DGCR5 attenuates neuropathic pain through sponging miR-330-3p and regulating PDCD4 in CCI rat models
Abstract
Neuropathic pain caused by somatosensory nervous system dysfunction is a serious public health problem. Some long noncoding RNAs (lncRNAs) can participate in physiological processes involved in neuropathic pain. However, the effects of lncRNA DGCR5 in neuropathic pain have not been explored. Therefore, in our current study, we concentrated on the biological roles of DGCR5 in neuropathic pain. Here, it was observed that DGCR5 was significantly decreased in chronic sciatic nerve injury (CCI) rat models. DGCR5 overexpression was able to alleviate neuropathic pain development including mechanical and thermal hyperalgesia. In addition, the current understanding of miR-330-3p function in neuropathic pain remains largely incomplete. Here, we found that miR-330-3p was greatly increased in CCI rats and DGCR5 can modulate miR-330-3p expression negatively. Upregulation of DGCR5 repressed inflammation-correlated biomarkers including interleukin 6 (IL-6), tumor necrosis factor α, and IL-1β in CCI rats by sponging miR-330-3p. The negative correlation between DGCR5 and miR-330-3p was confirmed in our current study. Inhibition of miR-330-3p suppressed neuropathic pain progression by restraining neuroinflammation in vivo. In addition, PDCD4 was predicted as a downstream target of miR-330-3p. Furthermore, PDCD4 was significantly increased in CCI rats and DGCR5 regulated PDCD4 expression through sponging miR-330-3p in CCI rat models. Taken these together, it was implied that DGCR5/miR-330-3p/PDCD4 axis participated in neuropathic pain treatment.
1 INTRODUCTION
Neuropathic pain due to nerve lesions or dysfunction is one of the most challenging neurological diseases. The prevalence of neuropathic pain in the gross population is about 7–10% (Bouhassira, Lantéri-Minet, Attal, Laurent, & Touboul, 2008; de Moraes Vieira, Garcia, da Silva, Mualem araújo, & Jansen, 2012). Therefore, it is crucial to focus on the underlying mechanisms of neuropathic pain.
Long noncoding RNA (lncRNA) belongs to a family of RNAs characterized with transcripts with more than 200 nucleotides in molecular length (Batista & Chang, 2013). Many lncRNAs are involved in neuropathic pain. NONRATT021972 is correlated with neuropathic pain scoring in type 2 diabetes patients (Yu, Zhao, Cao, Zhu, & Li, 2017). X-inactive specific transcript (XIST) is able to accelerate neuropathic pain progression through regulating miR-150 and ZEB1 in chronic sciatic nerve injury (CCI) rat models (T. Yan, Zhang, et al., 2018; X. T. Yan, Lu, et al., 2018). LncRNA MRAK009713 is able to regulate neuropathic pain in rats (Li et al., 2017). LncRNA DGCR5 is decreased in Huntington's disease (Johnson, 2012). However, the function of DGCR5 in neuropathic pain remains uninvestigated.
MicroRNAs are small noncoding RNAs, which can function through targeting the 3′-untranslated regions (3′-UTRs) of target genes (Lai, 2002). Emerging studies have reported that miRNAs are responsible for many biological processes (Pandey et al., 2015; Xu et al., 2016). Recently, miRNAs exert a critical role in neuroinflammation and neuropathic pain. miR-32-5p contributes to neuropathic pain by targeting Dusp5 (T. Yan, Zhang, et al., 2018; X. T. Yan, Lu, et al., 2018). MiR-142-3p can relieve neuropathic pain by targeting HMGB1 (Y. Zhang et al., 2018). MiR-200b/miR-429 inhibition leads to neuropathic pain by targeting ZEB1 (H. Yan, Rao et al., 2017; X. T. Yan, Zhao et al., 2017).
Currently, we observed that DGCR5 was dramatically decreased while miR-330-3p was increased in CCI rat models. Overexpression of DGCR5 inhibited neuropathic pain progression by targeting miR-330-3p. PDCD4 was predicted as a downstream target of miR-330-3p. Hence, it was hypothesized that DGCR5 played an inhibitory role in neuropathic pain progression by targeting miR-330-3p/PDCD4.
