Serenoa repens and N-acetyl glucosamine/milk proteins complex differentially affect the paracrine communication between endothelial and follicle dermal papilla cells
Abstract
Current treatments for hair follicle (HF) disruption are based on 5-α reductase inhibitors and prostaglandin modulators. Botanicals and nutraceutical compounds interfere with hair loss or stimulate its partial regrowth. Here, we used in vitro cocultures to investigate the activity of Serenoa repens ( SR) and N-acetyl glucosamine + milk proteins (NAG/Lac) on the paracrine interactions between human microvascular endothelial cells (HMVEC) and HF dermal papilla cells (FDPC). Both SR and NAG/Lac-induced endothelial tubulogenesis were enhanced by FDPC. SR promoted proliferation of both the cell types, while NAG/Lac was effective on endothelium. Vascular endothelial growth factor production, enhanced by SR, was further augmented by FDPC. In FDPC 5-α reductase-II and β-catenin expressions were modified by SR and less by NAG/Lac, with no additional effect by HMVEC. SR and NAG/Lac prevented lipid peroxidation, whereas NAG/Lac was effective on interleukin 1β production. Finally, SR and NAG/Lac differentially affected HMVEC permeability and tight junction proteins content. These data provide a mechanistic background for the potential use of these compounds as promoters of HF vascularization.
1 INTRODUCTION
The dermal papilla, is a cluster of mesenchymal cells located at the base of the hair follicle (HF), which have a number of important roles in the regulation of hair growth (Topouzi, Logan, Williams, & Higgins, 2017). HF dermal papilla controls hair growth and is characterized in the anagen phase by a highly developed vascular network with enhanced expression of angiogenic growth factors and cytokines (Yano, Brown, & Detmar, 2001). Nutritional deficiency may impact both hair structure and hair growth. Effects on hair growth include acute telogen effluvium (TE), a well-known effect of sudden weight loss or decreased protein intake (Mubki, 2014). Other studies reported a potential association between nutritional deficiency and chronic TE, androgenetic alopecia (AGA), female pattern hair loss, and alopecia areata (Guo & Katta, 2017). Clinical observations, as well as animal studies and in vitro techniques have been developed to investigate and promote hair reconstitution (Ohyama & Veraitch, 2013). Recently, new therapeutical options with topical 5-α reductase inhibitors, prostaglandin modulators, and treatments with stem cells have been proposed to overcome the limitations of oral finasteride and topical minoxidil (MXD), the current gold standard for AGA treatment (Valente Duarte de Sousa & Tosti, 2013).
A number of botanicals or dietary supplements are available that can effectively slow or reduce hair loss and inflammation observed in AGA or stimulate partial hair regrowth (Prager, Bickett, French, & Marcovici, 2006; Rondanelli, Perna, Peroni, & Guido, 2016). Serenoa repens (SR) is a low-growing palm tree that is endemic to all counties of Florida. Permixon, a pharmaceutical extract from fruit SR, is antiandrogenic and has been used to relieve symptoms of benign prostate hyperplasia (Ooi & Pak, 2017). SR is one among the many naturally occurring 5-α-reductase inhibitors which has gained popularity as a remedy for AGA (Murugusundram, 2009). Liposterolic extract of SR (LSESr) and its glycoside, β-sitosterol, were tested in subjects with AGA, which showed a highly positive response to treatment. The blockade of inflammation using a composition containing LSESr as well as two anti-inflammatory agents (carnitine and thioctic acid) alters the expression of molecular markers of inflammation in in vitro system. These findings suggest that 5-α reductase inhibitors combined with blockade of inflammatory processes could represent a novel two-pronged approach in the treatment of AGA with improved efficacy over current modalities (Chittur, Parr, & Marcovici, 2011). Notably, in vitro assays revealed the ability of LSESr to inhibit 5-α-reductase expression 3-fold better than Finasteride (Chittur et al., 2011). Recently, SR has been proposed as a “natural” alternative to conventional treatments for male AGA as well as for other hair disorders (Wessagowit et al., 2016).
