Immunological biomarkers of tolerance in human kidney transplantation: An updated literature review
Abstract
The half-life of transplanted kidneys is <10 years. Acute or chronic rejections have a negative impact on transplant outcome. Therefore, achieving to allograft tolerance for improving long-term transplant outcome is a desirable goal of transplantation field. In contrast, there are evidence that distinct immunological characteristics lead to tolerance in some transplant recipients. In contrast, the main reason for allograft loss is immunological responses. Various immune cells including T cells, B cells, dendritic cells, macrophages, natural killer, and myeloid-derived suppressor cells damage graft tissue and, thereby, graft loss happens. Therefore, being armed with the comprehensive knowledge about either preimmunological or postimmunological characteristics of renal transplant patients may help us to achieve an operational tolerance. In the present study, we are going to review and discuss immunological characteristics of renal transplant recipients with rejection and compare them with tolerant subjects.
1 INTRODUCTION
Kidney transplantation is the treatment of choice for patients suffering from end-stage renal disease, leading to improved quality of life and also reduced costs (Cherukuri et al., 2017; Le Texier et al., 2011; Shiu et al., 2015). Kidney graft is immediately damaged by the immune response during graft rejection, which is considered as the major cause of graft loss (J. Lu & Zhang, 2016; N. Lu et al., 2011). Therefore, patients need to receive immunosuppressive drug regimens (J. Lu & Zhang, 2016). Despite the introduction of modern immunosuppressive drug regimens, which result in a remarkable control of acute rejection, chronic rejection remains a major problem (Stolp, Turka, & Wood, 2014). In addition, lifelong use of immunosuppression due to their severe side effects is associated with high risk of malignancies, opportunistic infections, cardiovascular diseases, renal failure, and metabolic disorders like diabetes (Braza, Soulillou, & Brouard, 2012; Sagoo et al., 2010; Shiu et al., 2015; Wekerle, Segev, Lechler, & Oberbauer, 2017). Moreover, immunosuppressive drugs not only dont prevent chronic rejection (Elias, Cosimi, & Kawai, 2015) but also hardly affect chronic rejection, an important cause of long-term graft loss (Dalloul, 2013; Pallier et al., 2010).
Thus, there is a need to achieve great strategies for improving the long-term survival of allograft (Wekerle et al., 2017). This strategy could be considered as induction of operational tolerance, which is the main goal of transplant medicine (Behnam Sani & Sawitzki, 2017; Braza et al., 2012; Pallier et al., 2010; Wekerle et al., 2017), where the allograft remains functional for at least 1-year after stopping the immunosuppression (Chesneau et al., 2015, 2014; Newell et al., 2010; Vanikar, Trivedi, & Thakkar, 2017). Interestingly, allograft/operational tolerance has been reported notably (20%) in human liver and rarely (7%) in kidney transplantation upon distinct immunological features of these patients (Behnam Sani & Sawitzki, 2017; Louis et al., 2006; Lozano et al., 2011; Sagoo et al., 2010), suggesting that allograft tolerance can be achieved in humans (Braza et al., 2012).
The half-life of transplanted kidneys is still ~9 years (Dalloul, 2013; Wekerle et al., 2017) and 30–40% of patients lose their transplant in <10 years (Shiu et al., 2015). Transplanted kidneys lose their function due to both immunologic (Girmanova, Hruba, & Viklicky, 2015) and metabolic mechanisms (Dalloul, 2013; Wekerle et al., 2017) but the most predominant cause of graft loss is alloimmune response (Hernandez-Fuentes & Lechler, 2010; J. Lu & Zhang, 2016; Wekerle et al., 2017). Various immune cells contribute to graft rejection and failure (Ordonez et al., 2013) with the major role of T-cell- and antibody-mediated rejection usually in acute and chronic rejection (ABMR), respectively (Ordonez et al., 2013; Wekerle et al., 2017). Fibrosis plays a central role in the late graft loss upon alloimmune responses. It has been shown that no fibrosis is found in the allograft-tolerant patients (Wekerle et al., 2017), suggesting a key role of immunological tolerance in the survival of graft in the absence of immunosuppression therapy. The aim of the current study is to review immunological characteristics such as biomarker in the patients with operational tolerance and functionally long-term survival of kidney graft and compare them with patients with rejection or graft loss.
2 ADAPTIVE IMMUNITY IN KIDNEY TRANSPLANTATION OUTCOME
2.1 B cell in kidney transplant outcome
B cells can both induce and suppress the immune response (Clatworthy, 2014; Vitale, Mion, & Pucillo, 2010). In contrast, B cells play a central role in graft rejection by producing high levels of alloantibodies and stimulating CD4+ T cells to produce cytokines (Durand & Chiffoleau, 2015; Wortel & Heidt, 2017). The alloantibodies lead to the graft loss by means of two main mechanisms. The first is the complement proteolytic cascade activation, and the second, the antibody-dependent cellular cytotoxicity (Durand & Chiffoleau, 2015). Moreover, evidence suggested that B cells not only contributed to acute and chronic antibody-mediated rejection (Bigot et al., 2016), which caused graft loss in the clinic (Carpio et al., 2014; Chong & Khiew, 2017) but might also lead to acute cellular rejection (Clatworthy, 2014). Thus, B-cell-depleting monoclonal antibody, rituximab, had been introduced (Carreras-Planella, Borràs, & Franquesa, 2016). In contrast, regulatory B (Breg) cells through cytokine production like interleukin (IL)-10, IL-35, granzyme B (GzmB), transforming growth factor (TGF)-β, and production of immunoglobulin G4 (IgG4), act as suppressive cells and inhibit the proliferation of CD4+T cells and their differentiation into Th1 and Th17 phenotypes (Mauri & Menon, 2015; Rincón-Arévalo, Sanchez-Parra, Castaño, Yassin, & Vásquez, 2016; Wortel & Heidt, 2017; Figure 1). As a result, rituximab may also deplete these regulatory cells and inhibit their suppressive function (Carreras-Planella et al., 2016; Durand & Chiffoleau, 2015). Moreover, a clinical trial has demonstrated a high risk for acute cellular rejection upon decrease of B cells using rituximab before transplantation (Clatworthy et al., 2009). In addition, several studies have demonstrated a tolerogenic role for B cells and associated transcripts that will be discussed below.