2 MATERIALS AND METHODS
2.1 Animal studies
Sprague–Dawley rats (adult, female, weighed 190–210 g) were obtained from Shanghai Animal Laboratory Center (Shanghai, China). They were maintained in cages with an indoor-constant temperature of 25°C. Rats were administered at 9:00 every morning, which lasted for 10 days. CCI animal model was established as according to the previously reported method (Bennett & Xie, 1988). Based on the previous method (Richner, Jager, Siupka, & Vaegter, 2017), the dorsal spinal cords of L4–L6 were isolated and kept in liquid nitrogen tank (−80°C) for further research. The experimental procedures were strictly carried out under the requirements in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health.
2.2 Cell culture
Rat microglial cells and HEK-293T cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA). Cells were grown in Dulbecco's modified Eagle medium (Lonza Inc., Walkersville, MD) added with 10% heat-inactivated fetal bovine serum (Invitrogen, Carlsbad, CA) and 1% penicillin/streptomycin.
2.3 Intrathecal injection
PE-10 polyethylene catheter was inserted. Paralysis of bilateral hind limbs was carried out using an injection of lidocaine and after that, the intrathecal implantation was used. Then the catheter was fixed and the incision was sealed. For gene delivery, LV-DGCR5 (RiboBio, Guangzhou, China) and anti-miR-330-3p (RiboBio) or their corresponding scrambled controls at a multiplicity of infection of 20 were injected through a microinjection syringe linked with the intrathecal catheter 3 days before CCI surgery daily. MiR-330-3p mimics, inhibitors or their negative controls (NCs; RiboBio, Guangzhou, China) were transfected into the rat microglial cells.
2.4 Quantitative real-time polymerase chain reaction (PCR)
Total RNA were extracted by using the RNAiso Plus (Takara Biotechnology, Dalian, China). RNA reverse-transcribed by the Prime Script™ RT Master Mix and quantitative PCR was carried out by SYBR Premix Ex Taq II (Takara Biotechnology). The primers used in this study were manifested in Table 1. The 7500 Real-Time PCR System (Applied Biosystems, Foster City, CA) was used.
| Genes | Forward (5′~3′) | Reverse (5′~3′) |
|---|---|---|
| GAPDH | CAAGGTCATCCATGACAACTTTG | GTCCACCACCCTGTTGCTGTAG |
| U6 | CTCGCTTCGGCAGCACA | AACGCTTCACGAATTTGCGT |
| DGCR5 | CACGAGTGTAGTGCCCAGTT | GGTCAGGGACCTTTGTCGTT |
| PDCD4 | TATGATGTGGAGGAGGTGGATGTGA | CCTTTCATCCAAAGGAAAAACTACAC |
2.5 Pain threshold assessment
Paw withdrawal threshold (PWT) was used to evaluate mechanical allodynia. The plantar surface of the left hind paw was exposed to pressure created by the calibrated Electronic Von Frey Filament (Electronic Von Frey 2393; IITC, Woodland Hills, CA). The time of paw withdrawal responding to the pressure was observed. Paw withdrawal latency (PWL) was used to assess thermal hyperalgesia responding to radiant heat. The duration between stimuli starting and paw withdrawal was recorded.
2.6 Enzyme-linked immunosorbent assay (ELISA)
The protein expression of tumor necrosis factor α (TNF-α), interleukin 6 (IL-6), and IL-1β was tested using ELISA kits (R&D Systems, Minneapolis, MN).
2.7 Luciferase activity assay
The wild type (WT) or mutant (MUT) DGCR5-binding miR-330-3p was subcloned into a pGL3 Basic Vector (Promega, Madison, WI). MiR-330-3p mimics (RiboBio) were cotransfected with 10 μg pLUC-WT-DGCR5 or pLUC-MUT-DGCR5 into HEK-293T cells. The WT or MUT-PDCD4 binding miR-330-3p was subcloned into the pGL3 Basic Vector in a similar way.
