Pre-cold acclimation improves the immune function of trachea and resistance to cold stress in broilers
Abstract
Acclimation can alleviate the damage caused by adverse environmental factors. To investigate the effects of cold stimulation on immunity in tracheal of broilers, 360 one-day-old chicks were raised at normal temperatures during 1–7 days. From 8 day, G1 (control) continued to be raised at normal temperatures, whereas G2 and G3 (treatment groups) were cold-stimulated at 3°C and 12°C below the temperature of G1, respectively. At 42 day, all the groups were subjected to a 24-hr acute cold stress, designated as S1, S2, and S3. Tracheal tissues were collected to detect gene levels of immunoglobulins, antimicrobial peptides, Hsps, and cytokines, and oxidative stress-related indicators at 14 day, 42 day, and 43 day, and protein levels of Hsps and proinflammatory cytokines as well as morphology changes at 42 day and 43 day. The results showed that, compared with 42G1, tracheal structure of 42G2 was basically intact, and gene levels of immunoglobulins and antimicrobial peptides increased (p < 0.05), whereas tracheal structure of 42G3 was destroyed, with decreased levels of immunoglobulins ( p < 0.05), and increased levels of Hsps and proinflammatory cytokines ( p < 0.05). At 43 day, tracheal damage was visible and gene levels of immunoglobulins and antimicrobial peptides decreased in S1 ( p < 0.05). Tracheal structure was relatively intact and gene levels of antimicrobial peptides increased in S2 ( p < 0.05). Compared with S1 and S3, immune-related gene levels in S2 were higher, and Hsps and proinflammatory cytokines levels were lower. The results demonstrate that cold stimulation of lower 3°C from 8 to 42 day led to cold acclimation, which improved immunity of tracheal mucosa and resistance to cold stress in broilers.
Abbreviations
-
- ACS
-
- Acute cold stress
-
- Hsps
-
- Heat shock proteins
-
- IgY (G)
-
- Immunoglobulin Y (G)
-
- IgM
-
- Immunoglobulin M
-
- IgA
-
- Immunoglobulin A
-
- TNF-α
-
- Tumor necrosis factor α
-
- IL-1β
-
- Interlenkin-1β
-
- IL-6
-
- Interlenkin-6
-
- IFN-γ
-
- Interferon-γ
-
- SOD
-
- Superoxide dismutase
-
- GSH-Px
-
- Glutathione peroxidase
-
- GR
-
- Glutathione reductase
-
- CAT
-
- Catalase
-
- MDA
-
- Malondialdehyde
-
- OH−
-
- Hydroxyl radical
-
- H2O2
-
- Hydrogen peroxide
-
- AvBD
-
- Avian beta-defensin
-
- SDS-PAGE
-
- Sodium dodecyl sulfate-polyacrylamide gel electrophoresis
-
- PBST
-
- Phosphate-buffered solution with Tween-20; TBST, tris-buffered saline with Tween-20
-
- HRP
-
- Horseradish peroxidase
-
- ANOVA
-
- Analysis of variance
1 INTRODUCTION
Repeated adaptive training can mitigate the damage caused by adverse environmental factors to the organisms. Similar to the theory of hormesis, a small amount of seemingly harmful or stressful factors are beneficial to the health and life span of the organism (Shevchuk & Radoja, 2007). Lind (1771) pointed out that heat acclimation allowed the Europeans to enjoy a pretty good healthy state. Some of the studies also indicated that heat adaptation contributed to the improvement of heat resistance and the reduction in risk of heat-related diseases (Sawka, Wenger, & Pandolf, 1996). Similarly, repeated adaptive training for animals can also enhance their immune function and disease resistance. The cell-mediated immune function and resistance to disease of mice were remarkably enhanced after their adaptation to cold (Banerjee, Aviles, Fox, & Monroy, 1999).
The respiratory tract is the first place of organisms to contact the inhaled pathogenic microorganisms. The mucous membrane of the respiratory tract forms the first line of defense against the invasion of the exotic pathogenic microorganisms. The tracheal mucosal immune function plays an important role in the resistance to adverse stimulus. Antibacterial peptides are important components of the innate defense system. They show antimicrobial activities against a variety of pathogens including bacteria, fungi, and some viruses. Antibacterial peptides act as effector molecules that play an important role in the innate immune system of the respiratory tract. Human tracheal epithelial cells can express a peptide of about 4 kDa (HTAP), which exhibits bactericidal action against Pseudomonas aeruginosa (Ko, Delannoy, & Pedersen, 1997). Two breeds of African cattle (Boran and N’Dama) infected with Trypanosoma congolense, the gene expression of antimicrobial peptide TAP in their trachea was increased, which was one of the reasons for the ability to remain productive after infected (Meade et al., 2009). Perozo, Finol, and Mavárez (2007) pointed out that the evaluation of local antibody levels, such as the trachea and the gut could help study the antiviral response of the host. Immunoglobulin G4 (IgG4) plays an important role in the antidiastole of tracheal-related disease (Kobraei, Song, Mathisen, Deshpande, & Mark, 2013). Serum IgG levels of Spaniels with pneumonia were significantly decreased (Watson et al., 2006). Research has shown that cytokines play a critical role in respiratory diseases (Van Reeth & Nauwynck, 2000). Levels of cytokine tumor necrosis factor α (TNF-α) was significantly increased in some tracheal diseases (Hodge, Reynolds, Holmes, & Hodge, 2012). Heat shock proteins take part in the immune function of the organisms. Hsp70 expression was significantly depressed in the trachea of quails after 42 days of infected with Ornithobacterium rhinotracheale (Kapakin et al., 2013). Cold stress increased the expression of heat shock proteins (Hsp90, Hsp70, Hsp60, Hsp40, and Hsp27) in immune organs of chicken (Zhao et al., 2014). Oxidative stress can reduce immune function. A single exposure to cryogenic temperature (−130°C) for 3 min can cause oxidative stress in healthy people, leading to a decrease in activities of catalase (CAT) and T-glutathione (GSH) (Lubkowska, Dolegowska, Szygula, & Klimek, 2009). Cold stress could induce oxidative stress and reduce the messenger RNA (mRNA) levels of IgA and IgG in the duodenum, jejunum, and ileum of broilers (Zhao et al., 2013). Cold stress enhanced the levels of cytokine interlenkin (IL)-6 in Wistar rat serum (Guo et al., 2012).
