, changes in the guinea pig skin were observed.
2.11 Statistical analysis
The SPSS19.0 software was used for all statistical analysis. All experiments were performed in triplicate at the minimum. Data are presented as mean ± standard deviation. Significance tests were conducted on the data groups using analysis of variance followed by a comparison between the specific groups using Student's t test. p < 0.05 in all cases was considered statistically significant. ****p < 0.0001, ***p < 0.001, **p < 0.01, *p < 0.05.
3 RESULTS
3.1 GLP antagonizing UVB-induced melanogenesis
In this study, the toxic effects of GLP on B16F10 cells were first explored by MTT assay, and it was found that low concentrations of GLP (<160 ug/ml) had no obvious toxic effects on B16F10 cells. But when treated with whitening agent kojic acid (20 ug/ml) on a regular basis, B16F10 cells showed a significant decrease in viability (Figure 1a). MTT results showed that a low dose of UVB (≦60 mJ/cm2) had no significant influence on cell viability after treatment with different doses of UVB for B16F10 cells. When the UVB dosage was more than 60 mJ/cm2, however, cell viability decreased significantly in a dose-dependent manner (Figure 1b). Because UVB at a dose of 60 mJ/cm2 did not produce toxic effects on B16F10 cells, a total dose of 60 mJ/cm2 UVB was chosen for the subsequent experiments (Figure 1b). After B16F10 cells were irradiated with 20 mJ/cm2 UVB for three consecutive days (a total dose of 60 mJ/cm2), the cells were collected. Compared with those in the control group, the cells in UVB group were significantly darker. In comparison with the UVB group, the cells in the UVB + GLP group fade in color (Figure 1c). The pigment content of B16F10 cells were further determined by the NaOH method. The results showed that compared with the control group, the pigment content of the UVB group increased significantly; compared with the UVB group, the pigment content of the UVB + GLP group decreased (Figure 1d). After the melanosomes were stained with the immunofluorescence technique, there was an upward trend in the number of melanosomes in B16F10 cells which were irradiated with UVB, and they tended to be distributed to the cell membrane. Compared with the UVB group, melanosome in B16F10 cells in the UVB+GLP group decreased, and they were more likely to be distributed around the nucleus (Figure 1e). After the treatment of cells with only GLP, both the distribution of melanosomes and melanin content experienced no obvious change.
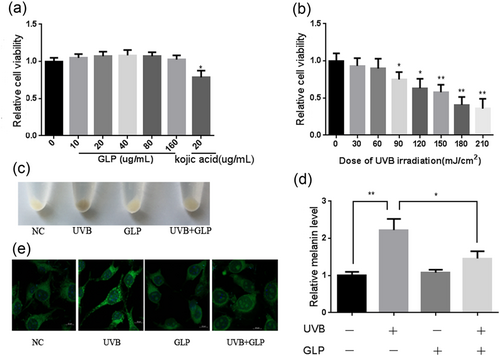
GLP antagonizing UVB-induced melanogenesis. The toxic effects of GLP and kojic acid on B16F10 cells (a). Detection of the toxic effects of different doses of UVB on cells with MTT assay after 24-hr irradiation (b). B16F10 cells were treated with 20 mJ/cm2 UVB×3d (total dosage of 60 mJ/cm2), 40 μg/ml GLP, or UVB + GLP. Then, cell color was observed after digestion and centrifugation (c), and melanin content was determined with NaOH assay (d). Melanosomes were labeled with NKI-beteb antibody, and melanosome distribution was observed with immunofluorescence (e). GLP: Ganoderma lucidum polysaccharide; MTT: methyl thiazolyl tetrazolium; UVB: ultraviolet B [Color figure can be viewed at wileyonlinelibrary.com]
3.2 GLP antagonizing the expression of genes related to UVB-induced melanin synthesis, and transport
Because GLP can inhibit UVB-induced melanogenesis, the melanin synthesis and transport genes were further determined by RT-PCR and western blotting. It was found that, after B16F10 cells were exposed to repeated UVB irradiation, the expression of both synthesis-related genes such as MITF, TYR, TYRP1, and TYRP2, and transport-related genes like Myosin and Rab27A showed a remarkable upward trend. Compared with the UVB group, the expression of MITF, TYR, TYRP1, TYRP2, Myo5a, and Rab27A in the UVB+GLP group was downregulated. Meanwhile, treatment of cells with GLP only indicated no significant impact on the expression of melanin synthesis and transport genes (Figure 2a,b). To exclude the possibility of nonspecific physical blockage of UVB by GLP, we added Maltodextrin (Malto), whose molecular weight and chemical structure is similar to that of GLP, as another control substance. Malto is another kind of polysaccharide, which is formed during the degradation of starch or glycogen. We found that Malto had no significant effect on UVB-induced melanogenesis (Supporting Information Figure 2).