In this study we compare the effects of SR to a mixture of N-acetyl-glucosamine (NAG) and milk proteins (β-lactoglobulin +α-lactalbumin; Lac). The precursor for hyaluronic acid, NAG, is a hexosamine sugar that is metabolized by the hexosamine biosynthesis pathway and stimulates O-linked N-acetylglucosamine (O-GlcNAc) modification (O-GlcNAcylation) of cytosolic and nuclear proteins (DeAngelis, Liu, & Linhardt, 2013). Clinical tests of formulations containing NAG have shown that it can also reduce the appearance of facial hyperpigmentation, especially when used beside niacinamide (Sarkar, Arora, & Garg, 2013). Due to the versatile functions of glucosamine and its derivative NAG, they are considered valuable ingredients for accelerating wound healing, improving skin hydration, and decreasing wrinkles and colors (Bissett et al., 2007; Vournakis, Eldridge, Demcheva, & Muise-Helmericks, 2008). Interestingly, poly-N-acetyl glucosamine nanofibers have been recently reported to regulate endothelial cell migration leading to angiogenesis (Vournakis et al., 2008). The hair apparatus contains considerable amounts of complex carbohydrates with different saccharide residues (α-d-mannose, β-d-glucose, α-l-fucose, and NAG). Moreover, the presence of glycogen in outer root sheath cells might enable these cells to provide other hair cells with energy when necessary (Ishii, Tsukise, & Meyer, 2001).
Milk is considered one of the most nutritionally complete foods available, it is mainly composed of water, lipids, lactose, and proteins, and their relative shares widely vary among species (Kuligowski et al., 2017). The two most prominent and abundant proteins of whey protein fraction are β-lactoglobulin and α-lactalbumin (Li et al., 2017). β-Lactoglobulin is the major whey protein in ruminant species; for its amino-acid sequence and three-dimensional structure it belongs to lipocalins, a widely diverse family of proteins, most of which bind small hydrophobic ligands and thus may act as specific transporters, similar to serum retinol binding protein (Brownlow et al., 1997). In addition, β-lactoglobulin from buffalo colostrum inhibits cell proliferation, microvessel sprouting, cell migration, and tube formation of human umbilical vein endothelial cells (Chougule, Shilpa, Salimath, & Aparna, 2013). A recent study reported the ability of Gly-Leu-Phe (GLF), an immune-stimulating peptide derived from α-lactalbumin, to prevent alopecia induced by the anticancer agent etoposide in a neonatal rat model after intraperitoneal injection (Tsuruki & Yoshikawa, 2005).
We previously established transwell-based cocultures to show that FDPC promote viability, proliferation and tubulogenesis of human microvascular endothelial cell (HMVEC) as well as to test the effects of biomimetic peptide Sh-Polypeptide 9-vascular endothelial growth factor (CG-VEGF; Bassino, Gasparri, Giannini, & Munaron, 2015; Bassino, Zanardi, Gasparri, & Munaron, 2016). Here, we took advantage of this approach to evaluate the bioactivity of both SR and NAG/Lac.
2 MATERIALS AND METHODS
2.1 Compounds
S. repens (Euromed S.A., Barcelona, Spain), NAG (Polichimica Srl, Bologna, Italy), milk proteins (β-lactoglobulins+α-lactalbumin: Lac; Davisko Foods International Inc. (Le Sueur, MN); milk proteins: 75% β-lactoglobulins and 22.03% α-lactalbumin, ΝΑG/milk proteins ratio: 1:1) were used at different concentrations (2.5 mg/ml, 25 μg/ml, and 2.5 μg/ml).
2.2 Cell culture
FDPC were obtained from PromoCell (Germany; lot 2040301.35; PromoCell). FDPC were grown in Follicle Dermal Papilla Cell Medium (PromoCell) with 1% antibiotic/antimycotic (Invitrogen, Grand Island, NY). To avoid phenotypical changes occurring during cell culture, only FDPC at 2–7 passages were used for all experiments. HMVEC was purchased from Lonza and grown in EGM 2-MV medium (Lonza, Basel, Switzerland). All cell types were maintained at 37°C in a humidified atmosphere of 5% CO2.