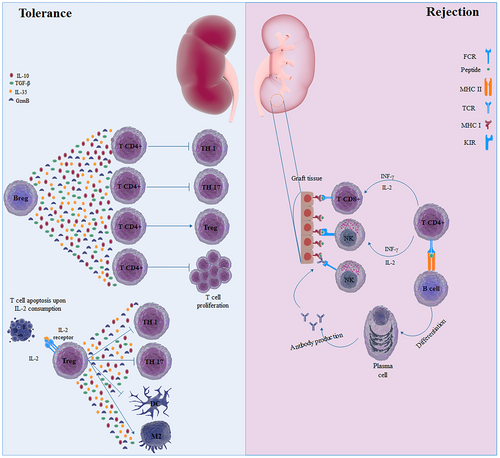
Immune system platform during allograft rejection and successful transplantation. In patients with tolerance to the allograft, several immune cells are involved in suppressing adverse immune response. Regulatory B (Breg) cells produce immunosuppressive mediators, such as transforming growth factor (TGF)-β and interleukin (IL)-10, which inhibit differentiation of CD4+ T cells with inflammatory phenotype and proliferation of T cells. Moreover, these mediators modulate the development of regulatory T (Treg) cells, which are the main suppressor cells of the immune system. These cells through several mechanisms conferred suppression on the immune system, including immunosuppressive cytokines, development of M2-macrophages, and consumption of IL-2. In contrast, CD8+ T cells and natural killer (NK) cells attack the allograft through T cell receptor (TCR) and by activating killer cell immunoglobulin-like receptor (KIR), respectively. CD4+ T cells, by producing cytokines like interferon (IFN)-γ and IL-2, may further activate CD8+ T cells and NK cells. CD4+ T cells, also activate B cells to be developed into plasma cells, which produce antibodies against alloantigen. NK cells, through FCγIII, recognize the fragment crystallizable (FC) of antibodies bound to alloantigen, leading to a liking of transplant by a phenomenon called antibody-dependent cellular cytotoxicity (ADCC) [Color figure can be viewed at wileyonlinelibrary.com]
Newell et al. (2010) have shown that B-cell gene signatures distinguished operationally tolerant (TOL) phenotype from recipients with stable graft phenotype in cases under immunosuppression. In particular, three genes including IGKV4-1, IGLL1, and IGKV1D-13, which encode λ or κ light chains during the differentiation of B cells from pre-B cells to mature B cells and also during the class switch and receptor editing after encountering antigen, obviously discriminated TOL group from stable graft patients. Using urinary cell sediments, they also indicated that CD20 transcript was significantly higher in the TOL group than stable graft patients. Significantly increased numbers of total B cells (CD19+) and naïve B cells (CD19+CD27−IgM+IgD+) in the TOL group compared with stable graft patients may have an important role in inducing immunological tolerance in the TOL patients (Newell et al., 2010). Sagoo et al. (2010) demonstrated that the proportion of transitional and memory B cells in tolerant patients were significantly higher and lower than patients with chronic rejection, respectively. In addition, B cells associated genes as well as the percentage of B cells had an obvious role in inducing immunological tolerance (Sagoo et al., 2010). Biopsies, as well as, blood samples are studied to get a fact about the transplant outcome. Immunostaining for B cells and plasma cells indicated the role of these cells in antibody-mediated rejection (ABMR) and T-cell-mediated rejection (TCMR). A greater density of CD20+ B cells and CD138+ plasma cells were observed in TCMR and ABMR, respectively (Carpio et al., 2014). At the first instance, it seemed that B cells only participated in ABMR but, here, the fact that B cells with help of T cells also contributed to TCMR got revealed, and this confirmed our above statement in which we said B cells also contributed to TCMR.
The regulatory transitional B cells are divided into two: T1 (CD24highCD38highCD5+) and T2 (CD24highCD38highCD5low) cells, based on their surface phenotype, and are found in peripheral blood and spleen (Cherukuri et al., 2017; Nouël et al., 2014). T1 cell produced a significantly higher ratio of IL-10 to tumor necrosis factor (TNF)-α compared with T2 cells, suggesting the anti-inflammatory role of T1 cells (Cherukuri et al., 2017). Nouël et al. (2014) have shown that both absolute counts of T1 and T2 cells in patients with chronic antibody-mediated rejection (cAbMR) were significantly lower when compared with stable patients and healthy volunteers. They have also demonstrated that B cells of cAbMR patients displayed significantly less suppressive activity than healthy volunteers and stable patients (Nouël et al., 2014). Further analysis by Cherukuri et al. (2017) indicated that patients at the time of biopsy for rejection (before any therapeutic strategy) had significantly lower T1/T2 ratio when compared with patients with stable kidney graft, as well as, greater reduction in the absolute numbers of both T1 and T2 cells (Cherukuri et al., 2017; Table 1), implying a central role of transitional B cells with a better suppressive activity for the T1 cell in induction of immunological tolerance.
| Phenotype | Situation in blood | Related to | References |
|---|---|---|---|
| Transitional B cell | |||
| ND | Increase | Tolerance | Sagoo et al (2010) |
| Type 1 & 2 | Decrease | Rejection | |
| ND | Increase | Tolerance | Chesneau et al (2014) |
| Memory B cell | |||
| ND | Decrease | Tolerance | Sagoo et al (2010) |
| Bm5/Bm5 | Increase | Tolerance | Pallier et al (2010) |
| CD19+CD27+ | Increase | Tolerance | Pallier et al (2010) |
| CD20+CD27+ | Increase | Tolerance | Chesneau et al (2014) |
| Plasma cell | |||
| CD19+CD38+CD138+ | Decrease | Tolerance | Chesneau et al (2014) |
| Naïve B cell | |||
| CD19+CD27−IgM+IgD+ | Increase | Tolerance | Newell et al (2010) |
| Bm2 | Increase | Tolerance | Pallier et al (2010) |
| CD20+CD24lowCD38low | Increase | Tolerance | Chesneau et al (2014) |
- Note. ND: not determined; IgM: immunoglobulin M; IgD: immunoglobulin D.