In summary, cold stress can reduce the immune function of animals, but appropriate cold stimulation could improve the immune function and disease resistance of organisms (Hangalapura et al., 2003). In this study, cold stimulation is one kind of external low-temperature condition. When the intensity of cold stimulation is oversize, the balance of heat production and heat dissipation can be disrupted, which leads to cold stress response accompanied by a series of physiological and functional adverse reactions in animals. The results of our previous studies showed that intermittent cold stimulation of 3°C lower than the conventional rearing temperature, applied 6-hr periods at 2-day intervals from 22 to 42 days of age, not only had no adverse effect on the performance of broilers (Wang et al., 2017), but also improved the antioxidant capacity of the birds (Li et al., 2017). Therefore, this study based on the previous results to further evaluate the impact of potential acclimation strategies for copying cold stress in broiler production. After having been raised at air temperatures of 3°C or 12°C (for establishing the cold stress model) below the normal level for 34 days, the broilers were exposed to acute cold stress at 7°C for a period of 24 hr. The oxidative stress-related indicators (glutathione peroxidase [GSH-Px], superoxide dismutase [SOD], CAT, malondialdehyde [MDA], and so on), the mRNA expression levels of immunoglobulins (IgA, IgM, and IgY), antimicrobial peptides (AvBD1, AvBD6, AvBD7, AvBD9, AvBD10, and AvBD12), heat shock proteins (Hsp27, Hsp40, Hsp60, Hsp70, and Hsp90), and cytokines (interferon-γ [IFN-γ], IL-1β, IL-6, and TNF-α), the protein levels of heat shock proteins (Hsp60, Hsp70, and Hsp90) and proinflammatory cytokines (IL-6 and TNF-α) as well as the histopathological changes in chicken trachea were examined. The purpose was to further explore whether long-term, sustained mild cold stimulation (3°C lower than normal rearing temperature) could promote the establishment of cold acclimation, and the effect of prior cold stimulation on immunity and antioxidant capacity in the trachea of cold-stressed broilers.
2 MATERIALS AND METHODS
2.1 Animals and experimental design
All the experiments and procedures described in this study comply with the Guidelines of Northeast Agricultural University Rules concerning Animal Care and Use and have been approved by the Northeast Agricultural University Animal Care and Use Committee (IACUCNEAU20150616). A total of 360 one-day-old healthy Arbor Acres female broiler chicks were randomly divided into three equal groups: Group I (G1), Group II (G2), and Group III (G3) each with 120 birds, and were housed in three climatic chambers, respectively. Each group had four replicates of 30 birds that were housed in cages, yielding an average stocking density of 10 birds/m2. The experimental thermal conditions are given in Table 1. As the control group, the birds in G1 were managed under normal room temperature scheme from 1 to 42 days, namely 34°C for 1–3 days, 33°C for 4–7 days, then reduced gradually by 1°C every two days till 20°C at 32 day and maintained at 20°C until 42 day (07:00 hr). G2 and G3 were the cold stimulation groups in which air temperature was the same as in G1 for the first 7 days of age (1–7 days), and cold stimulation started at 07:00 hr at 8 day. The cold stimulation conditions in G2 and G3 were 3°C and 12°C lower than that of G1. Therefore, the temperature in G2 and G3 was reduced to 17°C at 07:00 hr at 32 day and 14 day, respectively, and maintained at 17°C until 07:00 hr at 42 day. Commencing at 07:00 hr on 42 day, chicks in all the groups were subjected to acute cold stress (ACS) of 7°C for 24 hr. The three groups after cold stress were designated as S1 (G1 + ACS), S2 (G2 + ACS), and S3 (G3 + ACS). During the experiment, chicks were given ad-libitum access to water, complete starter diet (crude protein [CP] of 21% and metabolic energy [ME] of 12.1 MJ/kg for first 3 weeks of age) and growing-finishing diet (CP of 19.0% and ME of 12.6 MJ/kg for 4–6 weeks of age). Relative humidity of the chambers was 55–65%. Daily photoperiod was 23 hr light:1 hr dark (23L:1D) from 1 to 3 days at 25 lux and 16:8D from 4 day to the end of the experiment at 20 lux.
| Bird age (days) | Group I (G1) | Group II (G2) | Group III (G3) |
|---|---|---|---|
| 1–3 | 34°C | Same as in G1 | |
| 4–7 | 33°C | Same as in G1 | |
| 8–13 | −1°C/2 day | G1−3°C | G1−12°C |
| 14–31 | −1°C/2 day | G1−3°C | 17°C |
| 32–42 | 20°C | 17°C | 17°C |
| Acute cold stress (43 days) | 7°C (S1) | 7°C (S2) | 7°C (S3) |
- Note. Relative humidity varied from 55% to 65%. Daily photoperiod was 23 hr light:1 hr dark from 1 to 3 days and 16 hr light:8 hr dark from 4 day to the end of the experiment.
Two birds in each replicate were randomly selected and euthanized by cervical dislocation at 07:00 hr on 14 day, 42 day, and after ACS (43 day). Their middle trachea tissues were rapidly excised for later analyses. The oxidative stress-related indicators (GSH-Px, SOD, CAT, MDA, H2O2, and OH−) and the gene levels of immunoglobulins (IgA, IgM, and IgY), antimicrobial peptides (AvBD1, AvBD6, AvBD7, AvBD9, AvBD10, and AvBD12), heat shock proteins (Hsp27, Hsp40, Hsp60, Hsp70, and Hsp90), and cytokines (IFN-γ, IL-1β, IL-6, and TNF-α) at 14 day, 42 day, and 43 day were detected, respectively. At the same time, the protein levels of Hsp60, Hsp70, Hsp90, IL-6, and TNF-α, as well as morphology changes at 42 day and 43 day, were detected, respectively. The trachea tissues used for histopathological examination were immediately fixed in 4% polyformaldehyde solution. The trachea tissues used for detecting oxidation and antioxidant indicators were immediately homogenized for later analyses. The rest of the tracheal tissues were immediately frozen in liquid nitrogen and stored at −80°C for later quantitative real-time polymerase chain reaction (PCR) and western blot analysis.
2.2 Histopathological examination
Tissue specimens of the trachea were fixed in 4% polyformaldehyde solution and routinely processed in paraffin, stained with hematoxylin and eosin (H&E). Histological slides were examined under a light microscope (Nikon Eclipse E400, Chiyoda-ku, Tokyo, Japan).
2.3 Oxidation and antioxidant index determination
The oxidation and antioxidant indexes in the trachea were determined using diagnostic kits produced by Nan Jing Jian Cheng Bioengineering Institute (Nanjing, China) following the manufacturer's instructions. The diagnostic kits used in this experiment are as follows: MDA (type: A003-1), H2O2 (type: A064), SOD (type: A001-1), CAT (type: A007-1), GSH-Px (type: A005), and the producing ability of OH− (type: A018).
2.4 Quantitative real-time PCR (qPCR)
2.4.1 Primer synthesis
Primer sequences for β-actin, IgA, IgM, IgY, AvBD1, AvBD6, AvBD7, AvBD9, AvBD10, AvBD12, Hsp27, Hsp40, Hsp60, Hsp70, Hsp90, IFN-γ, IL-1β, IL-6, and TNF-α of chicken published in GenBank were designed and synthesized by the Sangon Biotech Co. Ltd (Shanghai, China). The housekeeping gene β-actin was the internal reference. The primer sequences are shown in Table 2.