2.3 Cocultures
HMVEC were thawed from frozen stock and seeded in 24 well plates at a density of 2.5 × 103 cells/well, medium was discarded after 18 hr, then complete medium was replaced with Dulbecco’s modified Eagle’s medium (DMEM) 2% fetal calf serum (FCS) to reduce cell proliferation for 24 hr and finally replaced with DMEM 0% + compounds (NAG/Lac or SR, used at different concentrations) alone or in the presence of the insert with FDPC. For cell proliferation in coculture, FDPC were thawed, centrifuged, recounted and seeded at a density of 2.5 × 103 cells/well for 2 days in DMEM +10% FCS until 100% confluence on transwell clear polyester membrane inserts with 0.4-µm pore size and a 0.3-cm2 area (Corning, Corning, NY). The proliferation of HMVEC (alone or in the presence of FDPC on the insert) was analyzed after 24 hr of coculture. As described above for FDPC, HMVEC were seeded on the transwell clear polyester membrane inserts and exposed to the same experimental protocol.
2.4 Cell viability
For proliferation assays in cocultures, HMVEC or FDPC (2.5 × 103 cells/well) were seeded in 24-well plates following the protocols described in the previous chapter. Cell number was evaluated by the CellTiter 96® AQueous Nonradioactive Cell Proliferation Assay (Promega, Madison, WI), using 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt (MTS). MTS conversion into the aqueous soluble formazan product is accomplished by dehydrogenase enzymes found in metabolically active cells. Formazan product was measured with a F5 FilterMax microplate reader (Molecular Devices, Sunnyvale, CA) at 490 nm, as absorbance is directly proportional to the number of viable cells.
2.5 Tubulogenesis
Matrigel (Collaborative Biomedical Products) was used to make up a basement membrane matrix gel solution for cell suspension. Subconfluent HMVEC were trypsinized and 5.0 × 104 cells were added to each well of a chilled 24-well plate and allowed to gel for 30 min at 37°C in a humidified 5% CO2 atmosphere. HMVEC, with or without insert containing FDPC at the different experimental conditions, were maintained in a stable humidified 5% CO2 atmosphere for all the experimental time course (18 hr). Image acquisition and statistical analysis were performed with MetaFluor and ImageJ softwares, respectively. Tubulogenic indexes were obtained with AngioTool software and include total vessel length and total number of junctions.
2.6 Enzyme-linked immunosorbent assay (ELISA) assays
β-Catenin, VEGF, occludin, claudin-5 and interleukin 1β (IL-1β; in cell lysates or medium) were assessed by ELISA using commercially available kits (ELISA Kit; Sigma-Aldrich, St. Louis, MO). Briefly, 100 µl of medium (for VEGF and IL-1β) or cell lysate (for occludin, and nuclear cell lysate for β-catenin; see below “Section 2.7”) were incubated into an antibody-coated 96-well plate at room temperature for 2.5 hr. The wells were washed four times with wash buffer solution. Then 100 µl antihuman β-catenin or IL-1α antibody was added and the samples were again incubated for 1 hr at room temperature. The plate was washed four times, 100 µl of streptavidin–peroxidase conjugated was applied for 1 hr at room temperature. After a final washing, 100 µl tetramethylbenzidine substrate was added and allowed to develop for 30 min in the dark at room temperature. After stopping the reaction with 50 µl stop solution containing citric acid 2.0 mmol/L, absorbance was read at 450 nm using a F5 FilterMax Microplate Reader (Molecular Devices). Sample concentration was calculated from the standard curve.