2.8 RNA immunoprecipitation (RIP) assay
RIP assay was carried out using Magna RIP Kit (EMD Millipore, Billerica, MA). Cells were lysed with RIP lysis buffer and magnetic beads conjugated to human anti-Ago2 antibody (Millipore) or isotype-matched control antibody (normal mouse IgG; Millipore) was used to incubate the cell lysis.
2.9 RNA pull-down assay
RNAs were labeled using biotin using Pierce RNA 3′ End Desthiobiotinylation Kit (Thermo Fisher Scientific, Waltham, MA). Cell lysates were indicated with the positive control (biotin-labeled WT miR-330-3p and miR-330-3p-Bio), NC (mutant miR-330-3p and miR-330-3p-Bio-mut), and biotinylated RNAs (NC-Bio).
2.10 Western blot analysis
Proteins were separated on 10% sodium dodecyl sulfate gels. Polyvinylidene difluoride membrane was used to transfer the proteins on. After incubated in 5% defatted milk for 1 hr, primary antibodies were used to incubate the membrane. Next day, after membranes were washed by TBST, secondary antibodies conjugated with horseradish peroxidase were used. The primary antibodies: anti-PDCD4 and anti-GAPDH (Abcam, Cambridge, UK) were used.
2.11 Statistical analysis
All data were indicated as the mean ± standard deviation. Comparisons between quantitative variables were conducted using Student's t test and one-way analysis of variance. Differences were significant when p < 0.05. The SPSS 19.0 (SPSS Inc., Chicago, IL) was used in our study.
3 RESULTS
3.1 DGCR5 was decreased and miR-330-3p was increased in CCI rats
To investigate the role of DGCR5 and miR-330-3p in neuropathic pain development, DGCR5 and miR-330-3p in the spinal cords were measured. DGCR5 was significantly downregulated in CCI rats (Figure 1a). In addition, miR-330-3p was greatly overexpressed in CCI rats (Figure 1b). These manifested that DGCR5 and miR-330-3p played crucial roles in neuropathic pain progression in vivo.
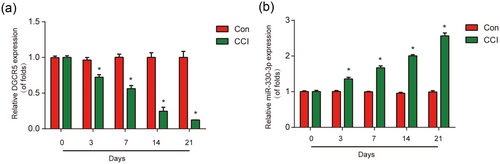
Expression of DGCR5 and miR-330-3p. (a) DGCR5 expression in the L4–L6 dorsal spinal cord of rats was detected by quantitative real-time polymerase chain reaction at postoperative Days 0, 3, 7, 14, and 21. (b) Expression of miR-330-3p in the L4–L6 dorsal spinal cord of rats. n = 8 for each group. Three independent experiments were carried out. Error bars stand for the mean ± SD of at least triplicate experiments. *p < 0.05. CCI: chronic sciatic nerve injury [Color figure can be viewed at wileyonlinelibrary.com]
3.2 DGCR5 inhibited neuropathic pain behaviors in CCI rats
To explore whether DGCR5 can affect neuropathic pain development in vivo, DGCR5 was overexpressed in CCI rats. As exhibited in Figure 2a,b, both mechanical allodynia and thermal hyperalgesia were strongly restrained by DGCR5 overexpression. These indicated that DGCR5 demonstrated a suppressive role in neuropathic pain progression in CCI rats.
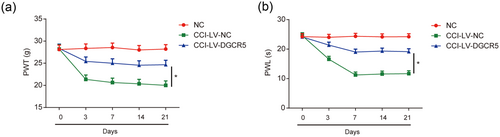
Overexpression of DGCR5 inhibited neuropathic pain development. (a) Effect of DGCR5 on mechanical allodynia was assessed by PWT. (b) Effect of DGCR5 on thermal hyperalgesia was evaluated by PWL. n = 8 for each group. Three independent experiments were carried out. Error bars stand for the mean ± SD of at least triplicate experiments. *p < 0.05. CCI: chronic sciatic nerve injury; NC: negative control [Color figure can be viewed at wileyonlinelibrary.com]
3.3 Overexpression of DGCR5 repressed neuroinflammation in vivo by targeting miR-330-3p
Next, to explore whether DGCR5 can influence miR-330-3p, DGCR5 was upregulated in CCI rats. In Figure 3a, it was shown that DGCR5 was greatly increased in CCI rats by LV-DGCR5. Besides these, we can observe that miR-330-3p was decreased by LV-DGCR5 in Figure 3b. Since neuroinflammation has been reported to participate in neuropathic pain, the expression of TNF-α, IL-6, and IL-1β were tested. ELISA results exhibited that DGCR5 overexpression significantly decreased the expression of TNF-α, IL-6, and IL-1β in CCI rats (Figure 3c–e). These indicated that DGCR5 restrained neuroinflammation in vivo through regulating miR-330-3p.