GLP antagonizing the expression of genes related to UVB-induced melanin synthesis, and transport. B16F10 cells were treated with 20 mJ/cm2 UVB × 3d (total dosage of 60 mJ/cm2), 40 μg/ml GLP, or UVB + GLP. Expressions of genes related to melanin synthesis and transport such as MITF, TYR, TYRP1, TYRP2, Myosin, and Rab27A were determined with RT-PCR (a), and protein levels of MITF, TYR, TYRP1, TYRP2, Myosin, and Rab27A were determined with western blotting (b). GLP: Ganoderma lucidum polysaccharide; MITF: microphthalmia-associated transcription factor; Rab27A: Ras-related protein Rab-27A; RT-PCR: reverse transcription polymerase chain reaction; TYR: tyrosine; TYRP1, tyrosinase related protein 1; TYRP2: tyrosinase related protein 2; UVB: ultraviolet B
3.3 GLP inhibits UVB-induced melanogeneisis by antagonizing PKA and MAPK signaling pathways
It has been reported that UVB mediates melanogenesis by activating the PKA signaling pathway (Drira & Sakamoto, 2016; Ren et al., 2016). Consistent with previous studies, it was found in our study that CREB phosphorylation levels, key molecules of the PKA signal pathway, in UVB-stimulated B16F10 cells increased. Compared with the UVB group, the phosphorylation level of CREB in the UVB+GLP group dropped significantly. GLP alone had no effect on CREB phosphorylation level (Figure 3a). The MAPK signaling pathway also played a key role in melanogenesis. With UVB irradiation, the phosphorylation levels of ERK, JNK, P38 experienced a significant increase. Compared with the UVB group, ERK, JNK, P38 phosphorylation levels in the UVB+GLP group were significantly lowered. Treatment with GLP alone had no significant effect on MPAK family proteins (Figure 3a). The above results suggest that GLP can antagonize UVB-induced pigmentation by inhibiting the PKA and MAPK signaling pathways.