2.7 Nuclear protein extraction for β-catenin quantification
FDPC were grown to 70–80% confluence. Afterwards, cells were scraped using fresh phosphate-buffered saline (PBS), collected into an appropriate conical tube and centrifuged (5 min at 450g). Then the supernatant was discarded and 1 ml lysis buffer (10 mM Tris–HCl, pH 7.5, 2 mM MgCl2, 3 mM CaCl2, 0.3 M sucrose, including dithiothreitol [DTT] and protease inhibitors) added to 200 µl of packed cell volume (PCV) for 15 min. Suspended cells were centrifuged for 5 min at 420g. Pellet of packed cells was resuspended in 400 µl (2× PCV) of lysis buffer and fragmented using a syringe with a narrow gauge. The disrupted cells in suspension were centrifuged for 20 min at 10,000g. The supernatant was transferred into a fresh tube and this fraction corresponds to the cytoplasmic fraction. Subsequently the pellet was resuspended in 140 µl of extraction buffer (20 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid [HEPES], pH 7.9, with 1.5 mM MgCl2, 0.42 M NaCl, 0.2 mM ethylenediaminetetraacetic acid [EDTA], 25% (vol/vol) glycerol added with 1.5 µl of the 0.1 M DTT solution and 1.5 µl of the protease inhibitor cocktail) and centrifuged for 5 min at 20,000g. The resulting supernatant is the nuclear protein extract. It is finally collected to a clean tube and analyzed with β-catenin ELISA assay.
2.8 Fluorescence microscopy
5-α Reductase II expression was detected using immunofluorescence assay. Briefly, after the treatments cells were fixed in 4% paraformaldehyde for 25 min at room temperature. Then, the fixed cells were washed three times with ice cold PBS solution, incubated 20′ with 0.3% Triton, and 1% bovine serum albumin (BSA; Sigma-Aldrich) in PBS, and stained with the primary antibody 24 hr at 4°C. Cover slides were washed twice with PBS and incubated 1 hr at room temperature with the secondary antibody. After two washes in PBS, cover slides were mounted on standard slides with DABCO (Sigma-Aldrich, Germany) and observed after 24 hr with by fluorescence microscopy (Nikon T-E Microscope, ×10 objective; Nikon). For each experiment, we randomly acquired three fields/sample evaluating the mean of fluorescence intensity/cell/field (about 30 cells/field).
2.9 Lipid peroxidation (LP)
LP was analyzed using Click-iT® LAA reaction kit (Life Techologies). Briefly, linoleic acid is the most abundant polyunsaturated fatty acid found in mammals and its LP products likely account for the majority of lipid-derived protein carbonyls. When incubated with cells, linoleamide alkyne (LAA) incorporates into cellular membranes. Upon LP, LAA is oxidized and produces 9- and 13-hydroperoxy-octadecadienoic acid (HPODE). These hydroperoxides decompose to multiple α,β-unsaturated aldehydes, which readily modify proteins at nucleophilic side chains. These alkyne-containing modified proteins can be subsequently detected using Click-iT® chemistry and multiplexed with other probes appropriate for fixed cells. Briefly, HMVEC were grown and recovered overnight at 37°C alone or with FDPC and treated with H2O2 used alone or with NAG/Lac or SR, respectively. Then we added Click-iT® LAA stock solution to the cells in complete growth medium at a final concentration of 50 μM and we treated the cells with the compound of interest for the desired time. Cells were washed three times with PBS to remove free Click-iT® LAA from the cells, and immediately fixed and permeabilized. Finally we added 0.5 ml of Click-iT® reaction cocktail to each well containing a coverslip. Cells were incubated for 30′ at room temperature, protected from light. Fluorescence (Alexa Fluor® 488, ThermoFisher scientific, Waltham, MA) was acquired using a F5 FilterMax microplate reader (excitation emission maxima: 495/519 nm; Molecular Devices).
2.10 Transendothelial resistance (TEER) measurement
The transendothelial resistance (inverse of permeability) of HMVEC monolayer treated with the different compounds was evaluated with TEER measurements, using an Endohm 12-electrode chamber and an endothelial volt/ohm meter EVOM2 (World Precision Instruments, 175 Sarasota Center Blvd., Sarasota). TEER values were obtained from three independent experiments.