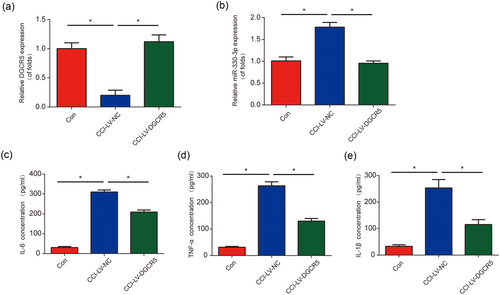
Increase of DGCR5 suppressed neuroinflammation in chronic sciatic nerve injury (CCI) rat models. (a) DGCR5 expression in the L4–L6 dorsal spinal cord of rats was detected by quantitative real-time polymerase chain reaction at postoperative Day 7. Rats were infected with LV-DGCR5. (b) Expression of miR-330-3p in the L4–L6 dorsal spinal cord of rats. Protein levels of interleukin 6 (IL-6; c), tumor necrosis factor α (TNF-α; d) and IL-1β (e) in the L4–L6 dorsal spinal cord of rats were detected by enzyme-linked immunosorbent assay at postoperative Day 7. n = 8 for each group. Three independent experiments were carried out. Error bars stand for the mean ± SD of at least triplicate experiments. *p < 0.05 [Color figure can be viewed at wileyonlinelibrary.com]
3.4 DGCR5 served as a sponge of miR-330-3p
The association between lncRNAs and microRNAs has drawn increasing attention in neuropathic pain. In our current study, ChipBase, LncRNAdb, and StarBase were used to predict the correlation between DGCR5 and miR-330-3p (Figure 4a). Dual-luciferase reporter assay was conducted to validate that miR-330-3p was a target of DGCR5 (Figure 4b). The luciferase reporter plasmid containing the wild type of DGCR5 with miR-330-3p mimics were cotransfected into HEK-293T cells and significantly decreased reporter activity was exhibited (Figure 4c). Next, to assess whether DGCR5 can sponge miR-330-3p directly, RIP assay was carried out. DGCR5 and miR-330-3p were much more enriched in Ago2 pellet compared with IgG pellet (Figure 4d). Additionally, RNA pull-down assay using biotinylated miR-330-3p (miR-330-3p-Bio) probe increased the level of DGCR5 than control (NC-Bio) or miR-330-3p probes (Figure 4e) in rat microglial cells. These results implied that DGCR5 can serve as a sponge of miR-330-3p in neuropathic pain.