Pallier et al. (2010) used Bm1–Bm5 classification system to detect B cell subpopulations in patients. Bm1 and Bm2 naïve B cells with CD38–IgD+ and CD38+IgD+ phenotype, respectively, early Bm5/Bm5 memory B cells with IgD–CD38+/– (Leibler et al., 2014) but not Bm3/Bm4 cells (nonexistent in the blood) were assessed. They demonstrated that CD19+ cells in TOL patients had significantly higher frequency compared with stable graft patients but not patients with chronic rejection (CR) and also had significantly higher absolute numbers compared with both patients with stable graft and chronic rejection. Further analysis indicated that both Bm2 and eBm5/Bm5 had greater increased absolute numbers in the TOL group compared with stable graft group but not chronic rejection group. When absolute counts of CD19+ CD27+ memory B cells were analyzed, the tangible increase was shown in the TOL group compared with both stable graft and chronic rejection groups, suggesting a tolerogenic role for these cells. IgD–CD38+/– memory B cells of TOL patients also expressed significantly higher levels of costimulatory molecules, including CD80 and CD86 compared with other transplant patients and CD40 only compared with chronic rejection group but not stable graft patients (Pallier et al., 2010; Table 1).
To analyze the immunological characteristics of TOL patients in the rejection-free patients on immunosuppression, expression of eight selected tolerance-related genes (MS4A1, CD79B, TCL1A, TMEM176B, FOXP3, TOAG-1, MAN1A1, and TLR5) and total B-cell (CD45+CD19+CD3−) counts of kidney transplant recipients were evaluated in the follow-up. B-cells (CD19+) counts in the peripheral blood of rejection-free patients on immunosuppression were significantly higher than in patients with acute rejection. Expression levels of all three B-cell-related genes including MS4A1 (CD20), TCL1A, and CD79B, as well as, FOXP3 and TOAG-1 genes were significantly higher in the patients without rejection when compared with patients with acute rejection. Inconsistently, MAN1A1 and TLR5 genes did not have an increase in rejection-free patients when compared with patients with acute rejection (Viklicky et al., 2013).
B-cell compartment might be influenced by therapeutic strategy. Leibler et al. (2014) indicated that Belatacept-treated patients had remarkably higher frequency and absolute numbers of transitional (CD19+CD24hiCD38hi) and naïve B cells when compared with a calcineurin inhibitor (CNI)-treated patients. They also demonstrated that B-cell activating factor (BAFF) and BAFF-receptor (BAFF-R) levels in Belatacept group had significant reduction compared with CNI group. Consistent with this result, Bm1–Bm5 classification system showed a significantly increased frequency of naïve Bm2 and transitional Bm2 cells as well as decreased the frequency of memory Bm5 cells in the Belatacept-treated patients compared with CNI-treated patients (Leibler et al., 2014). These observations suggested that therapeutic strategies might have a pivotal role in the transplant outcome, but a high-quality systematic review and meta-analysis are needed to clarify the pure effect of Belatacept on the transplant outcome.
Chesneau et al. (2014) similar to some studies mentioned above demonstrated that the TOL group had a significantly higher frequency of transitional B cells (CD20+CD24hiCD38hi) as well as naïve B cells (CD20+CD24loCD38lo). However, the significantly lower frequency of CD19+CD38+CD138+ differentiated plasma cells compared with stable graft group, but the negligible increased frequency of memory B cells (CD20+CD27+) in the TOL group was observed. It has been shown that IRF4 and PRDM1, which are involved in differentiation into plasma cells, were downregulated in the TOL compared with cases with stable graft (Chesneau et al., 2014).
The ability of B cells from three groups of a healthy volunteer (HV), TOL and stable to inhibit T-cell proliferation, Th1 cell proinflammatory cytokine (interferon [IFN]-γ, TNF-β) production, and induced T-cell apoptosis were assessed in vitro. These data showed that B cells from all three groups considerably inhibited proliferation and induced apoptosis of autologous CD4+CD25− T cell but without the effect on the production of a proinflammatory cytokine by Th1 cell. B cells-produced GzmB in the three groups (TOL, stable graft, and HV) had a central role in the prevention of T-cell proliferation, but the greater increase of GzmB+ B cells in the TOL group compared with stable graft and HV, particularly, demonstrated a tolerogenic role of these cells in the TOL group (Chesneau et al., 2015). B cells from TOL patients produced a higher amount of IL-10 and a comparable concentration of IL-6 and TGF-β compared with stable graft and HV subjects (Chesneau et al., 2014). Blocking IL-10 and TGF-β in the coculture assay had no effect on the suppressive activity of B cells on T-cell proliferation (Chesneau et al., 2015). Several studies also highlighted the role of B cells and their transcripts in the survival of kidney graft. Altogether, these data suggested a suppressive activity and indispensable tolerogenic role for B cells and associated gene signature in inducing operational tolerance (Haynes et al., 2012; Lozano et al., 2011; Shabir et al., 2015; Shiu et al., 2015).