2.11 Statistical analysis
Statistical significance in all experiments was evaluated by KaleidaGraph software (Synergy) using nonparametric Wilcoxon test. The Wilcoxon test was chosen for the survival assays analysis because for each condition in each experiment five biological replicates were done and they were not normally distributed. The Wilcoxon test was also used for cell tubulogenesis analysis because the percentages of tubulogenesis were not normally distributed. All values are presented as the mean ± standard error (SEM). For each experimental condition five technical replicates were performed; three biological replicates were done for each experiment: N = 3. Results with p < 0.05 were considered statistically significant: *p < 0.05; **p < 0.01; ***p < 0.001.
3 RESULTS
3.1 Effects of SR and NAG/Lac on tubulogenesis and VEGF production by HMVEC grown alone or cocultured with FDPC
SR enhanced HMVEC tubulogenic potential measured by endothelial tube formation assay both in terms of total tubule length and number of junctions (Figure 1b–f, see Figure 1a for set-up configurations); FDPC significantly increased both tubulogenic indexes only in presence of the highest dose of SR (Figure 1, see Figure 1a for set-up configurations). All concentrations of NAG/Lac enhanced HMVEC tubulogenic indexes (Figure 1b–f, see Figure 1a for set-up configurations), although the effect was more prominent for the highest dose of NAG/Lac (2.5 mg/ml).
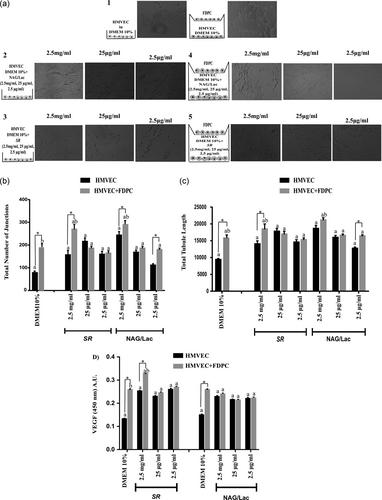
SR and NAG/Lac promote tubulogenesis and VEGF production by HMVEC. (a) Scheme of the experimental setup and relative contrast phase images of tubule formation at 18 hr. Arabic numbers (1–5) are referred to the monoculture and coculture configurations. (1) HMVEC in DMEM 10% or HMVEC in DMEM 10% + FDPC; (2) HMVEC in DMEM 10% + NAG/Lac (2.5 mg/ml, 25 μg/ml, and 2.5 μg/ml); (3) HMVEC in DMEM 10% + SR (2.5 mg/ml, 25 μg/ml, and 2.5 μg/ml); (4) HMVEC in DMEM 10% + NAG/Lac (2.5 mg/ml, 25 μg/ml, and 2.5 μg/ml) and in presence of FDPC; (5) HMVEC in DMEM 10% + SR (2.5 mg/ml, 25 μg/ml, and 2.5 μg/ml) and in presence of FDPC. (b) Total number of junctions were evaluated in HMVEC (grown alone or cocultured with FDPC) treated with SR or NAG/Lac (2.5 mg/ml, 25 μg/ml, and 2.5 μg/ml). (c) Total tubule length was analyzed on HMVEC (grown alone or in coculture with FDPC) treated with SR or NAG/Lac (2.5 mg/ml, 25 μg/ml, and 2.5 μg/ml). (d) VEGF quantification in HMVEC (grown alone or cocultured with FDPC) treated with SR and NAG/Lac (2.5 mg/ml, 25 μg/ml, and 2.5 μg/ml). DMEM: Dulbecco’s modified Eagle’s medium; FDPC: hair follicle dermal papilla cells; HMVEC: human microvascular endothelial cells; SR: Serenoa repens; NAG/Lac: N-acetyl glucosamine + milk proteins; VEGF: vascular endothelial growth factor
The presence of FDPC further increased tubulogenic indexes, the effect was statistically significant only for the lowest dose (Figure 1b–f). Accordingly, SR and NAG/Lac were both able to significantly promote VEGF production from endothelial cells; coculture with FDPC produced a significant additional enhancement only upon SR treatment (Figure 1g).