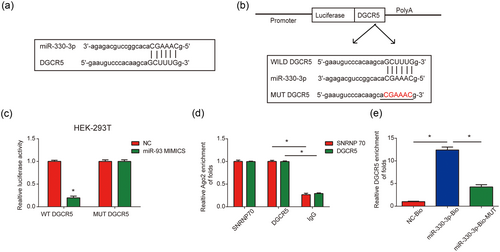
DGCR5 sponged miR-330-3p. (a) Binding region between miR-330-3p and DGCR5. (b) The luciferase reporter constructs with the wild type (WT-DGCR5) or mutant DGCR5 (MUT-DGCR5). (c) WT-DGCR5 or MUT-DGCR5 was cotransfected into HEK-293T cells with miR-330-3p mimics or their corresponding negative controls. (d) The correlation between DGCR5 and Ago2 was evaluated by RIP assay. Cellular lysates were immunoprecipitated using Ago2 antibody or IgG. SNRNP70 expression served as a positive control. (e) RNA pull-down assay indicated the direct interaction between miR-330-3p and DGCR5. Cellular lysates were pulled down using biotinylated control (NC-Bio), miR-330-3p (miR-330-3p-Bio), or miR-330-3p probe containing mutations in the DGCR5-binding site (miR-330-3p-Bio-mut). Three independent experiments were carried out. Error bars stand for the mean ± SD of at least triplicate experiments. *p < 0.05 [Color figure can be viewed at wileyonlinelibrary.com]
3.5 Silence of miR-330-3p inhibited neuropathic pain development in vivo
Next, to investigate whether miR-330-3p can influence neuropathic pain development, miR-330-3p was downregulated in CCI rats. In Figure 5a, it was indicated that miR-330-3p was greatly decreased in CCI rats by anti-miR-330-3p. Meanwhile, DGCR5 was upregulated by anti-miR-330-3p in CCI rats (Figure 5b). Both mechanical allodynia and thermal hyperalgesia were repressed by miR-330-3p inhibition (Figure 5c,d). In addition, IL-6, TNF-α, and IL-1β were significantly inhibited by downregulation of miR-330-3p (Figure 5e–g). These data indicated that the silence of miR-330-3p repressed neuropathic pain development in vivo.
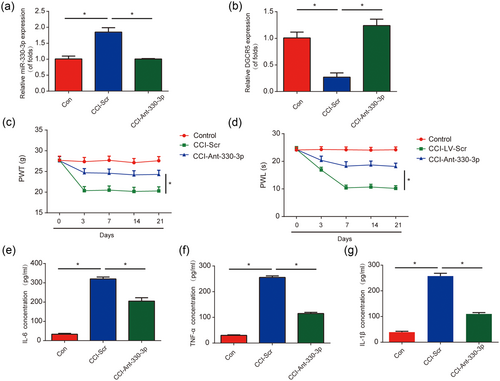
Inhibition of miR-330-3p inhibited neuropathic pain development in vivo. (a) MiR-330-3p expression in chronic sciatic nerve injury (CCI) rat models. Rats were infected with anti-miR-330-3p. (b) DGCR5 expression in CCI rat models. (c) Effect of anti-miR-330-3p on mechanical allodynia was assessed by PWT. (d) Effect of anti-miR-330-3p on thermal hyperalgesia was evaluated by PWL. Protein levels of interleukin 6 (IL-6; e), tumor necrosis factor α (TNF-α; f) and IL-1β (g) in the L4–L6 dorsal spinal cord of rats were detected by enzyme-linked immunosorbent assay at postoperative Day 7. n = 8 for each group. Three independent experiments were carried out. Error bars stand for the mean ± SD of at least triplicate experiments. *p < 0.05 [Color figure can be viewed at wileyonlinelibrary.com]
3.6 PDCD4 was a direct target of miR-330-3p
To find out the direct target gene of miR-330-3p, bioinformatic analysis including TargetScan, Starbase, miRanda, and miRDB database was performed. Interestingly, it was observed that PDCD4 3′-UTR had a conserved binding region of miR-330-3p (Figure 6a). The 3′-UTR of PDCD4 harboring the WT-PDCD4 or MUT-PDCD4 was designed (Figure 6b). Cotransfection of miR-330-3p mimics with the luciferase vector containing WT-PDCD4 repressed the luciferase activity in HEK-293T cells (Figure 6c). In addition, PDCD4 expression was significantly downregulated by miR-330-3p mimics while upregulated by miR-330-3p inhibitors (Figure 6d,e). These implied that miR-330-3p directly targeted PDCD4.