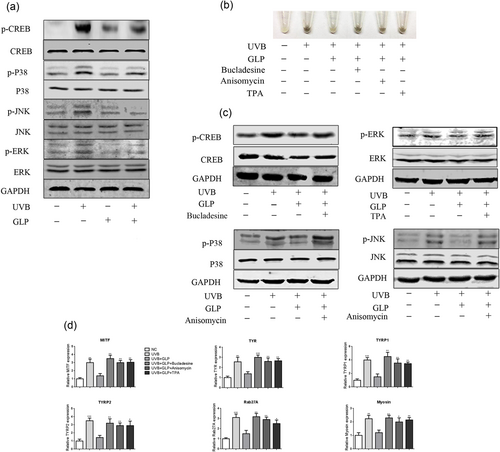
GLP inhibits UVB-induced melanogeneisis by antagonizing PKA and MAPK signaling pathways. B16F10 cells were treated with 20 mJ/cm2 UVB × 3d (total dosage of 60 mJ/cm2), 40 μg/ml GLP, or UVB+GLP. In comparison, the phosphorylation levels of CREB, JNK, ERK, and P38 in UVB-irradiated cells were significantly higher than those in the control group, and compared with UVB group, the phosphorylation levels of CREB, JNK, ERK, and P38 in UVB+GLP group were lower (a). After treatment of cells with UVB+GLP, the cells were further treated with PKA, ERK, and JNK/P38 signaling pathway activators (Bucladesine, TPA, and Anisomycin respectively), and then cell sediment images were shot with a digital camera (b). Expression of MAPK family proteins was determined by western blotting (c), and expression of genes related to melanin synthesis and transport was determined with RT-PCR (d). CREB: cAMP response element-binding protein; ERK: extracellular signal regulated kinase; GLP: Ganoderma lucidum polysaccharide; JNK: c-Jun N-terminal kinase; MAPK: mitogen-activated protein kinase; PKA: protein kinase A; RT-PCR: reverse transcription polymerase chain reaction; UVB: ultraviolet B [Color figure can be viewed at wileyonlinelibrary.com]
To further verify that GLP antagonized UVB-induced pigmentation through the PKA and MAPK signaling pathways, the cells were treated with PKA, ERK, and P38/JNK activators, respectively. WB results showed the activation of PKA, ERK, P38, and JNK pathways, respectively (Figure 3c). Compared with the UVB+GLP group, the color of B16F10 cells in each group deepened (Figure 3b). The RT-PCR results showed that all the activators could promote melanogenesis-related gene expression (Figure 3d).
3.4 GLP reduces UVB-induced ROS and cAMP
Our previous studies found that after exposure of fibroblasts to UVB irradiation, intracellular ROS levels increased significantly (Zeng et al., 2017). The current study found that B16F10 intracellular ROS levels experienced a significant increase as well after repeated UVB irradiation. Compared with the UVB group, the intracellular ROS levels in UVB+GLP group decreased, whereas GLP alone had no significant effect on ROS levels in B16F10 cells (Figure 4a,b). UVB irradiation could lead to mitochondrial damage, which may result in the generation of large amounts of ROS (Swalwell, Latimer, Haywood, & Birch-Machin, 2012). After exposure of B16F10 cells to UVB irradiation, a JC-fluorescent probe was used to determine mitochondrial membrane potential, the results of which showed that mitochondrial membrane potential decreased significantly, suggesting mitochondrial damage. Compared with the UVB group, the mitochondrial membrane potential of the UVB+GLP group increased (Figure 4c,d). Because cAMP is capable of activating the PKA signaling pathway, the level of cAMP was determined, which showed that repeated UVB irradiation of cells would lead to a significant increase in intracellular cAMP levels. Compared with the UVB group, the cAMP content in the UVB+GLP group dropped significantly, whereas GLP alone had no significant effect on the intracellular cAMP content (Figure 4e).