| Gene | Serial number | Primer sequences (5′–3′) |
|---|---|---|
| β-actin | NM_205518.1 | F: CCGCTCTATGAAGGCTACGCR: CTCTCGGCTGTGGTGGTGAA |
| IgA | S40610 | F: GTCACCGTCACCTGGACTACAR: ACCGATGGTCTCCTTCACATC |
| IgM | X01613.1 | F: GCATCAGCGTCACCGAAAGCR: TCCGCACTCCATCCTCTTGC |
| IgY (IgG) | X07174.1 | F: ATCACGTCAAGGGATGCCCGR: ACCAGGCACCTCAGTTTGG |
| AvBD1 | NM_204993.1 | F: CCCAGGCTCTAGGAAGGAAGR: TGAGCATTTCCCACTGATGA |
| AvBD6 | NM_001001193.1 | F: TTCCAGCCCTATTCATGCTTR: CCTGTTCCTCACACAGCAAG |
| AvBD7 | NM_001001194.1 | F: TTTCCAGGGATCTGTCGAAGGR: GAATGGAGTTGGAGTGCCAGA |
| AvBD9 | NM_001001611.2 | F: TACAGCCAAGAAGACGCTGAR: CATTTCAGCTTCCCACCAC |
| AvBD10 | NM_001001609.2 | F: AGACCCACTTTTCCCTGACAR: GGAATCTTGGCACAGCAGTT |
| AvBD12 | NM_001001607.2 | F: CCAGACAGCTGTAACCACGAR: GTGGGAGTTGGTGACAGAGG |
| Hsp27 | NM_205290.1 | F: ACACGAGGAGAAACAGGATGAGR: ACTGGATGGCTGGCTTGG |
| Hsp40 | NM_001199325.1 | F: GGGCATTCAACAGCATAGAR: TTCACATCCCCAAGTTTAGG |
| Hsp60 | NM_001012916.1 | F: AGCCAAAGGGCAGAAATGR: TACAGCAACAACCTGAAGACC |
| Hsp70 | NM_001006685.1 | F: CGGGCAAGTTTGACCTAAR: TTGGCTCCCACCCTATCTCT |
| Hsp90 | NM_001109785.1 | F: TCCTGTCCTGGCTTTAGTTTR: AGGTGGCATCTCCTCGGT |
| IFN-γ | NM_205149.1 | F: GAACTGGACAGGGAGAAATGAGAR: ACGCCATCAGGAAGGTTGTT |
| IL-1β | NM_204524.1 | F: ACTGGGCATCAAGGGCTACAR: GCTGTCCAGGCGGTAGAAGA |
| IL-6 | NM_204628.1 | F: AAATCCCTCCTCGCCAATCTR: CCCTCACGGTCTTCTCCATAAA |
| TNF-α | NM_204267.1 | F: GCCCTTCCTGTAACCAGATGR: ACACGACAGCCAAGTCAACG |
2.4.2 Total RNA extraction and reverse transcription
Total RNA was extracted from frozen trachea samples using an RNAiso Plus (TaKaRa, Dalian, China) according to the manufacturer’s protocol. The RNA integrity and quality were assessed using 1% agarose gel electrophoresis. The RNA concentration and purity were determined with spectrophotometry at 260/280 nm (Gene Quant 1300/100). Complementary DNA (cDNA) was synthesized using the RR047 kit (TaKaRa, Dalian, China) according to the manufacturer's instructions.
2.5 Real-time quantitative PCR
Real-time quantitative PCR was performed using a LightCycler®96 (Roche, Switzerland) according to the manufacturer’s instructions. Reactions were performed in a 10-μL mixture including 1 μl of diluted cDNA, 5 μl of SYBR green master, 0.3 μl of forward primer, 0.3 μl of reverse primer, and 3.4 μl of PCR-grade water. The PCR program was 95°C for 10 min, and repeated 40 cycles of 95°C for 15 s and 60°C for 60 s. The melting curve showed a single peak for each PCR product. The relative abundance of mRNA was calculated according to the methods of Pfaffl (2001) and Jin, Jia, Liu, and Xu (2018).
2.6 Western blot analysis
Western blot analysis was performed to detect the protein expression levels. Briefly, equal amounts of total protein (40 μg/condition) were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions on 12% gels. Separated proteins were transferred to nitrocellulose membranes using tank transfer for 2 hr at 200 mA in Tris-glycine buffer containing 20% methanol. The membranes were blocked with 5% skim milk in Tris-buffered saline with Tween-20 (TBST) for 16–24 hr. The membranes were incubated overnight at 4°C with diluted primary chicken antibodies, and the diluted ratio was shown in Table 3. The polyclonal antibodies of heat shock proteins were produced by Veterinary Internal Medicine Laboratory, Northeast Agricultural University. The antibodies of IL-6 and TNF-α were produced by Santa Cruz Biotechnology, Inc. (California). After three 10-min washes in phosphate buffered solution with Tween-20 (PBST), followed by a horseradish peroxidase (HRP)-conjugated secondary antibodies against rabbit IgG (1:1000, Santa Cruz). The membrane was incubated with a monoclonal β-actin antibody (1:1000, Santa Cruz Biotechnology) to verify equal sample loading, followed by incubation with an HRP-conjugated goat antimouse IgG (1:1000). The signal was detected by the enhanced chemiluminescence system (Cheml Scope5300, Clinx Science Instruments, Shanghai, China). The relative abundance of proteins was expressed as the ratios of the optical density of each of these proteins to that of β-actin, respectively.
| Antibody name | Dilution ratio |
|---|---|
| Hsp60 | 1:1400 |
| Hsp70 | 1:500 |
| Hsp90 | 1:500 |
| IL-6 | 1:1000 |
| TNF-α | 1:500 |
| β-actin | 1:1000 |
2.7 Statistical analysis
IBM SPSS Statistics software (version 21.0) was used for the statistical analysis. The Kolmogorov–Smirnov procedure was used to examine data normal distribution. All data showed homogeneity of variance. Indicators related to oxidative stress were analyzed by two-factor (cold stimulation intensity and age) analysis of variance (ANOVA), and the differences among the three groups at the same measuring time point as well as the differences of each group at a different measuring time point were analyzed, respectively. The remaining indicators were analyzed by one-way ANOVA. Duncan's multiple comparison tests were used to compare means. Data are shown as the mean ± standard error (mean ± SEM). Probability values <0.05 were considered significant.
3 RESULTS
3.1 Histopathology
At 42 day, G1 and G2 had normal tracheal morphology, and the mucosal structure was intact (Figure 1a,b). The tracheal morphology of G3 occurred lesions, such as the damaged mucosal structure, intraluminal cells shedding, and the serious inflammatory infiltration (Figure 1c,d). After ACS, compared with G1, the tracheal structure of S1 had obvious pathological changes. The mucosal structure was damaged with intraluminal cells shedding and inflammatory infiltration (Figure 1e,f). Compared with S1, the tracheal structure of S2 was relatively intact and the inflammatory infiltration was minor (Figure 1g). Compared with G3, the trachea structure of S3 had no significant changes (Figure 1h).
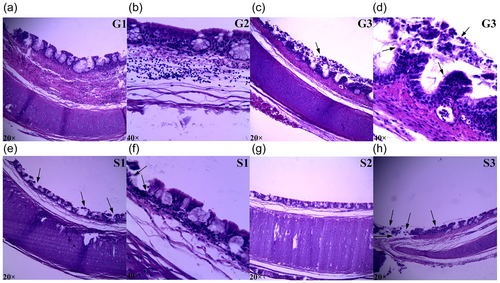
Trachea morphologies of 42-day-old AA broilers before and after a 24-hr acute cold stress at 7°C (S1, S2, and S3). The broilers had been raised either under normal (thermal conform) temperatures (G1) or under cold stimulation of 3°C or 12°C below normal levels (G2 and G3) from 8 to 42 days of age. Images in a, c, e, g, and h were magnified ×20, and images in b, d, and f have magnified ×40 [Color figure can be viewed at wileyonlinelibrary.com]
3.2 Changes in free radical levels and antioxidant enzyme activities
Effects of the cold stress on levels of MDA and H2O2, OH− producing ability, and the activities of GSH-Px, SOD, and CAT in the trachea of the broilers with or without cold stimulation are shown in Table 4. At 14 and 42 days of age, there were no differences in levels of MDA and H2O2 as well as OH− producing ability of trachea between G1 and G2 (p > 0.05), and these levels in both groups were significantly lower than those of G3 (p < 0.05). After ACS, the levels of MDA and H2O2 and OH− producing ability in S1 and S2 were significantly higher than those of 42G1 and 42G2, respectively (p < 0.05), and these levels in S3 were not different from those of 42G3 (p > 0.05). The levels of free radicals in S2 were markedly lower than those of S1 and S3 (p < 0.05).