2.2 T cell in kidney transplant outcome
T-cell, as well as B-cell responses, mainly culminated in graft rejection (Wekerle et al., 2017). Alloreactive T cells are involved in graft failure through direct and indirect pathway recognition of allograft (Turka & Lechler, 2009). Nevertheless, a subpopulation of T cells has been recognized that specifically had a suppressive activity. These cells included natural regulatory T (Treg) cells (CD4+CD25+FOXP3) and induced peripheral Treg, which are subdivided into T regulatory 1 (Tr1), T helper 3 (TH3), and Tr35 cells (Alessandrini & Turka, 2017; Hosseini, Dolati, Hashemi, Abdollahpour-Alitappeh, & Yousefi, 2018). Natural Treg cells through different mechanisms like suppressive cytokines production, metabolite and molecule consumption, and utilization of inhibitory molecules, suppress the immune response and maintain immune tolerance (Braza, Durand, Degauque, & Brouard, 2015; Figure 1). Moreover, another classification of T cells has been identified that play an important role in kidney transplantation outcome. Many studies showed an important role of different subsets of Treg cells in the tolerance to kidney graft.
Louis et al. (2006) demonstrated that patients with rejection had significantly lower absolute counts and frequencies of CD25hiCD4+ Treg cells compared with TOL and HV groups. CD4+ and CD8+ T cells from stable graft patients had greater reduction in the TGF-β and programmed cell death (PD)-1 transcript levels, respectively, compared with TOL patients (Louis et al., 2006). It was found that a slight pretransplant expression of CD28 molecules on CD4+ lymphocytes could significantly predict acute rejection. As well, 15 days and first-month post-transplant measurement of CD28 on CD4+ lymphocytes from acute patients with acute rejection were remarkably higher than its pretransplant value. However, no change in the expression of CD28 molecules on circulating CD4+ lymphocytes was observed in the nonrejected patients (Boix et al., 2017), suggesting that acute rejection may affect the level of CD28 molecules expression. In another study, Akoglu et al. (2014) identified that CD8+ T cells with a high amount of intracellular IL-2 were significantly associated with rejection. They also observed that patients with one or more rejection episodes had significantly higher frequency of CD8+ T cells with detectable intracellular IL-2 compared with nonrejected patients. The frequency of CD8+ T cells with detectable intracellular IL-2 did not change upon therapy with different immunosuppressive drugs (Akoglu et al., 2014). Several studies highlighted the role of the lymphocytes migration in the transplant outcome, since the lymphocyte infiltration into the allograft, which is related to chemokines and chemokine receptors, may result in allograft dysfunction and finally its rejection. It was found that T cells expressing chemokine (C–X–C motif) receptor 3 (CXCR3), chemokine (C–C motif) receptor (CCR) 1, and CCR4 might have predictive value for transplant outcome (Caballero et al., 2014; Foroughi et al., 2016).
The CD45 molecule is a transmembrane glycoprotein and a member of Src-family kinases with different isoforms (CD45RA, CD45RB, and CD45RC), which are produced through alternative splicing (Altin & Sloan, 1997; Henson, Riddell, & Akbar, 2012; Ordonez et al., 2013). CD45 signaling is required for efficient activation of T cells after T-cell receptor engagement (Ordonez et al., 2013). CD45 isoforms are differentially expressed on T cells, according to different stages of T cells differentiation and function (Altin & Sloan, 1997; Ordonez et al., 2013). Here, we highlighted the association of CD45 molecule isoforms with acceptance of kidney graft. On the basis of FoxP3 and cell surface marker expression, Miyara et al. (2009) defined subpopulations of Treg cells including resting or naïve (CD45RA+FoxP3lo) Treg cells, activated or memory (CD45RA−FoxP3hi) Treg cells, and cytokine-secreting (CD45RA− FoxP3lo) non-Treg cells (Miyara et al., 2009). It was found that activated Treg cells had highly suppressive activity (San Segundo et al., 2014) and regulatory molecules, such as cytotoxic T-lymphocyte associated protein 4 (CTLA4), lymphocyte activating 3 (LAG3), TNF receptor superfamily member 18 (TNFRSF18 [GITR]), CD39, and CD73, were highly expressed on these Treg cells leading to better suppressive activity (Schmidl et al., 2014). It was reported that pretransplant increased percentage of activated Tregs could be considered as a biomarker to predict acute rejection (San Segundo et al., 2014). In addition, Braza, Dugast, et al. (2015) demonstrated that the count of activated Treg cells (CD45RA−Foxp3hiCD25hiCD127lo) in the TOL group were significantly higher than statin intolerance patients, chronic kidney rejection, and HV. They also reported that activated Treg cells from TOL patients had greater suppressive activity and higher demethylation of the Treg-specific demethylated region when compared with other three groups (Braza, Dugast, et al., 2015). Consistent with this study, Schaier et al. (2012) highlighted an important role of activated Treg cells in acceptance of kidney graft (Schaier et al., 2012; Table 2). Moreover, according to CD45RC expression, CD4+ and CD8+ T cells were divided into CD4+CD45RClow, CD45RChigh and CD8+CD45RClow, CD45RCint and CD45RChigh subsets. There was no association between graft rejection and CD4+CD45RClow or CD45RChigh subpopulations. Nevertheless, both CD8+CD45RChigh and CD45RCint but not CD45RClow subpopulations substantially predicted the rejection of kidney graft (Ordonez et al., 2013), suggesting that expression level of a molecule on a specific cell may contribute to graft acceptance or rejection.
| Natural Treg | Phenotype | Situation in blood | Related to | References |
|---|---|---|---|---|
| Activated/memory Treg | CD4+CD25high | Decrease | Rejection | Louis et al (2006) |
| CD45RA−FOXP3hiCD25hi CD127low | Increase | Tolerance | Braza et al (2015) | |
| DRhiCD45− | Increase | Tolerance | Schaier et al (2012) | |
| CD4+CD25+CD39+ | Decrease | Rejection | McRae et al (2017) | |
| Memory effector T cell | CD4+CD25−CD39+ | Decrease | Rejection | McRae et al (2017) |
| CD4+ T cell | HLA-G+ | Decrease | Rejection | N. Lu et al (2011) |
- Note. Treg: regulatory T cells.