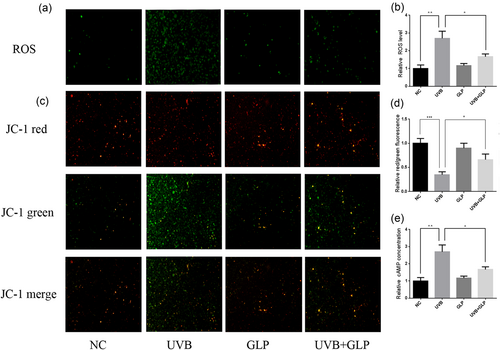
GLP reduces UVB-induced ROS and cAMP. B16F10 cells were treated with 20 mJ/cm2 UVB × 3d (total dosage of 60 mJ/cm2), 40 μg/ml GLP, or UVB + GLP. Intracellular ROS levels were determined by DCFH-DA probe method, and images were shot under a fluorescent microscope (a). The fluorescence intensity was measured with a multifunctional microplate reader (b). The mitochondrial membrane potential was determined by JC-1 fluorescent probe method. The red/green ratio represented the mitochondrial membrane potential: the smaller the membrane potential, the greater the mitochondrial damage, and images were shot under a fluorescent microscope (c). A multifunctional microplate reader was used to determine the fluorescence intensity (d). Intracellular cAMP levels were determined with cAMP-GloTM Assay (e). cAMP: cyclic adenosine monophosphate; GLP: Ganoderma lucidum polysaccharide; ROS; reactive oxygen species; UVB: ultraviolet B [Color figure can be viewed at wileyonlinelibrary.com]
3.5 GLP inhibits UVB-induced melanogenesis by antagonizing PKA and MAPK signaling pathways in PIG1 cells
To further verify that GLP could antagonize UVB-induced melanogenesis, human melanocyte PIG1 cells were used for reconfirmation. After exposure of PIG1 cells to UVB irradiation, the expression of MITF, TYR, TYRP1, TYRP2, Myosin, and Rab27A were upregulated (Figure 5b), and the phosphorylation levels of CREB, ERK, JNK, and P38 increased significantly. In comparison, an opposite trend was found in the UVB+GLP group. GLP alone had no significant effect on the melanogenesis genes and phosphorylation levels of CREB, ERK, JNK, and P38 in PIG1 cells. These results suggest that GLP can antagonize UVB-induced melanogenesis in PIG1 cells by inhibiting the PKA and MAPK signaling pathways.
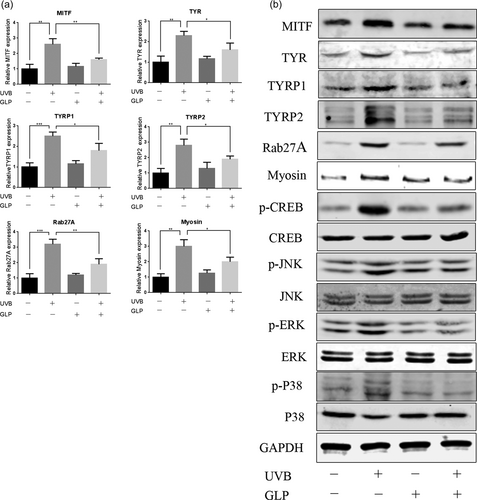
GLP inhibits UVB-induced melanogenesis by antagonizing PKA and MAPK signaling pathways in PIG1 cells. PIG1 cells were treated with 20 mJ/cm2 UVB × 3d (total dosage of 60 mJ/cm2), 40 μg/ml GLP, or UVB+GLP. Expression of genes related to melanin synthesis and transport such as MITF, TYR, TYRP1, TYRP2, Myosin, and Rab27A was determined with RT-PCR (a). Proteins related to melanin synthesis and transport and proteins related to PKA and MAPK signaling pathways were determined with western blotting assay (b). GLP: Ganoderma lucidum polysaccharide; MAPK: mitogen-activated protein kinase; MITF: microphthalmia-associated transcription factor; PKA: protein kinase A; Rab27A: Ras-related protein Rab-27A; RT-PCR: reverse transcription polymerase chain reaction; TYR: tyrosine; TYRP1, tyrosinase related protein 1; TYRP2: tyrosinase related protein 2; UVB: ultraviolet B
3.6 GLP antagonizing UVB-induced skin pigmentation in vivo
To explore whether GLP can antagonize UVB-induced pigmentation in vivo, a UVB-induced pigmentation model was constructed by treating zebrafish with UVB irradiation. After treatment of zebrafish juveniles with UVB irradiation for 5 days, increase in melanin content was observed. Compared with the UVB group, the melanin content in the UVB+GLP group experienced a downward trend (Figure 6a). To explore whether GLP was capable of sunscreen, depilated guinea pigs were irradiated with UVB, which resulted in obvious sunburn damage and erythema reaction. Compared with the UVB group, the guinea pigs in the UVB+GLP group suffered less sunburn damage and erythema reaction (Figure 6b). The results obtained in vivo confirmed GLP could antagonize UVB-induced pigmentation and sunburn reactions. At the same time, GLP could also antagonize UVB-induced pigmentation in KM mice (Supporting Information Figure 1).