3.2 SR and NAG/Lac affect proliferation of HMVEC and FDPC grown separately or in coculture
NAG/Lac (25 μg/ml) promoted proliferation of FDPC grown alone, without any additional effect of the coculture with HMVEC (Figure 2b, see Figure 2a for set-up configurations). All concentrations of SR significantly increased FDPC number (Figure 2b). The presence of HMVEC, in coculture configuration, significantly enhanced the response of 25 μg/ml SR (Figure 2b). NAG/Lac significantly increased cell number of HMVEC grown alone, and the coculture with FDPC further enhanced the response in presence of 2.5 μg/ml NAG/Lac (Figure 2c). All concentrations of SR promoted HMVEC proliferation (Figure 2c). An additional gain of the response to SR (2.5 μg/ml) was observed in the presence of FDPC (Figure 2c).
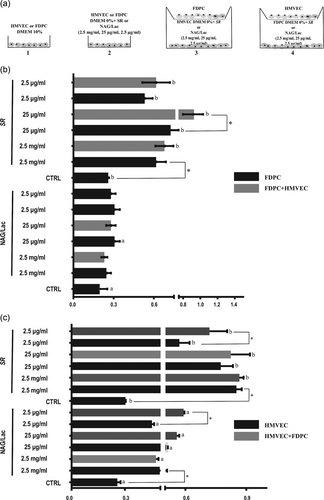
SR and NAG/Lac promotes proliferation. (a) Scheme of the experimental setup. Arabic numbers (1–4) are referred to the monoculture and coculture configurations. (1) FDPC or HMVEC in DMEM 10%; (2) FDPC or HMVEC in DMEM 0% + SR or NAG/Lac (2.5 mg/ml, 25 μg/ml, and 2.5 μg/ml); (3) HMVEC in coculture with FDPC + SR or NAG/Lac (2.5 mg/ml, 25 μg/ml, and 2.5 μg/ml); (4) FDPC in coculture with HMVEC + SR or NAG/Lac (2.5 mg/ml, 25 μg/ml, and 2.5 μg/ml). (b) Cell proliferation (24 hr) of FDPC grown alone or in coculture with HMVEC and in presence of SR or NAG/Lac (2.5 mg/ml, 25 μg/ml, and 2.5 μg/ml). (c) Cell proliferation (24 hr) of HMVEC grown alone or in coculture with FDPC and in presence of SR or NAG/Lac (2.5 mg/ml, 25 μg/ml, and 2.5 μg/ml). CTRL: control; DMEM: Dulbecco’s modified Eagle’s medium; FDPC: hair follicle dermal papilla cells; HMVEC: human microvascular endothelial cells; SR: Serenoa repens; NAG/Lac: N-acetyl glucosamine + milk proteins; VEGF: vascular endothelial growth factor
3.3 Effects of SR and NAG/Lac on the expression of 5-α reductase II and β-catenin by FDPC
The 5-α reductase II enzyme catalyzes the conversion of testosterone to dihydrotestosterone, the most potent male hormone that causes AGA (Jang et al., 2007). Here, we tested the role of both compounds in the modulation of 5-α reductase II expression in testosterone treated cells. As indicated by the graph, in coculture configuration, HMVEC drastically reduced 5-α reductase II expression in FDPC treated with testosterone. SR significantly prevented 5-α reductase II expression triggered by 100 nM testosterone in FDPC (Figure 3a,b (i, ii); 24 hr). NAG/Lac completely failed to counteract the response to testosterone.