Over the last decade, Treg cells (CD4+CD25+FoxP3+) with high suppressive activity was identified that express human leukocyte antigen (HLA)-DR, which is a major histocompatibility complex (MHC) class II molecule (Baecher-Allan, Wolf, & Hafler, 2006). DR+ Treg cells have raised suppressor ability compared with DR− Treg cells but are more sensitive to GzmB-related apoptosis than DR− Treg cells (Kisielewicz et al., 2010). Kisielewicz et al. (2010) indicated an important role for the HLA-DR molecule in acceptance of the kidney graft. They showed that percentage of DR+ Treg cells was not different between patients with stable kidney graft and patients with acute rejection but interestingly, stable kidney graft recipients had significantly higher expression level of HLA-DR on the Treg cells compared with cases with acute rejection (Kisielewicz et al., 2010). This finding suggested that a molecule but not count of cells might participate in the survival of kidney graft. In addition, a newly classification divide CD4+ T cells based on different expression of CD25 and CD39 into four subsets, including CD4+CD25+CD39+ (activated/memory Treg cells), CD4+CD25+CD39− (proinflammatory T helper subsets), CD4+CD25−CD39+ (memory effector T cells), and CD4+CD25−CD39− T cells (naïve/effector T cells; Dwyer et al., 2010). It was found that percentages of CD4+CD25+CD39+ (activated/memory Tregs) and CD4+CD25−CD39+ (memory effector T cells) were lower both before and after transplantation in the patients with acute rejection than patients without rejection, which the posttransplant differences were only significant at the time of indicated biopsy (McRae, Chia, Pommey, & Dwyer, 2017; Table 2).
The end-stage renal disease is accompanied by immunological abnormalities, in which T cell terminally differentiates and, thereby, T cells response is reduced (Betjes, Meijers, de Wit, Weimar, & Litjens, 2012; Yoon, Gollapudi, Pahl, & Vaziri, 2006). Thus, the reduced T-cell response may cause a lowered risk for acute rejection of kidney graft (Betjes et al., 2012). A terminally differentiated T cell is divided into central memory T cells CCR7+CD45RO+ (Tcm), effector memory T cells CCR7−CD45RO+ (Tem), and extra effector memory T cells CCR7−CD45RO−CD45RA+ (Temra); and this differentiation is associated with loss of costimulatory molecule CD28 on their surface (Betjes et al., 2012; Litjens, van Druningen, & Betjes, 2006) and reduced telomere length. It was reported that pretransplant numbers of both CD4+CD28null and CD8+CD28null T cells in the patients with early acute rejection were lower than nonrejected patients, in which differences remained only significant for CD4+CD28null T cells (Dedeoglu et al., 2016). Consistently, Betjes et al. (2012) found that the pretransplant percentage and absolute counts of CD4+CD28null and CD8+CD28null T cells were significantly higher in the nonrejected patients compared with patients with acute rejection. They also demonstrated that the pretransplant percentage of highly differentiated CD8+ Temra cells modified the risk for acute rejection (Betjes et al., 2012). In contrast, a study by Vondran et al. (2014) displayed that patients with rejection had higher CD4+CD28null/CD8+CD28null T cells frequencies compared with rejection-free patients (Vondran et al., 2014). The contrary results may stem from different demographic status and clinical circumstances of the subjects. To demonstrate the effect of infusion of donor bone marrow cells (DBMCs) on allograft tolerance, in our previous study we showed that the frequency of Tregs (CD4+CD25+FoxP3+) and CD3+CD8+CD28null cells was not significantly different in infused patients compared with noninfused patients using flow cytometry. We also demonstrated that infused patients possessed a higher ratio of IL-10/IFN-γ-producing cells than noninfused patients using enzyme-linked immunosorbent assay spot analysis (Ranjbar et al., 2012). However, this study and our other studies showed that DBMCs infusion decreased acute rejection episodes and increased patients’ survival (Mohammadnia et al., 2011; Solgi et al., 2013).
Recent reports demonstrated that HLA-G was important in the transplantation and could protect allograft, thereby, led to tolerance of the transplanted tissues (Jin et al., 2012; N. Lu et al., 2011). The HLA-G molecule was recognized in 1990 (Pabón et al., 2014). It is a nonclassical HLA class I molecule and alternative splicing of its single primary transcript resulted in seven isoforms. These isoforms comprised membrane-bound (HLA-G1 to G4) and soluble isoforms (HLA-G5 to G7). HLA-G1 by proteolytic cleavage activity of metalloproteinase enzymes could be released in blood, called shed HLA-G1 (Bardi et al., 2011; Le Rond et al., 2006; Pankratz, Ruck, Meuth, & Wiendl, 2016). Both membrane-bound and soluble isoforms have suppressive function by binding to the inhibitory receptors, such as inhibitory killer immunoglobulin-like receptor (KIR) expressed on NK cells, Ig-like transcript 2 (ILT2) expressed on all immune cells, and ILT4 expressed on myeloid lineages (Bahri et al., 2009; Krongvorakul et al., 2015; Naji et al., 2007). However, membrane-bound/shed HLA-G1 and soluble HLA-G5 possess more immunosuppressive activity than others (Pankratz et al., 2016). To evaluate the role of an inhibitory molecule like HLA-G in the field of transplantation and its relation to acceptance of allograft, items like shed HLA-G in sera, HLA-G gene expression in the graft, HLA-G-positive cells counts, and the expression level of HLA-G on cells could be assessed. Regarding the inhibitory role of HLA-G molecule, including inhibition of CD4+ T-cell proliferation, induction of CD4+ T cell-ignorance, or unresponsiveness, it is expected that increased expression of this molecule in serum and graft is associated with better graft acceptance. Moreover, it was reported that HLA-G molecule could induce Treg cells with lower expression of CD4 and CD8 molecule and higher expression of CD45RA and HLA-DR (Naji et al., 2007).