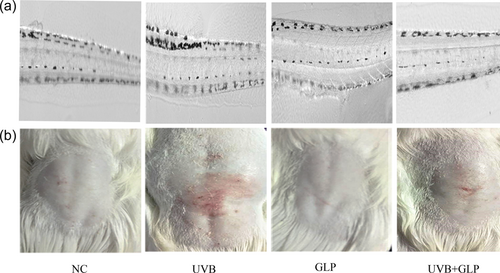
GLP antagonizing UVB-induced skin pigmentation in vivo. On Day 1 after hatching of zebrafish embryos, the control group was treated with PBS, the UVB group with 300 mJ/cm2 UVB, the GLP group with 40 mg/ml GLP, and the UVB+GLP group with both 300 mJ/cm2 UVB and 40 mg/ml GLP. The experiments were performed once a day for five consecutive days. Changes of pigment content in zebrafish were observed (a). The control group was treated with PBS, the UVB group with 500 mJ/cm2 UVB, the GLP group with 40 mg/ml GLP, and the UVB+GLP group with both 500 mJ/cm2 UVB and 40 mg/ml GLP. Changes in the back skin of guinea pig were observed (b). GLP: Ganoderma lucidum polysaccharide; UVB: ultraviolet B [Color figure can be viewed at wileyonlinelibrary.com]
4 DISCUSSION
Previous studies have found that GLP is effective in treating a variety of skin diseases, such as promoting refractory wound healing (Tie et al., 2012), inhibiting skin photo aging (Zeng et al., 2017), inhibiting melanoma (Sun et al., 2014) and so on. In this experiment, it has been found for the first time that GLP can antagonize UVB-induced melanogenesis by inhibiting the cAMP/PKA and ROS/MAPK signaling pathways.
It has also been confirmed in previous studies that UVB can induce skin pigmentation (Yun et al., 2015). Short-term UVB exposure can induce oxidation and redistribution of melanin granules in the stratum corneum, resulting in short-term pigmentation of the skin (Kelly, Seed, Young, & Walker, 2003). In comparison, repeated exposure to UVB for a long time can stimulate melanogenesis of melanocytes, upregulate the expression of genes related to melanin synthesis and transport, then increase the production of melanin granules, and accelerate the transport of melanin granules to keratinocytes (Chanchal & Swarnlata, 2008; He et al., 2012). In this study, more melanin was found to be transported to the peripheral membrane from the nucleus after repeated exposure of B16F10 cells to UVB irradiation for three consecutive days. Moreover, genes related to melanin synthesis and transport such as MITF, TYR, TYRP1, TYRP2, Rab27A, and Myosin were upregulated in expression. The results suggest that repeated UVB irradiation induces melanogenesis in cells. Meanwhile, no significant changes were found in the melanin content and melanogenesis-related gene expression after the cells were treated with GLP alone, but GLP was found to be capable of antagonizing UVB-induced melanogenesis and the expression of related genes. It was further confirmed that GLP could antagonize UVB-induced skin pigmentation in zebrafish and mice pigmentation models induced by repeated irradiation of UVB. In addition, GLP was found to relieve erythema in UVB-induced sunburn reactions model. The results suggest that GLP is a potential agent for whitening and sunscreen.