| Indicator | Age (day) | G1 (S1) | G2 (S2) | G3 (S3) | Intensity (I) p-value | Age (A) p-value | I × A p-value |
|---|---|---|---|---|---|---|---|
| MDA (nmol/mgprot) | 14 | 6.42by ± 0.91 | 7.10by ± 0.34 | 11.84ax ± 1.41 | <0.01 | <0.01 | <0.01 |
| 42 | 6.74by ± 0.85 | 7.05by ± 1.02 | 9.08ay ± 0.79 | ||||
| 43 | 11.18ax ± 0.82 | 8.97cx ± 0.51 | 9.84by ± 0.33 | ||||
| H2O2 (mmol/gprot) | 14 | 17.25by ± 2.43 | 18.95bx ± 2.72 | 28.79ax ± 4.58 | <0.01 | <0.01 | <0.01 |
| 42 | 16.25by ± 2.24 | 15.36by ± 1.53 | 18.12ay ± 1.99 | ||||
| 43 | 22.49ax ± 2.94 | 17.90cx ± 1.85 | 20.12by ± 2.16 | ||||
| OH− (U/mgprot) | 14 | 417.01by ± 12.61 | 427.92by ± 9.89 | 517.93ax ± 23.72 | <0.01 | <0.01 | <0.01 |
| 42 | 423.32by ± 4.32 | 426.40by ± 4.01 | 475.97ay ± 5.21 | ||||
| 43 | 489.83ax ± 5.35 | 460.01bx ± 8.62 | 484.08ay ± 6.58 | ||||
| GSH-Px (U/mgprot) | 14 | 929.15by ± 18.90 | 914.33bz ± 8.44 | 1075.44ax ± 35.95 | <0.01 | <0.01 | <0.01 |
| 42 | 1017.19bx ± 15.52 | 1253.85ax ± 30.12 | 839.47cy ± 13.79 | ||||
| 43 | 836.30bz ± 19.87 | 1053.05ay ± 40.26 | 831.25by ± 8.68 | ||||
| SOD (U/mgprot) | 14 | 117.57by ± 5.94 | 120.35bz ± 5.01 | 131.36ax ± 5.38 | <0.01 | <0.01 | <0.01 |
| 42 | 132.18bx ± 6.31 | 145.61ax ± 5.80 | 119.59cy ± 5.96 | ||||
| 43 | 117.45by ± 5.52 | 135.13ay ± 5.94 | 115.45by ± 5.86 | ||||
| CAT (U/mgprot) | 14 | 103.23bx ± 5.54 | 101.70bz ± 6.21 | 114.38ax ± 6.31 | <0.01 | <0.01 | <0.01 |
| 42 | 100.12bx ± 3.49 | 116.23ax ± 9.16 | 96.32by ± 3.90 | ||||
| 43 | 87.82by ± 5.79 | 108.22ay ± 4.41 | 91.32by ± 7.38 |
- Note. a, b, and c represent the significant difference comparison between different groups at the same time point. x, y, and z represent the significant difference comparison of the same group at different time points. Different small letter superscripts mean significant difference (p < 0.05), whereas with no letter or the same letter superscripts mean no significant difference (p > 0.05). The data are presented as means ± SEM. ACS: acute cold stress; CAT: catalase; GSH-Px: glutathione peroxidase; H2O2: hydrogen peroxide; MDA, malondialdehyde; OH−, hydroxyl radical; SOD, superoxide dismutase.
At 14 days of age, there were no differences in GSH-Px, SOD, and CAT activities between G1 and G2 (p > 0.05), and these levels in both groups were remarkably lower than those of G3 (p < 0.05). At 42 days of age, compared with G1, the activities of antioxidant enzymes in G2 increased significantly (p < 0.05); however, the activities of SOD and GSH-Px in G3 decreased remarkably (p < 0.05), and the CAT activity was not different between G1 and G3 (p > 0.05). After the ACS, the activities of GSH-Px, SOD, and CAT in S1 and S2 were significantly lower than those of 42G1 and 42G2, respectively (p < 0.05). There were no differences between S3 and 42G3 in the activities of antioxidant enzymes (p > 0.05). The activities of antioxidant enzymes in S2 were significantly higher than those of S1 and S3 (p < 0.05).
The interaction of cold stimulation intensity and age had a significant effect on the above indicators related to oxidative stress (p < 0.05).
3.3 Changes in mRNA expression levels of immunoglobulins
Effects of the cold stress on mRNA expression levels of IgA (Figure 2a), IgM (Figure 2b), and IgY (Figure 2c) in the trachea of the broilers with or without cold stimulation are shown in Figure 2. At 14 days of age, no differences were detected between G1 and G2 in IgA and IgM mRNA expression levels (p > 0.05), and these levels in both groups were significantly lower than those of G3 (p < 0.05). The IgY mRNA expression level in G1 was markedly lower than that of G2 and G3 (p < 0.05), and there was no difference in gene level of IgY between G2 and G3 (p > 0.05). At 42 days of age, the mRNA expression levels of IgA, IgM, and IgY in G1 were remarkably lower than those of G2 (p < 0.05); however, these levels in G3 were the lowest. After ACS, the mRNA expression levels of three immunoglobulins in S1 and S2 were significantly lower than those of 42G1 and 42G2, respectively (p < 0.05). The gene levels of IgA and IgM in S3 had no difference with those of 42G3 (p > 0.05), and the IgY gene level was significantly lower than that of 42G3 (p < 0.05). The mRNA expression levels of three immunoglobulins in S2 were significantly higher than those of S1 and S3 (p < 0.05).