It was shown that HLA-G+CD4+ T cells and the expression level of HLA-G on CD4+ T cells were significantly decreased after acute rejection. It is worthwhile to note that when acute rejection occurred, HLA-G levels decreased compared with before and during the follow-up phase after acute rejection (N. Lu et al., 2011). Other study reported that high-level sHLA-G after transplantation was independently related to graft acceptance. They showed that patients with acute rejection had a significantly lower both pretransplant and posttransplant sHLA-G levels than patients without rejection (Rebmann et al., 2009). In consistence with this study, some studies reported the association of high sHLA-G concentration with better graft acceptance (Jin et al., 2012; Poláková et al., 2015). However, Žilinská et al. (2015) demonstrated that patients with acute rejection had significantly higher expression of HLA-G messenger RNA (mRNA) in their biopsies compared with patients without rejection (Žilinská et al., 2015). Several studies have found no significant relation between sHLA-G level and graft acceptance (Bardi et al., 2011; Krongvorakul et al., 2015). From these data, it can be concluded that increased HLA-G expression was associated with better graft acceptance but applied immunosuppressive drugs, sampling after transplantation, and clinical characteristics of patients might cause conflicting results.
3 INNATE IMMUNITY IN KIDNEY TRANSPLANTATION OUTCOME
3.1 Dendritic cell (DC) in kidney transplant outcome
DC, which are regarded as professional antigen-presenting cells, have pivotal role in initiation and regulation of immune system, particularly with priming T cells (Fangmann et al., 2007; Lim, Kireta, Thomson, Russ, & Coates, 2006; Nikoueinejad et al., 2014). There are at least two distinct types of dendritic cells with different properties. Myeloid DCs (mDCs) with an expression of CD13 and CD33, and plasmacytoid DC (pDCs) with lack of cell markers of the myeloid lineage and expressing CD123 (α-chain receptor of IL-3) are two subtypes of DCs (Pontrelli et al., 2015; Womer et al., 2010). DCs have a central role in the allograft rejection through triggering the direct and indirect recognition pathways (Fangmann et al., 2007). In the direct recognition pathway during transplantation, donor DCs that are present in the allograft migrate to regional lymph nodes of recipients and active recipient T cells. Direct recognition occurs shortly after transplantation, which whole MHC molecules of donor DCs are recognized by recipient T cells, and may contribute to acute rejection. In the indirect recognition pathway that occurs late after transplantation, recipients DCs migrate to the allograft and take up donor MHC alloantigen. These DCs then circulate into the regional lymph nodes and present alloantigen (donor MHC molecule) to the recipient T cells, which contribute to the chronic rejection and alloantibody response (Turka & Lechler, 2009; Zhuang et al., 2016). Many studies have evaluated DC signature in the kidney transplant recipients.
Nikoueinejad et al. (2014) demonstrated that mDCs and pDCs had different modifications in the patients with normal graft function after transplantation. The frequency of mDCs increased 2 weeks after transplantation and then decreased to its baseline within 6 months. Unlike mDCs, pDCs decreased 2 weeks after transplantation and increased to its baseline within months after transplantation. They also reported that regulatory CD4+CD25+FoxP3+ and CD8+CD28− T cells changed similar to pDCs but not mDCs after transplantation (Nikoueinejad et al., 2014). Batal et al. (2015) used dendritic cell-specific intercellular adhesion molecule-3-grabbing nonintegrin (DC-SIGN) marker, which distinguished mDCs from macrophages, to evaluate the role of DCs located in the biopsy of kidney transplant recipients. They indicated that patients with a high number of DC-SIGN+ cells had worse graft survival compared with patients with a low count of DC-SIGN+ cells. In addition, when DC-SIGN+ cells were adjusted for clinical but not histology variables, it was observed that DC-SIGN+ cell count independently predicted the graft loss. Patients with high total inflammation in their graft had worse graft survival than patients with low total inflammation and DC-SIGN+ cell frequency strongly predicted allograft loss in the former patients (Batal et al., 2015). Moreover, secretion of sHLA-G from DCs upon CTLA4-Ig (Belatacept) treatment and its suppressive properties was demonstrated. CTLA4-Ig-treated patients had significantly higher plasma concentration of sHLA-G than patients under therapy with calcineurin-inhibitors or healthy donors. In vitro analysis displayed that DCs were one of the cellular sources of sHLA-G in patients treated with CTLA4-Ig (Bahri et al., 2009). With respect to the role of DC infiltration in the graft loss, blockade of their migratory receptors may contribute to long-term graft survival. In addition, therapeutic strategies along with demographic and clinical data may have an important role in the allograft outcome, which commonly is not adjusted in most studies.
3.2 Macrophage in kidney transplant outcome
Macrophages are the phagocytic cells that belong to the innate immune system and modulate inflammation, contribute to adaptive and innate immune response as well as tissue repair (Bergler et al., 2016; Ginhoux & Jung, 2014). Macrophages in response to environment, innate, and adaptive immune cell signals can acquire three different phenotypes and functions. These include M1 classical macrophages, which contribute to removal of pathogens from the body; M2 wound-healing macrophages participate in tissue repair; and M2 regulatory macrophages have anti-inflammatory functions. In the field of transplantation, infiltration of macrophages within the kidney graft is associated with graft rejection (Bergler et al., 2016). In 1958, it was reported that T cells and macrophages were major immune cells in the transplanted kidney of patients with acute rejection (Brent, Brown, & Medawar, 1958). Moreover, acute cell-mediated rejection was reported in some patients after depletion of T cells, suggesting that macrophages are also involved in the acute cell-mediated allograft rejection (Bergler et al., 2016).