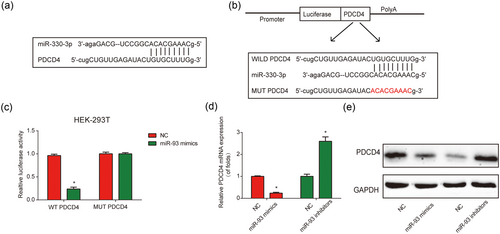
PDCD4 was a target of miR-330-3p in vitro. (a) Binding region between miR-330-3p and PDCD4. TargetScan, Starbase, miRanda, and miRDB database were used to predict putative microRNA targets of PDCD4. (b) The luciferase reporter constructs with the wild type (WT-PDCD4) or mutant PDCD4 (MUT-PDCD4). (c) Dual-luciferase reporter assay of miR-330-3p and PDCD4 3′-untranslated region. MiR-330-3p mimics were cotransfected with the dual-luciferase vector containing WT-PDCD4 or MUT-PDCD4 into HEK-293T cells. Dual-luciferase assay system was used to measure the relative luciferase activity. (d) Effect of miR-330-3p on mRNA expression of PDCD4. Real-time polymerase chain reaction was performed to measure the PDCD4 mRNA levels. (e) Effect of miR-330-3p on PDCD4 protein levels. Western blot assay was performed to investigate PDCD4 protein using glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as a protein loading control. Three independent experiments were carried out. Error bars stand for the mean ± SD of at least triplicate experiments. *p < 0.05. NC: negative control [Color figure can be viewed at wileyonlinelibrary.com]
3.7 PDCD4 was modulated by DGCR5 in CCI rats
Furthermore, PDCD4 expression in CCI rats was increased significantly in a time-dependent course (Figure 7a,b). In addition, it was exhibited that PDCD4 expression was greatly inhibited by LV-DGCR5 in CCI rats infected with LV-DGCR5 (Figure 7c,d). These results suggested that PDCD4 was elevated in CCI rats and it can be regulated by DGCR5.
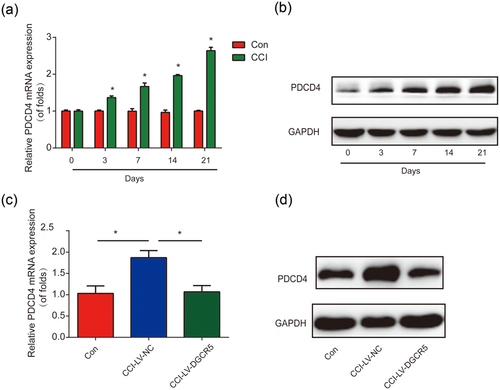
PDCD4 was modulated by DGCR5 in vivo. (a) Messenger RNA (mRNA) levels of PDCD4 in rat chronic sciatic nerve injury (CCI) models. Quantitative real-time polymerase chain reaction (qRT-PCR) was performed. (b) Protein expression of PDCD4 in CCI rat models. (c) mRNA levels of PDCD4 in rat CCI models. CCI rats were infected with LV-DGCR5 or LV-NC. qRT-PCR was performed at postoperative Day 7. (d) Protein expression of PDCD4 in CCI rat models. n = 8 for each group. Three independent experiments were carried out. Error bars stand for the mean ± SD of at least triplicate experiments. *p < 0.05. GAPDH: glyceraldehyde 3-phosphate dehydrogenase; NC: negative control [Color figure can be viewed at wileyonlinelibrary.com]
4 DISCUSSION
Recently, increasing roles of noncoding RNAs have been revealed in neuropathic pain development. Here, it was observed that DGCR5/miR-330-3p/PDCD4 axis was involved in neuropathic pain of CCI rats. DGCR5 was downregulated in CCI rat models and its overexpression can inhibit neuropathic pain progression through targeting miR-330-3p. In addition, miR-330-3p was upregulated and knockdown of miR-330-3p restrained neuroinflammation. Moreover, PDCD4 was predicted as a downstream target of miR-330-3p and miR-330-3p regulated Zeb1 PDCD4 expression negatively. Subsequently, DGCR5 can serve as a sponge of miR-330-3p and modulate PDCD4 levels in neuropathic pain.