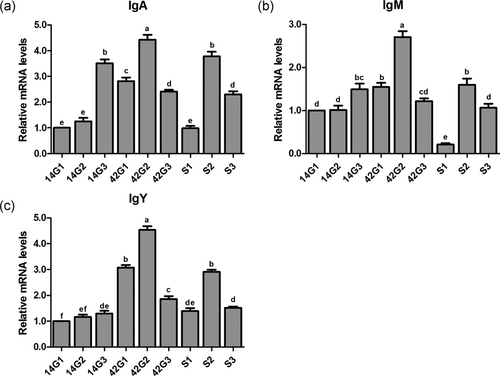
mRNA expression levels of immunoglobulin in the trachea of broilers before and after a 24-hr acute cold stress at 7°C (S1, S2, and S3). The broilers had been raised either under normal (thermal conform) temperatures (G1) or under cold stimulation of 3°C or 12°C below normal levels (G2 and G3) from 8 to 42 days of age. Bars with different small letter superscripts mean significant difference (p < 0.05), whereas with the same letter superscripts mean no significant difference (p > 0.05). The data are presented as means ± SEM. mRNA, messenger RNA, Ig, immunoglobulin
3.4 Changes in mRNA expression levels of antimicrobial peptides
Effects of the cold stress on mRNA expression levels of AvBD1 (Figure 3a), AvBD6 (Figure 3b), AvBD7 (Figure 3c), AvBD9 (Figure 3d), AvBD10 (Figure 3e), and AvBD12 (Figure 3f) in trachea of the broilers with or without cold stimulation are shown in Figure 3. At 14 days of age, the mRNA expression levels of AvBD1, AvBD7, AvBD10, and AvBD12 in G1 were remarkably lower than those of G2 and G3 (p < 0.05). There were no differences in mRNA expression levels of AvBD6 and AvBD9 between G1 and G2 (p > 0.05), and these levels in both groups were significantly lower than those of G3 (p < 0.05). At 42 days of age, the mRNA expression levels of the six kinds of antimicrobial peptides in G1 were significantly lower than those of G2 (p < 0.05). The mRNA expression levels of AvBD1, AvBD6, AvBD7, AvBD9, and AvBD10 in G1 were significantly higher than those of G3 (p < 0.05), and no difference was detected between G1 and G3 in AvBD12 mRNA expression level (p > 0.05). After ACS, compared with 42G1, the mRNA expression levels of the six kinds of antimicrobial peptides in S1 decreased significantly (p < 0.05). Compared with 42G2, the mRNA expression levels of AvBD1, AvBD6, AvBD7, AvBD9, and AvBD12 in S2 increased remarkably (p < 0.05). Compared with 42G3, the mRNA expression levels of AvBD1, AvBD6, AvBD7, and AvBD9 in S3 increased significantly (p < 0.05), and there was no difference in AvBD12 mRNA expression level between 42G3 and S3 (p > 0.05). The AvBD10 gene levels of all the three groups decreased significantly (p < 0.05) after ACS, but that level in S2 was the highest. The mRNA expression levels of the six kinds of antimicrobial peptides in S2 were markedly higher than those of S1 and S3 (p < 0.05).
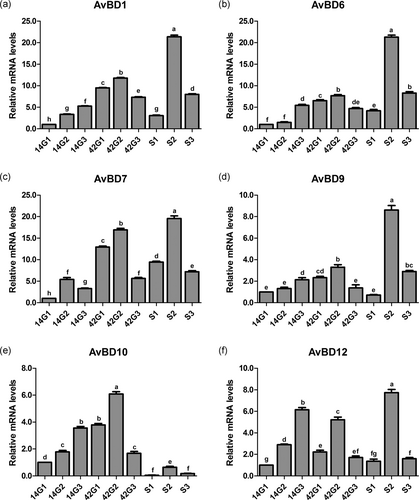
mRNA expression levels of the antimicrobial peptide in the trachea of broilers before and after a 24-hr acute cold stress at 7°C (S1, S2, and S3). The broilers had been raised either under normal (thermal conform) temperatures (G1) or under cold stimulation of 3°C or 12°C below normal levels (G2 and G3) from 8 to 42 days of age. Bars with different small letter superscripts mean significant difference (p < 0.05), whereas with the same letter superscripts mean no significant difference (p > 0.05). The data are presented as means ± SEM. mRNA, messenger RNA
3.5 Changes in mRNA expression levels of heat shock proteins
Effects of the cold stress on mRNA expression levels of Hsp27 (Figure 4a), Hsp40 (Figure 4b), Hsp60 (Figure 4c), Hsp70 (Figure 4d), and Hsp90 (Figure 4e) in the trachea of the broilers with or without cold stimulation are shown in Figure 4. At 14 and 42 days of age, there were no differences in mRNA expression levels of the five kinds of heat shock proteins between G1 and G2 (p > 0.05), and these levels in both groups were markedly lower than those of G3 (p < 0.05). After ACS, the mRNA expression levels of the five kinds of heat shock proteins in S1 were significantly higher than those of 42G1 (p < 0.05). No differences were detected between S2 and 42G2 in mRNA expression levels of Hsp27, Hsp40, Hsp60, and Hsp90 (p > 0.05), and the gene level of Hsp70 in S2 was significantly higher than that of 42G2 (p < 0.05). Compared with 42G3, the mRNA expression levels of Hsp27 and Hsp40 in S3 decreased significantly (p < 0.05), and the mRNA levels of Hsp60, Hsp70, and Hsp90 increased significantly (p < 0.05). The mRNA expression levels of the five kinds of heat shock proteins in S2 were remarkably lower than those of S1 and S3 (p < 0.05).
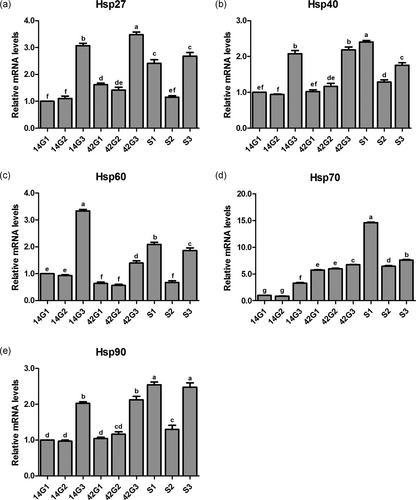
mRNA expression levels of heat shock protein in the trachea of broilers before and after a 24-hr acute cold stress at 7°C (S1, S2, and S3). The broilers had been raised either under normal (thermal conform) temperatures (G1) or under cold stimulation of 3°C or 12°C below normal levels (G2 and G3) from 8 to 42 days of age. Bars with different small letter superscripts mean significant difference (p < 0.05), whereas with the same letter superscripts mean no significant difference (p > 0.05). The data are presented as means ± SEM. mRNA, messenger RNA
3.6 Changes in mRNA expression levels of cytokines
Effects of the cold stress on mRNA expression levels of IFN-γ (Figure 5a), IL-1β (Figure 5b), IL-6 (Figure 5c), and TNF-α (Figure 5d) in trachea of the broilers with or without cold stimulation are shown in Figure 5. At 14 days of age, there was no difference in IFN-γ mRNA expression level between G1 and G2 (p > 0.05), and this level in both groups was remarkably lower than that of G3 (p < 0.05). At 42 days of age, there was no difference in IFN-γ mRNA expression level between G1 and G3 (p > 0.05), and this level in both groups was significantly lower than that of G2 (p < 0.05). After the ACS, the IFN-γ mRNA expression level in S1 and S2 was significantly higher than that of 42G1 and 42G2 (p < 0.05), respectively. The mRNA expression level of IFN-γ between S3 and 42G3 was not different (p > 0.05). The IFN-γ gene level in S2 was remarkably higher than that of S1 and S3 (p < 0.05). At 14 days of age, the gene levels of IL-1β and TNF-α in G1 and G2 were not different (p > 0.05), and these levels in both groups were significantly lower than those of G3 (p < 0.05). The IL-6 mRNA expression level in G1 was significantly lower than that of G2 and G3 (p < 0.05). At 42 days of age, the IL-1β gene level in G2 was markedly lower than that of G1 and G3 (p < 0.05), and the level of G3 was the highest. There were no differences in gene levels of IL-6 and TNF-α between G1 and G2 (p > 0.05), and these levels in both groups were markedly lower than those of G3 (p < 0.05). After ACS, compared with all three groups at 42 days of age, respectively, the gene levels of IL-1β, IL-6, and TNF-α in S1, S2, and S3 increased significantly (p < 0.05). However, the gene levels of IL-1β, IL-6, and TNF-α in S2 were significantly lower than those of S1 and S3 (p < 0.05).