Several studies have reported that macrophages contribute to renal fibrosis and poor graft function. Toki et al. (2014) found that enhanced macrophages infiltration was related to less graft function. They also, with regard to expression of CD163 and CD206, which are associated with regulatory and tissue repair macrophages, respectively, demonstrated that the majority of macrophages displayed M2 phenotype even in grafts with acute cell-mediated rejection (Toki et al., 2014). Consistent with this study, Ikezumi et al. (2015) indicated that CD68+CD163+CD206+ M2 macrophages participated in renal fibrosis (Ikezumi et al., 2015). Moreover, it was reported that number of infiltrating macrophages not only correlated with long-term graft survival but also correlated with acute rejection severity (Bergler et al., 2016). Now a question arises that, can reduced infiltrating macrophages participate in graft survival? A study that was done by Özdemir, Özdemir, Sezer, Colak, and Haberal (2011) answering this question. They showed that patients who were treated with vitamin D, which had immunomodulatory effects, compared with patients who did not receive vitamin D had lower interstitial and peritubular capillaries infiltrating macrophages and, thereby, had better graft acceptance and long-term graft survival (Özdemir et al., 2011). Altogether, these data suggested that macrophage infiltration was associated with graft loss. However, there are poor data to show the role these cells in the better function of kidney graft. Nonetheless, injection of regulatory macrophages into two patients resulted in induction of allograft tolerance (Rowshani & Vereyken, 2012).
3.3 Natural killer (NK) cell in kidney transplant outcome
NK cells pertain to the arm of the innate immune system and belong to the innate lymphoid cells, that initially mediate immune responses against intracellular pathogens and allograft through the production of proinflammatory cytokines and cytotoxic properties (Hoffmann et al., 2015; Trojan et al., 2017). NK cells with their receptors (Ig-like superfamily or C-type-lectin-like receptors superfamily) interact with HLA class I molecules. KIRs possess an important role in activation and inhibition of NK cell (Jafari et al., 2017; Littera et al., 2017). After kidney transplantation, NK cells trigger immune response through ligation of CD16 (FCγRIII) with donor-specific antibodies (DSA) bound to HLA molecules on the allograft and, thereby, lead to graft rejection (Hoffmann et al., 2015; Jafari et al., 2017; Littera et al., 2017; Figure 1). NK cell-related gene transcripts were found in the biopsies of DSA-positive patients with ABMR but not TCMR. In DSA-positive patients with ABMR, immunostaining of biopsies revealed an increased number of NK cells in the peritubular capillaries (Hidalgo et al., 2010). Moreover, another papers highlighted the association of NK-cell-related gene transcripts with ABMR (Sellarés et al., 2013; Venner, Hidalgo, Famulski, Chang, & Halloran, 2015).
However, there was evidence that NK-cell signature may have a tolerogenic role in the transplant recipients (Hoffmann et al., 2015). NK cell frequency was assessed by Sagoo et al. (2010), demonstrating significantly increased number of these cells in tolerant drug-free patients compared with immunosuppressed recipients (Sagoo et al., 2010). This result was validated in the rejection-free patients but not tolerant drug-free recipients in comparison with patients with rejection (Vondran et al., 2014). However, Trojan et al. (2017) reported that NK cell numbers were increased after transplantation in the recipients with good graft outcome and this increase was independent of both occurrences of acute rejection or infection and doses of immunosuppressive drugs (Trojan et al., 2017). Altogether, these data suggest that, on the one hand, NK cells signature may contribute to ABMR and, on the other hand, good graft function is related to high numbers of NK cells in the peripheral blood. As was mentioned above, KIRs play a key role in either activation or inhibition of NK cell and polymorphisms in these genes may arise different alloreactive NK cells and, thereby, a different role in the transplant recipients. The KIRs along with immunosuppressive drugs may have an important role in the distinct effect of NK cell.
3.4 Myeloid-derived suppressor cell (MDSC) in kidney transplant outcome
Myeloid-derived suppressor cells, which was described more than 20 years ago in tumor-bearing mice and cancer patients, are a heterogeneous population of cells produced during normal hematopoiesis in the bone marrow and consisted of macrophages, DCs, granulocyte progenitors, and immature myeloid cells (IMCs; Boros, Ochando, Chen, & Bromberg, 2010; Safarzadeh, Orangi, Mohammadi, Babaie, & Baradaran, 2018; Yamamoto et al., 2008; Yazdani et al., 2015). In healthy individuals, IMCs, which comprise ~0.5% of peripheral blood mononuclear cells (PBMCs), immediately differentiate into granulocyte, macrophages, or DCs in the different peripheral organs (Gabrilovich & Nagaraj, 2009; Yan, Xiaodong, Jianbin, & Zongyou, 2012). In human, MDSCs are divided into two subsets: granulocytic MDSCs, which are CD49d−CD14−HLA-DRlow/−CD15+CD66b+CD33+CD11b+ and monocytic MDSCs, with the marker of HLA-DRlow/−CD14+CD49d+CD15lowCD11b+ (Hock, McKenzie, Cross, & Currie, 2015; Yan et al., 2012). MDSCs inhibit proliferation and cytokine production of T, B, and NK cells through various mechanisms, promote the development of FoxP3+ Treg cells and induce T-cell apoptosis (Gabrilovich & Nagaraj, 2009; Lees, Azimzadeh, & Bromberg, 2011; Yan et al., 2012). In the field of transplantation, MDSCs is related to long-term survival of kidney allograft.