Existing studies show that cAMP/PKA, MAPK and other signaling pathways play an important role in melanogenesis, and the cAMP/PKA signaling pathway is the main regulatory pathway (Drira & Sakamoto, 2016; Ren et al., 2016). UVB irradiation can stimulate melanocytes and keratinocytes, fibroblasts to secrete α-melanocyte stimulating hormone and adrenocorticotropic hormone (Shin et al., 2015). The two hormones can stimulate increase the intracellular cAMP content in melanocytes through autocrine or paracrine stimulation and then activate PKA. The activated PKA further phosphorylates CREB, which in turn enhances the expression of MITF. Upregulation of MITF expression promotes the expression of melanin synthesis-related genes such as TYR (Park et al., 2009). MITF is also a key transcription factor for Rab27A (Chiaverini et al., 2008) and plays a central regulatory role in melanosome transport. Therefore, the cAMP/PKA signaling pathway can affect the synthesis and transport of melanin by regulating MITF. It has been found that herbal extracts of hesperidin (H.J. Kim, Yonezawa, Teruya, Woo, & Cha, 2015) can reduce the melanin production by inhibiting the cAMP/PKA signaling pathway, and thus play a role in whitening. It was found in our study that the treatment of cells with GLP alone had no significant effect on the cAMP/PKA signaling pathway, but GLP could inhibit UVB-induced cAMP levels and p-CREB levels, suggesting that GLP can antagonize UVB-induced cAMP/PKA signaling pathway activation. However, the specific mechanism of GLP inhibiting the increase of UVB-induced cAMP needs to be further explored.
The MAPK signaling pathway is another major pathway regulating melanogenesis (Choi, Kim, Park, Park, & Kang, 2015; Regazzetti et al., 2015). After exposure of melanocytes to UV irradiation, ERK/MAPK, JNK/MAPK, and p38/MAPK signaling pathways can be activated, which further enhances expression of MITF and promotes melanin synthesis and transport (Chiaverini et al., 2008; Park et al., 2009). In addition, studies have shown that the MAPK signaling pathway is involved in the regulation of melanogenesis through the direct regulation of TYR expression (A. Kim et al., 2015). ROS is a general term for a group of molecules or ions with higher oxidative activity, which can serve as a second messenger to affect various cellular functions through a variety of signaling pathways (Y.H. Kim, Kumar, & Chang, 2017; Liu et al., 2012; Wang & Zhang, 2017). UV-induced ROS plays an important role in activating melanogenesis mediated by the MAPK signaling pathway (H.N. Kim, Gil, Kim, Shin, & Choi, 2016). Our preliminary study has also demonstrated that ROS inhibitor NAC can antagonize UVB-induced activation of MAPK signaling pathway in melanocytes (Zeng et al., 2016). In fibroblasts, GLP can clear UVB-induced ROS (Zeng et al., 2017). In this study it was found that GLP could also remove UVB-induced ROS in B16F10 cells and antagonize the UVB-induced generation of melanin by inhibiting the MAPK signaling pathway. Mitochondria, endoplasmic reticulum, cell membrane, and nucleus all produce ROS, and mitochondria are the main sites of ROS production (Idelchik, Begley, Begley, & Melendez, 2017; Zhu, Lu, Zhang, & Hu, 2017). It has been found that UVB irradiation can lead to mitochondrial damage, which can produce a large amount of ROS (Swalwell et al., 2012). It was found in this study that UVB irradiation would lead to mitochondrial damage and ROS increase in B16F10 cells, whereas GLP could reduce UVB-induced mitochondrial damage and ROS. These results suggest that GLP may reduce ROS levels by antagonizing UVB-induced mitochondrial damage.
5 CONCLUSION
Our results show that GLP can antagonize UVB-induced melanogenesis by inhibiting the cAMP/PKA and ROS/MAPK signaling pathways and is a potential natural, safe whitening agent and sunscreen.
ACKNOWLEDGEMENTS
This study was supported by National Natural Science Foundation of China (No. 81703101), the New Xiangya Talent Projects of the Third Xiangya Hospital of Central South University (No. JY201623 and No. 20170301), and Natural Science Foundation of Hunan Province (No. 2018JJ3788 and No. 2018JJ3793).
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.