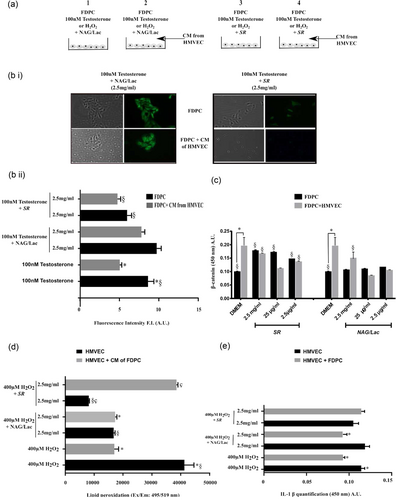
SR and NAG/Lac differently affect 5-α reductase II, β-catenin expression and proinflammatory processes. (a) Scheme of the experimental setup. Arabic numbers (1–4) are referred to the monoculture and coculture configurations. (1) FDPC + testosterone (100 nM) or H2O2 (400 μM) and treated with NAG/Lac (2.5 mg/ml); (2) FDPC were grown in presence of CM of HMVEC + testosterone (100 nM) or H2O2 (400 μM) and treated with the NAG/Lac (2.5 mg/ml); (3) FDPC + testosterone (100 nM) or H2O2 (400 μM) and treated with SR (2.5 mg/ml); (4) FDPC were grown in presence of CM of HMVEC + testosterone (100 nM) or H2O2 (400 μM) and treated with SR (2.5 mg/ml). (b) Representative images of FDPC grown alone or in coculture with HMVEC + 100 nM testosterone and NAG/Lac or SR (2.5 mg/ml). Images of 5-α reductase-II fluorescence intensity were taken with Olympus fluorescence microscope after 24 hr of treatment (i). Statistical analysis of 5-α reductase-II fluorescence intensity (ii). (c) β-Catenin quantification in FDPC grown alone or in coculture with HMVEC + SR or NAG/Lac (2.5 mg/ml, 25 μg/ml, and 2.5 μg/ml). (d) Lipid peroxidation in HMVEC (treated with H2O2) grown alone or in presence of FDPC + SR or NAG/Lac (2.5 mg/ml). (e) IL-1β production in HMVEC (treated with H2O2) grown alone or in presence of CM of FDPC + SR or NAG/Lac (2.5 mg/ml). FDPC: hair follicle dermal papilla cells; HMVEC: human microvascular endothelial cells; SR: Serenoa repens; NAG/Lac: N-acetyl glucosamine + milk proteins; VEGF: vascular endothelial growth factor [Color figure can be viewed at wileyonlinelibrary.com]
Moreover, SR increased the nuclear expression of β-catenin in FDPC with no further variations in coculture with the endothelium. Conversely, NAG/Lac was ineffective on β-catenin expression in FDPC; the presence of HMVEC raised this parameter only in the presence of NAG/Lac (2.5 mg/ml; Figure 3c).
3.4 Effects of SR and NAG/Lac on IL-1β production and LP by HMVEC alone or in coculture with FDPC
We asked whether SR and NAG/Lac could protect endothelium against oxidative stress typically occurring during aging, skin wounding, and inflammation. HMVEC were treated with H2O2 (400 μM, 24 hr) to induce a strong oxidative stress. Four hundred micromolar of H2O2 strongly induced endothelial LP (Figure 3d). The FDPC-derived CM significantly prevented LP (Figure 3d). NAG/Lac and SR drastically reduced oxidative stress, but the presence of FDPC-derived CM abolished SR effect (Figure 3d). NAG/Lac also prevented IL-1β production by HMVEC treated with H2O2 and in presence of FDPC. No changes were observed when endothelial cells were treated with SR (2.5 mg/ml) (Figure 3E).
3.5 SR and NAG/Lac modify endothelial permeability: evaluation of TEER and tight junction protein content
HMVEC were grown at confluence and TEER measurements were performed for 7 days (Figure 4a). As indicated by the graph, the highest concentration (2.5 mg/ml) of SR and NAG/Lac reduced endothelial resistance upon 24 hr of treatment. The TEER values did not recover up to 96 hr of treatment. Accordingly, NAG/Lac decreased the content of occludin, a well-known component of the tight junctions (24 hr), while no variations were induced by SR treatment (Figure 4b). Finally, both compounds reduced the cytosolic levels of claudin-5, another protein included in tight junctions (24 hr), although the effect was statistically significant for the only SR (2.5 mg/ml; Figure 4C).