DGCR5 is downregulated in Huntington's disease (Johnson, 2012). Increasing papers indicate that DGCR5 is closely related with several cancers. For example, downregulation of DGCR5 suggests poor prognosis of hepatocellular carcinoma (Huang et al., 2016). DGCR5 is reduced in pancreatic ductal adenocarcinoma tissues and cell lines and its downregulation can predict poor prognosis (Yong et al., 2017). In addition, DGCR5 can promote LUAD progression by inhibiting hsa-mir-22-3p (Dong, Wang, Jin, Zeng, & Pan, 2017). DGCR5 is involved in lung cancer progression by sponging miR-1180 (Chen, Zhang, Xu, Zhu, & Hu, 2017). Here, in our study, we focused on the biological role of DGCR5 in neuropathic pain progression. It was indicated that DGCR5 was strongly decreased in CCI rats and overexpression of DGCR5 significantly inhibited neuropathic pain behaviors. In addition, neuroinflammation was also repressed by DGCR5 upregulation since we observed that DGCR5 restrained NF-α, IL-6, and IL-1β protein levels obviously.
MiR-330-3p exerts oncogenic roles in various cancers. For instance it is greatly upregulated in prostate cancer patients and primary plasma cell leukemia (Lionetti et al., 2013; Medina-Villaamil et al., 2014). In addition, miR-330-3p is increased in non–small-cell lung cancer (NSCLC) patient tissues (Arora et al., 2011). MiR-330-3p promotes NSCLC cell invasion and metastasis by activating MAPK/ERK (Wei et al., 2017). MiR-330-3p is elevated in plasma samples from patients with gestational diabetes mellitus (Sebastiani et al., 2017). MiR-330-3p was predicted as a target of DGCR5 by using bioinformatics analysis. MiR-330-3p was increased in CCI rats in a time-dependent manner. DGCR5 can modulate miR-330-3p expression negatively and we confirmed the reverse interaction between them. It was indicated in our study that DGCR5 can inhibit neuropathic pain development through sponging miR-330-3p in vivo.
PDCD4 is a translation factor binding to eIF4A (Goke et al., 2002; Yang et al., 2003). It has been reported that PDCD4 deficiency can aggravate colitis and colorectal cancer through IL-6/STAT3 (Wang et al., 2016). Lipopolysaccharide can promote IL-10 production by inducing the degradation of PDCD4 (van den Bosch, Palsson-Mcdermott, Johnson, & O'Neill, 2014). PDCD4 stabilization can provide a target for tumors (Schmid et al., 2011). In addition, XIST can regulate PDCD4 by interacting with miR-21-5p in osteosarcoma (R. Zhang & Xia, 2017). MiR-155 can regulate inflammation response through SOCS1–STAT3–PDCD4 in atherogenesis (Ye et al., 2016). LncRNA MEG3 can regulate ischemic neuronal death via targeting miR-21/PDCD4 (H. Yan, Rao et al., 2017; X. T. Yan, Zhao et al., 2017). MiR-499 can protect endothelial cells against inflammatory damage by inhibiting NF-κB/TNF-α by targeting PDCD4 in coronary artery disease (Y. H. Zhang, He, & Shi, 2017). MiR-21/PDCD4 axis can act as a promising strategy for some neurological diseases (Su et al., 2017). PDCD4 was predicted as a downstream target of miR-330-3p and we found that PDCD4 was decreased in CCI rats. MiR-330-3p can regulate PDCD4 levels negatively. In addition, DGCR5 can modulate PDCD4 expression by sponging miR-330-3p in vivo.
In conclusion, a significant role of DGCR5/miR-330-3p/PDCD4 axis was exhibited in neuropathic pain progression. It was indicated that DGCR5 was able to alleviate neuropathic pain development in CCI rats via sponging miR-330-3p and regulating Zeb1. Apart from these, we observed that DGCR5 can inhibit neuroinflammation by reducing TNF-α, IL-6, and IL-1β by targeting miR-330-3p. MiR-330-3p was validated as a target of DGCR5 and PDCD4 was predicted as a downstream target of miR-330-3p, respectively. Overexpression of DGCR5 elevated PDCD4 levels via sponging miR-330-3p in vivo.
CONFLICTS OF INTEREST
The authors declare that there are no conflicts of interest.