It was reported that both subsets of MDSCs were increased rapidly following the transplantation and decreased in different time points after transplantation and reduced numbers of MDSCs might contribute to graft rejection (Hock et al., 2015). It was demonstrated that frequency of MDSCs was higher at 3, 6, and 12 months after transplantation compared to the time of transplantation (Luan et al., 2013; Meng et al., 2014). It was also reported that patients with high MDSC frequency had long-term graft survival when compared with patients with low MDSC frequency. In addition, as expected, the plasma levels of IFN-γ and TNF-α were markedly increased in the MDSCs low group compared to MDSCs high group, implying to cytokine signature of MDSCs in transplantation outcomes (Meng et al., 2014). In vitro analysis of MDSC properties showed that CD11b+CD33+HLA-DR− myeloid cells potentially inhibited proliferation of CD4+ T cells and produced TGF-β1 and IL-10, which were related to suppression of T cells activation (Luan et al., 2013). Moreover, patients with low MDSC frequency had lower plasma level of TGF-β compared with patients with high MDSCs frequency (Meng et al., 2014).
Expressions of human S100 calcium-binding protein A8 and S100 calcium-binding protein A9, which suppress immune responses, in the graft and PBMCs, are related to MDSCs marker and improved graft outcome. Rekers et al. (2016) indicated that S100 A8 and A9 proteins significantly increased during posttransplant phase versus a pretransplant period in the patients with stable graft function, but the differences were not significant in the patients with acute rejection. They also demonstrated that patients with acute rejection, expressing high levels of S100A9 mRNA, had better graft function for at least 6 years compared with patients with acute rejection, expressing decreased S100 A9 mRNA (Rekers et al., 2016). This observation suggested that overexpression of S100 A9 mRNA during acute rejection contributed to better graft outcome, while acute rejection was one of the main causes of graft loss. Altogether, a high amount of MDSCs after transplantation participate in long-term graft survival.
4 NEW VISTAS IN THE FIELD OF BIOMARKERS IN HUMAN KIDNEY TRANSPLANTATION
Here, we are going to briefly speak about exosomes as novel biomarkers in the field of transplantation. Exosomes are one type of extracellular vesicles with 30–120 nm diameter, which are formed within multivesicular bodies, also called late endosomes, of the secreting cells. Exosomes were described more than 40 years ago and are gaining the attention of researcher ,especially, in the last decade. Exosomes contained various proteins, specific and nonspecific one, and also various nucleic acids including mRNAs, microRNAs (miRNA), long noncodingRNAs, genomic and also mitochondrial DNA, each of them could separately be used as exosomal biomarker and a part from exosomes. Exosomes are released by any kinds of cells such as T cells, B cells, dendritic cells, fibroblasts, endothelials, and are found in different body fluids like blood, saliva, urine, seminal fluid, amniotic fluid, and breast milk (Bobrie, Colombo, Raposo, & Théry, 2011; Théry, Ostrowski, & Segura, 2009; Urbanelli et al., 2015).
As was mentioned above, exosomes are found in a wide range of biological fluids but generally, urinary exosomes are evaluated in the field of kidney transplantation, which are secreted by nephron segments and provide some information of kidney function (Alvarez et al., 2013). Lv et al. (2013) used urinary exosomal miR-29 and miR-200 families to clarify their role in renal fibrosis. They showed that miR-29a, miR-29c, mirR-200b, and miR-200c significantly were downregulated in chronic kidney disease patients with mild fibrosis with respect to moderate to severe fibrosis group (Lv et al., 2013). Two studies confirmed this study, out of which one of them was a meta-analysis report (Chun-Yan et al., 2018; Gholaminejad, Abdul Tehrani, & Gholami Fesharaki, 2018). In addition, Zununi Vahed et al. (2017) demonstrated that decreased levels of miR-200b which was isolated from urine and plasma sample in two separate studies was accompanied by kidney graft fibrosis (Vahed, Omidi, Ardalan, & Samadi, 2017; Zununi Vahed et al., 2017). However, to our knowledge there is no study to assess the role of urinary exosomal miR-29 or miR-200 families in kidney transplant recipients. If such study conducted with expectable results, it may be helpful for monitoring graft function and predicting allograft outcome.
Neutrophil gelatinase-associated lipocalin (NGAL) was considered as kidney injury biomarker, which was produced by nephrons (Alvarez et al., 2013; Peake et al., 2014). Alvarez et al. (2013) reported that urinary exosomal NGAL significantly higher in the delayed graft function (DGF), which was considered as a need for dialysis during 1 week after transplantation, than non-DGF group, but the results were not significant for urinary cellular NGAL (Alvarez et al., 2013). It is worthwhile to note that only NGAL in exosomal fraction discriminated DGF and non-DGF group and this confirmed that, here, exosomes approaches have acceptable efficacy with respect to cellular one. Other kidney injury molecules such as IL-18 and cystatin c also could be detectable in urinary exosomes and provide important information about kidney or transplant outcome (Peake et al., 2014). As it was mentioned, immune cells produced their specific exosomes. Infiltrating immune cells in the graft could result in the production of their specific exosomes which can be detectable in patients’ urine. A study conducted by Park et al. (2017) tells us whether T-cell-specific exosome is associated with biopsy- proven acute cellular rejection. The answer was yes. They reported that CD3+ exosomes were higher in the graft of rejected patients compared with nonrejected subjects (Park et al., 2017). We suggest that finding potential immune cell-specific exosomes of rejection might be used instead of gold standard and invasive biopsy to discriminate rejected from nonrejected subjects. However, several similar studies need to confirm the accuracy of the results.
5 CONCLUSION
Studies have shown that an immune cell can either induce or suppress the immune response in the transplant recipients. Regulatory cells signature is high or present in the tolerant patients compared with patients with rejection. What is the reason? With respect to the literature, clinical characteristics of patients, such as DGF, cold ischemia time, HLA-mismatches and other reasons like therapeutic strategies, sampling after transplantation may affect the graft outcome. We suggest that controlling the mentioned reasons, which contribute to rejection, may provide immune tolerance in such patients. In addition, further investigations to reduce adverse immune responses during transplantation should focus on the cells mentioned in the study with regulatory or suppressive activity. This strategy, along with current drug treatments, hopefully, will extend the survival of the transplanted organ.
CONFLICTS OF INTEREST
The authors have declare that there are no conflicts of interest.