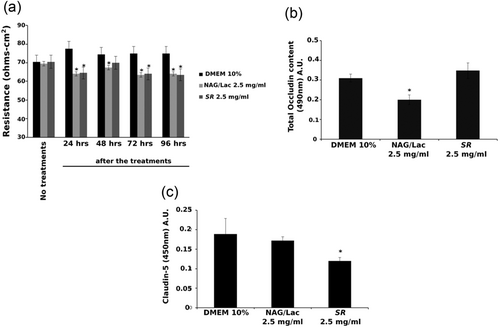
SR and NAG/Lac affect endothelial permeability of HMVEC monolayer and tight junctions content. (a) TEER measurement in HMVEC treated for 7 days with NAG/Lac (2.5 mg/ml) and SR (2.5 mg/ml). (b) Occludin quantification in HMVEC lysates. HMVEC were treated for 24 hr with NAG/Lac (2.5 mg/ml) or SR (2.5 mg/ml). (c) Claudin-5 quantification in HMVEC lysates. The cells were treated for 24 hr with NAG/Lac (2.5 mg/ml) or SR (2.5 mg/ml). DMEM: Dulbecco’s modified Eagle’s medium; HMVEC: human microvascular endothelial cell; SR: Serenoa repens; NAG/Lac: N-acetyl glucosamine + milk proteins; TEER: transendothelial resistance
4 DISCUSSION
We recently reported that human follicle dermal cells strongly support endothelial survival, proliferation, and tubulogenesis in transwell cocultures (Bassino et al., 2015). Here the same approach was used to investigate the activity of nutritional supplements. Recently, has been reported that the diet supplementation with omega-3 and -6 and antioxidants acts efficiently against female hair loss in improving hair density and reducing the telogen percentage and the proportion of miniaturized anagen hair (Le Floc’h et al., 2015). In this study, we tested the role of SR and NAG/Lac compounds in the regulation of the paracrine cross-talk between endothelium and follicle dermal papilla cells. Here, we show that SR and NAG/Lac exert differential activities. In particular, the results reveal that SR is more powerful than NAG/Lac in promoting endothelial production of VEGF and tubulogenesis; notably, the activity of SR is more effective than previously measured for the widely used topical drug MXD (see Bassino et al., 2016). SR enhances β-catenin expression by FDPC, protects vascular endothelium from oxidative stress and prevents 5-α reductase II expression induced by testosterone. Recently, the topical application of SR extract was suggested to be an alternative treatment in male patients who cannot tolerate the side-effects of standard medications (Wessagowit et al., 2016). Specifically, the lipidosterolic extract of SR, LSESr, highly enriched with fatty acids and phytosterols, was reported to block both isoforms of 5-α reductase (types I and II) in contrast to finasteride (Chittur et al., 2011). Moreover, LSESr promotes hair regeneration and repair in mouse models by activating TGF-β signaling and mitochondrial pathway (Zhu, Gao, Yang, Li, & Gao, 2018). Finally, we observed that both compounds affect endothelial permeability ad alter the expression of occludin and claudin-5, two critical components of the tight junction complexes. These results unveil a novel functional role for SR and NAG/Lac that could efficiently lower vascular endothelium resistance. In conclusion, our in vitro study points to SR an NAG/Lac as effective compounds; nonetheless, further mechanistic studies, strengthened by in vivo and clinical trials, are required to shed more light on their applicative potentiality.
CONFLICTS OF INTEREST
The author declare that there are no conflicts of interest.
AUTHOR CONTRIBUTIONS
E. B. designed the research study, performed the research, analyzed the data and wrote the paper. F. G. designed the research study and contributed essential reagents. L. M. designed the research study, wrote the paper and contributed essential reagents.