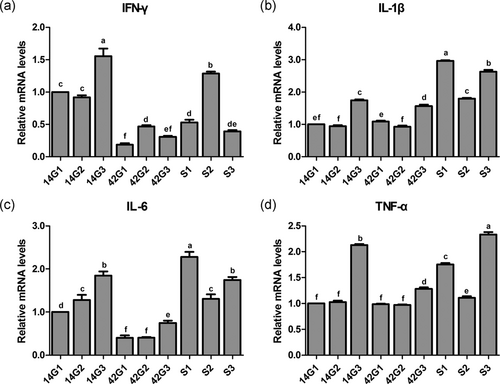
mRNA expression levels of cytokine in the trachea of broilers before and after a 24-hr acute cold stress at 7°C (S1, S2, and S3). The broilers had been raised either under normal (thermal conform) temperatures (G1) or under cold stimulation of 3°C or 12°C below normal levels (G2 and G3) from 8 to 42 days of age. Bars with different small letter superscripts mean significant difference (p < 0.05), whereas with the same letter superscripts mean no significant difference (p > 0.05). The data are presented as means ± SEM. IFN-γ, interferon-γ; IL-6, interlenkin-6; TNF-α, tumor necrosis factor α
3.7 Changes in protein levels of Hsp60, Hsp70, and Hsp90
Effects of the cold stress on protein expression levels of Hsp60 (Figure 6a), Hsp70 (Figure 6b), and Hsp90 (Figure 6c) in trachea of broilers with or without cold stimulation are shown in Figure 6. At 42 days of age, the protein level of Hsp60 in G1 was significantly higher than that of G2 (p < 0.05), and there were no differences in protein levels of Hsp70 and Hsp90 between G1 and G2 (p > 0.05). The protein levels of the three kinds of heat shock proteins in G3 were significantly higher than those of G1 and G2 (p < 0.05). After ACS, compared with G1, the protein levels of Hsp60, Hsp70, and Hsp90 in S1 increased significantly (p < 0.05). There were no differences in protein levels of Hsp60 and Hsp90 between S2 and G2 (p > 0.05), and the Hsp70 protein level in S2 was remarkably higher than that of G2 (p < 0.05). Compared with G3, the protein levels of Hsp60, Hsp70, and Hsp90 in S3 increased remarkably (p < 0.05). The protein levels of Hsp60, Hsp70, and Hsp90 in S2 were remarkably lower than those of S1 and S3 (p < 0.05).
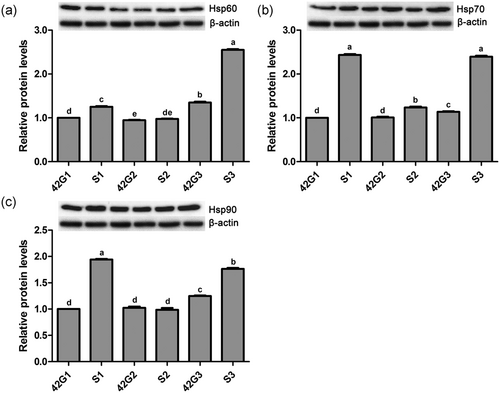
Protein expression levels of heat shock proteins in the trachea of broilers before and after a 24-hr acute cold stress at 7°C (S1, S2, and S3). The broilers had been raised either under normal (thermal conform) temperatures (G1) or under cold stimulation of 3°C or 12°C below normal levels (G2 and G3) from 8 to 42 days of age. Bars with different small letter superscripts mean significant difference (p < 0.05), whereas with the same letter superscripts mean no significant difference (p > 0.05). The data are presented as means ± SEM
3.8 Changes in protein levels of IL-6 and TNF-α
Effects of the cold stress on protein expression levels of IL-6 (Figure 7a), TNF-α (Figure 7b) in the trachea of broilers with or without cold stimulation are shown in Figure 7. At 42 days of age, there were no differences in protein levels of IL-6 and TNF-α between G1 and G2 (p > 0.05), and these levels in both groups were significantly lower than those of G3 (p < 0.05). After the ACS, the IL-6 protein level in the three groups increased significantly (p < 0.05). The TNF-α protein level in S1 and S3 was markedly higher than that of G1 and G3, respectively (p < 0.05), and no difference was detected in TNF-α protein level between S2 and G2 (p > 0.05). The protein levels of IL-6 and TNF-α in S2 were remarkably lower than those of S1 and S3 (p < 0.05).
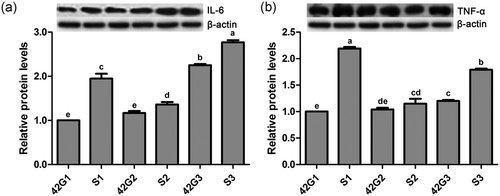
Protein expression levels of proinflammatory cytokines in the trachea of broilers before and after a 24-hr acute cold stress at 7°C (S1, S2, and S3). The broilers had been raised either under normal (thermal conform) temperatures (G1) or under cold stimulation of 3°C or 12°C below normal levels (G2 and G3) from 8 to 42 days of age. Bars with different small letter superscripts mean significant difference (p < 0.05), whereas with the same letter superscripts mean no significant difference (p > 0.05). The data are presented as means ± SEM. IL-6, interlenkin-6; TNF-α, tumor necrosis factor α
4 DISCUSSION
Environmental temperature, as a common source of stress, changes the immunity of many animal species and affects the frequency and severity of infections. Usually, appropriate low-temperature stimulation can improve the immune function and disease resistance of organisms. For example, after 2 weeks of cold acclimation in a 2°C environment, the cell-mediated immune function of mice was enhanced (Xu, Yang, & Su, 1992). After 6 weeks of the repeated cold water immersions (14°C for 1 hr, 3 times/week), human-built up cold acclimation, and the immune system was activated to a certain extent (Janský et al., 1996). The results of this study showed that cold stimulation environment of 12°C lower than the normal temperature (hereinafter referred to as lower 12°C) led to cold stress. However, cold stimulation environment of 3°C lower than the normal temperature (hereinafter referred to as lower 3°C) could enhance the immunity and antioxidant capacity. Appropriate cold stimulation could relieve the damage of the trachea caused by cold stress, and this is mainly due to the relative integrity of tracheal mucosa, the higher activities of antioxidant enzymes and higher expression levels of immunoglobulins as well as antimicrobial peptides, and the lower levels of free radicals, heat shock proteins as well as proinflammatory cytokines in S2.
Tracheal defense function mainly depends on the integrity of its mucosa and the secretion of protective proteins, such as immune globulins, cytokines, and antimicrobial peptides. Cold stress could cause mucosal damage. The respiratory mucosa of the horse was damaged after 5°C cold air inhalation (Davis, Lockard, Marlin, & Freed, 2002). Cold stress caused intestinal mucosal congestion, hemorrhage, and leukocyte infiltration in chicken (Zhao et al., 2013). However, the adaptation mechanism produced by one kind of stressor to stimulate the body could protect the body from the damage of a variety of stressors. Glavin, Lockhart, Rockman, Hall, and Kiernan (1987) pointed out that compared with the control group rats treated with water for 7 days, the gastric ulcer in the treatment group was significantly reduced when subjected to cold-restraint stress after they adapted to the concentration of 20% alcohol for 7 days. Wallace, Track, and Cohen (1983) also pointed out that compared with the rats in control group, the gastric mucosal injury of rats was significantly reduced when subjected to cold-restraint stress after they adapted to chronic mild restraint stress for 2–10 days. In this study, after cold stimulation of 34 days, the tracheal structure of G2 had no obvious lesions, whereas G3 was damaged seriously, and inflammatory cell infiltration was serious. This suggested that cold stimulation of lower 3°C was relatively mild for broiler chicks, and within the range of cold temperature the birds could adapt to, therefore there was no tracheal injury shown. Whereas cold stimulation of lower 12°C resulted in tracheal mucosal injury, this may be caused by cold stress, indicating that birds could not adapt to this kind of low-temperature stimulus. After ACS, the inflammatory injury in tracheal structure occurred for the birds in S1; however, the tracheal structure of S2 had no obvious lesions, similar to the results reported by Glavin et al. (1987) and Wallace et al. (1983). This probably because such mild cold stimulation so that 3°C lower than the normal one reduced the sensitivity to tracheal mucosa injury, and it suggested that moderate cold stimulation could produce cold acclimation without causing morphological damage. Compared with G3, the inflammatory injury of S3 was more serious. This may be that the previous cold temperature stimulation of lower 12°C had caused chronic cold stress shown by broilers, leading to the decline of resistance ability. Therefore, the degree of tracheal injury in S3 was even severe after ACS.
Immunoglobulin levels are important indicators to reflect the immune function of the body. The studies of Ring et al. (2000) and Choi et al. (2017) indicated that the cold stress reduced IgA level in human saliva and IgM level in serum of black rockfish Sebastes schlegelii. In our study, at 14 days of age, cold temperature stimulation of lower 3°C and 12°C than the normal temperature increased the expression levels of immunoglobulins in the trachea, and these levels in G3 were significantly higher than those of G1 and G2. This may be because the cold temperature condition of lower 12°C was intenser for broiler chicks, so the broilers in G3 mobilized immune system and increased the levels of immunoglobulins to against the low-temperature conditions. With the extension of cold stimulus time up to 42 days of age, the cold temperature condition of lower 12°C significantly reduced the expression levels of immunoglobulins in G3, which was consistent with the results of Ring et al. (2000) and Choi et al. (2017). This may suggest that the broilers failed to adapt to the temperature condition of lower 12°C, eventually resulting in cold stress. Therefore, the immune function of the broilers in G3 was inhibited and the levels of immunoglobulins significantly decreased. However, appropriate cold temperature condition can enhance the immune function of the body. Research showed that the IgM and IgG levels in serum of rabbits were increased after their 6 weeks of chronic cold exposure to 4°C environment (Sabiston & Rose, 1976). In this study, after cold stimulation of 34 days, the mRNA expression levels of immunoglobulins in G2 were significantly higher than those of G1, which was similar with the report of Zhao et al. (2013), who indicated that the IgA, IgM, and IgG mRNA expression levels in the small intestine of chicks exposed to chronic cold exposure at (12 ± 1)°C for 20 days increased. This may suggest that repeated stimulation of mild cold environmental condition can enhance the metabolic activity of the body (Sabiston & Rose, 1976), meanwhile could mediate immunoglobulin to regulate autoimmune function (Carr, Woolley, & Blalock, 1992), activate the immune system to a certain extent, thus improve the immune function and cold tolerance of the body (Janský et al., 1996). So, after ACS, the levels of immunoglobulins in S2 were higher than those of S1 and S3.
Antibacterial peptides are important components of the innate immune system and play a protective role against pathogen invasion. He, Qiang, Xu, and Zhu (2014) reported that low-temperature acclimation increased antimicrobial peptide mRNA levels in tilapia. This study was first to study the effect of cold stimulation on the expression levels of antimicrobial peptides in the chicken trachea. The results indicated that the levels of antimicrobial peptide genes in cold stimulation groups increased at 14 days of age. It suggested that the body could mobilize the immune system to against the cold environment at the early stage of cold stimulation. However, with the continuation of cold stimulation, levels of antimicrobial peptide genes in G3 were significantly lower than those of G1 at 42 days of age. It suggested that broilers failed to adapt to the cold condition of lower 12°C, and suffered from cold stress. However, the expression levels of antimicrobial peptides in G2 were significantly higher than those of G1 after 34 days of cold stimulation, and this was similar to the results reported by He et al. (2014). It indicated that broilers could adapt to the condition at the lower 3°C in temperature. Appropriate cold stimulation activated the immune system (Janský et al., 1996), and enhanced the tracheal immune function of broilers. At the same time, the higher levels of antimicrobial peptides helped to improve the ability of the body to resistance to low-temperature stress (He et al., 2014). So, the gene levels of antimicrobial peptides in S2 were significantly higher than those of S1 and S3 after ACS.
Stressors can regulate the secretion of cytokines, and the expression of cytokines can adjust the immune function of chickens during cold stress process (Zhao et al., 2013). So we detected levels of some cytokines in this study. Hangalapura, Kaiser, Poel, Parmentier, and Lamont (2006) suggested that cold stress could enhance IL-1β and IL-6 mRNA levels in peripheral blood leukocytes of chicken. Su et al. (2018) indicated that cold stimulation of lower 12°C significantly increased TNF-α gene level in the ileum of broilers. In this study, the levels of cytokines in cold stimulation groups showed an increasing trend at 14 days of age, especially levels in G3 with a significant increase. It showed that lower 3°C had little effect on broiler chicks in the early stage of cold stimulation. However, lower 12°C had a more severe effect on broilers. Over time, at 42 days of age, IL-1β, IL-6, and TNF-α gene levels in trachea of G3 were significantly higher than those of G1, consist with the results reported by Hangalapura et al. (2006) and Su et al. (2018). It suggested that broilers failed to adapt to the condition of the lower 12°C and suffered from cold stress. However, appropriate stress did not lead to the increase of proinflammatory cytokines. Mormede et al. (2002) reported that after 3 weeks of chronic mild stress, the expression levels of IL-1β and IL-6 in peripheral of mice decreased. Loizzo et al. (2002) pointed out that after the newborn mice adapted to mild pain training for 21-day, its IFN-γ release increased. In this study, at 42 days of age, the expression level of IFN-γ in G2 was significantly higher than that of G1, and the expression level of IL-1β in G2 was significantly lower than that of G1, similar to the results reported by Mormede et al. (2002) and Loizzo et al. (2002). IL-1β, IL-6, and TNF-α are the major proinflammatory cytokines in inflammation, playing an important role in many pathological processes, and their secretions can reduce the damage caused by cold stress to the body (Lu et al., 2017; Rajendran et al., 2018). The changing trends of various cytokines in this experiment showed that the cold tolerance and immune function of G2 were enhanced after 34 days of cold stimulation. Therefore, S2 did not rely on a large amount of secretion of proinflammatory cytokines to alleviate the damage caused by cold stress, so the levels of proinflammatory cytokines in S2 were significantly lower than those of S1 and S3. At the same time, cold stimulation of lower 3°C as a kind of mild stress applied to chicks may cause a long-term change in immune system and enhance cell-mediated immune function (Loizzo et al., 2002). This was reflected in the significant increase in IFN-γ expression of 42G2 and S2, which enhanced the immune function by upregulating the strongest function of T cells (Zhao et al., 2013).
Oxidative stress is closely related to the immune function of the organism. Oxidative stress can cause the release of various inflammatory cytokines, leading to the occurrence of inflammation along with decreased immune function as well as activities of T cells and macrophages (Srivastava, Singh, Patel, & Singh, 2017). Cold stress can cause oxidative stress and impair immune function by disrupting the balance of the oxidant/antioxidant system (Şahin & Gümüşlü, 2004). Ramnath and Rekha (2009) found that after chickens exposed to cold stress at 4°C for 10 days, SOD and GSH-Px activities in the blood decreased significantly, and MDA level in serum increased remarkably. Research by Şahin and Gümüşlü (2004) showed that cold stress of 5°C reduced CAT activity in the stomach of rats. In this study, at 14 days of age, there were no differences in free radical levels and antioxidant enzyme activities between G1 and G2. However, those of G3 were significantly higher than G1. It suggested that cold stimulation of lower 3°C was mild to broilers, and it did not cause oxidative stress. Cold stimulation of lower 12°C causes the increase of free radicals in broilers, so antioxidant system was activated and produced a large number of antioxidant enzymes to scavenge free radicals. With the continuation of cold stimulation up to 42 days of age, antioxidant enzyme activities in G3 were significantly lower than those of G1, and free radical levels were significantly higher than G1. It indicated that broilers eventually failed to adapt to the condition of the lower 12°C and suffered from cold stress. Cold stress broke the balance of oxidant/antioxidant system and caused oxidative damage in the trachea of G3. However, appropriate cold stimulation can enhance antioxidant capacity. Quiroga et al. (1991) pointed out that the rats after 21 days of cold-adapted training, the activities of SOD, CAT, GPx, and GR in brown adipose tissue increased. Research by Selman, McLaren, Himanka, and Speakman (2000) showed that after cold exposure of 61 days, the CAT activities in skeletal muscle and kidney of voles increased, and the activities of CAT and GPx in cardiac muscle increased significantly. The results of this study showed that, after 34 days of cold stimulation, the activities of antioxidant enzymes in G2 were significantly higher than those of G1, which were consistent with the results reported by Quiroga et al. (1991) and Selman et al. (2000), indicating that low temperature allowed the antioxidant defense system to be recombined at molecular level (Spasić et al., 1993), thereby activating the activities of antioxidant enzymes (Li et al., 2017), and this was the reason that the differences in free radical levels between G2 and G1 were not significant at 42 days of age. Cold stimulation of lower 3°C did not cause oxidative stress of the body, so the tracheal mucosa was complete and no inflammatory response. In general, the organisms exposing to low temperature for 21 days could achieve cold acclimation (Bukowiecki, Geloen, & Collet, 1986). Cold acclimation can promote cold tolerance of organisms (Bernabucci et al., 2010), prevent and reduce the damage from cold stress to a certain extent. After ACS, S1 occurred oxidative stress, the levels of free radicals significantly increased, and the activities of antioxidant enzymes decreased remarkably. The tracheal mucosal structure was damaged and the infiltration of inflammatory cells was obvious. Although oxidative stress in S2 was lighter, the levels of free radicals were significantly lower than those of S1. The activities of antioxidant enzymes were significantly higher than those of S1. The tracheal mucosa was relatively intact and the infiltration of inflammatory cells was minor. This probably because G2 has established cold acclimation after cold stimulation of 34 days, therefore resistance to cold stress was stronger. It indicated that appropriate cold stimulation can alleviate oxidative stress, the decrease of immune function and occurrence of inflammation induced by cold stress.
Heat shock proteins are a series of highly conserved molecular chaperone proteins being capable of modulating innate and adaptive immune responses (Srivastava, 2002). Cold stress can cause oxidative stress in organisms and increase the expression levels of heat shock proteins, which can lead to inflammatory response and decrease in immune function (Zhao et al., 2014). Numerous studies have shown that oxidative stress can lead to inflammation and tissue damage in the respiratory system, as well as subsequent immune damage (Greene, 1995). In addition, the occurrence of oxidative stress is often accompanied by high expressions of heat shock proteins such as Hsp27, Hsp40, Hsp60, Hsp70, and Hsp90 (Morici et al., 2017). Heat shock proteins, as typical damage markers, can protect cells from all types of stressors through their proliferation and differentiation. The injury process of multiple tissues and organs is often accompanied by high expression of heat shock protein (Khoso, Yang, Liu, & Li, 2015). Research by Zhao et al. (2014) showed that chronic cold stress at (12 ± 1)°C caused oxidative stress in immune organs of chickens and significantly increased expression levels of Hsp27, Hsp40, Hsp60, Hsp70, and Hsp90 in immune organs. In this study, at 14 days of age, there was no difference in heat shock protein levels between G1 and G2; however, those of G3 were significantly higher than G1. It indicated that the cold stimulation condition of lower 3°C was relatively mild to broiler chicks, so it did not cause oxidative stress. However, the cold stimulation of lower 12°C had the severe effect on broilers, so the body needed to synthesize heat shock proteins in large quantity to protect against the cold condition. With the continuation of cold stimulation, at 42 days of age, the levels of heat shock proteins in G3 were significantly higher than those of G1, which was consistent with the results of Zhao et al. (2014). It suggested that broilers eventually failed to adapt to the cold condition of lower 12°C and suffered from cold stress. However, there was no difference in the levels of heat shock proteins between G1 and G2. It showed that broilers could easily adapt to cold stimulation of lower 3°C. After the ACS, the levels of heat shock proteins in S1 increased significantly, whereas these levels in S2 were significantly lower than those of S1. It was probably due to the temperature stimulation condition of lower 3°C being milder for the birds, so it did not cause tracheal damage. After the chickens adapted to this kind of low-temperature condition, their resistance to cold stress increased. Therefore S2 had lighter oxidative stress and lower heat shock protein expression levels, and the immune function was still maintained at a higher level. Horowitz, Maloyan, and Shlaier (1997) reported that compared with the nonacclimated rats, the levels of heat shock proteins in heat-acclimated rats were lower when they were subjected to heat stress. The results of this experiment were consistent with the ones reported by Horowitz et al. (1997), which suggested that appropriate cold stimulation could enhance the immune function by alleviating oxidative stress and reducing the expression levels of heat shock protein.
5 CONCLUSION
In conclusion, the results of this study showed that cold stimulation environment of 12°C lower than the normal temperature led to the cold stress of the birds accompanied by inflammation, oxidative stress, and decreased immune function. However, the cold stimulation environment of 3°C lower than normal was in the range of cool temperature the broiler chicken could adapt to. The mild and prolonged cold stimulation of 3°C lower for 34 days could make the chicken establish cold acclimation. Cold acclimation improved immune function, the antioxidant capacity of the trachea and the resistance to cold stress of the birds to a certain extent, and reduced cold injury caused by cold stress. Cold acclimation effectively alleviated oxidative stress induced by cold stress and reduced levels of heat shock proteins, which may be a protective mechanism of cold acclimation.
ACKNOWLEDGMENT
This study was supported by the National Natural Science Foundation of China (Grant no. 31772647).
CONFLICTS OF INTEREST
The authors declare that there is no conflict of interest.




