Dental pulp stem cells senescence and regenerative potential relationship
Abstract
Uncomplicated treatments for pulpitis and periodontitis continues to be challenging and regenerative approaches could meet this contingency. Dental pulp stem cells (DPSCs) represent a good candidate for oral recovering therapies. Here, we investigated changes in morphology, proliferation, and in vitro differentiation toward mesenchymal and neuronal phenotypes of human DPSCs harvested from differently aged donors. Aging is a physiologic phenomenon occurring with time that hamper body’s capability to maintain homeostasis also affecting the functional reserve. Cytofluorimetric, immunohistochemical, quantitative reverse-transcription polymerase chain reaction (qRT-PCR), and western blot analyses were performed to gain insight for successful regenerative strategies in elderly. We observed a decline in DPSCs proliferation and differentiation potential with age. Interestingly, these cells behaved differently under osteogenic or odontogenic stimuli, showing different age-related mineralization capabilities. Similarly, neurogenic differentiation decreased with age. In conclusion, our observations represent a valid tool for the development of tailored regenerative strategies in an aging society.
1 INTRODUCTION
Cell and tissue biology studies have extended the focus on oral medical research to propose in vitro or in vivo approaches for a novel generation of biomimetic tissues structurally and functionally like the native tissue.
Dental pulp (DP) is a highly specialized ectomesenchymal tissue originating from migrating neural crest cells during development (Lindvall, Kokaia, & Martinez-Serrano, 2004). It is a vascularized tissue in the central pulp cavity of each tooth, “pulp chamber” (Trubiani, Tripodi, Delle Fratte, Caputi, & Di Primio, 2003), composed of soft connective tissue, mesenchymal cells, neural fibers, blood vessels, and lymphatics. The principal roles of DP are producing dentin and maintaining the biological and physiological viability of teeth (Liu, Gronthos, & Shi, 2006). Due to its easy accessibility and high cell proliferation potential, DP cells can be a good candidate for regenerative therapies (Gronthos et al., 2002). Dental pulp stem cells (DPSCs), first isolated by Gronthos, Mankani, Brahim, Robey, and Shi (2000) from DP of third molars, showed a great self-expansion and multilineage differentiation ability (Diomede et al., 2017; La Noce et al., 2014).
Aging is a physiologic phenomenon occurring with time (Morgan, Kunkel, & Alessio, 2001); it is affected by multiple factors at different rates, such as lifestyle, environment, and genetics. As people get older, the capability to respond to external or internal stresses (i.e., to maintain homeostasis) decreases, as well as the body’s functional reserve (Besdine, 2013). Hence, aging leads to a new set of problems also in the regenerative medicine field. Like the other tissues, DP undergoes to age-related modifications such as volume reduction, decreasing of vascularization, innervation, and cell availability (Balic, 2018). These processes begin around the middle age (40-years old), as noted by Bernick and Nedelman since 1975. Moreover, in molars from over 60-years old subjects an increase in the number of calcification loci was observed (Bernick & Nedelman, 1975).
At present, there are few and conflicting knowledge about the role of aging on DPSC regenerative potential (R. Feng & Lengner, 2013), consequently, it becomes crucial to further investigate on this process. Based on these considerations, in this study, we examined in vitro senescence features of human DPSCs (hDPSCs) and their possible role in the impairment of multipotency and differentiation potential. Harvested cells were divided in three group (young, middle age, and old). Cytofluorimetric, immunohistochemical, quantitative reverse-transcription polymerase chain reaction (qRT-PCR), and western blot analyses were performed to ascertain changes in hDPSC osteogenic, odontogenic, and neurogenic capability.
2 MATERIALS AND METHODS
2.1 Sample collection and cell culture
In collaboration with Odontostomatological and Special Surgery Unit, Ospedali Riuniti of Ancona, DP was obtained from third molars of 12 individuals gender matching (mean age, 43 years; range, 20–64 years), following approved guidelines set by the Local Ethics Committee. All patients were aware of the voluntariness of their participation and provided an informed consent in the form of verbal authorization. Samples were divided into three age groups: group A (21 years; 20–23 years), group B (43 years; 42–45 years) and group C (64 years; 62–66 years).
Cells were isolated from DP as described in the previous study (Gronthos et al., 2000). Briefly, tissue was gently removed and immersed in the digestive solution (3.0 mg/ml type I collagenase and 4.0 mg/ml dispase) for 1 hr at 37°C, then filtered by 70-μm cell strainers to obtain hDPSC suspension. Cells were plated in T25 flasks and cultured in a complete culture medium DMEM/F12 with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin at 37°C and 5% CO2.
2.2 Senescence-associated-β galactosidase assay (SA-β-Gal)
The SA-β-Gal activity was determined using a SIGMA kit (Sigma-Aldrich, Milan, Italy) according to manufacturer’s instructions. In brief, hDPSCs were cultured overnight on two-well Lab-Tek® Chamber Slides (Sigma-Aldrich) at a density of 2 × 104 cells/cm2 and fixed with 4% paraformaldehyde (PFA). After overnight incubation with SA-β-Gal, cells were washed with phosphate-buffered saline (PBS), counterstained with hematoxylin and then observed under Nikon Eclipse 600 light microscope. Images were captured with a Nikon DSVi1 digital camera and processed with NIS Elements BR 3.22 imaging software (all from Nikon Instruments, Florence, Italy). The senescent cells, blue stained, were counted in at least five randomly selected fields per sample and quantified as a percentage of the total counted cells. Mean ± SD was considered.
2.3 Immunocytochemistry (ICC)
For ICC staining, 15 × 104 cells per well hDPSCs were cultured on two-well Lab-Tek® Chamber Slide, fixed in 4% PFA in 0.1 M sodium phosphate buffer pH 7.4 for 20 min at room temperature (RT) and then permeabilized in 0.1% Triton X-100 in PBS for 10 min. Following overnight incubation at 4°C with mouse anti-human p16ink4A monoclonal antibody (1:50; Santa Cruz Biotechnology, Santa Cruz, CA), hDPSCs were immunostained using the streptavidin–biotin peroxidase technique (Envision Peroxidase Kit; DakoCytomation, Darmstat, Germany). After the incubation with 0.05% 3,3′-diaminobenzidine (Sigma-Aldrich) in 0.05 M Tris buffer, pH 7.6, with 0.01% hydrogen peroxide, cells were counterstained with hematoxylin, dehydrated in ethanol, and coverslipped with Eukitt mounting medium (Electron Microscopy Sciences, Hatfield, PA). For negative controls, the primary antibody was replaced with nonimmune serum. The immunocytochemical expression of p16ink4A was evaluated under Nikon Eclipse E600 light microscope by two independent observers. Images were captured and processed as above.
2.4 Population doubling time (PDT)
where T represents the duration of cell culture (in days) and Nf and N0 the final and the initial concentration, respectively (Roth, 2006).
2.5 Telomere length determination
Telomere length was assessed by Relative Human Telomere Length Quantification qPCR Assay Kit (ScienCell™, Carlsbad, CA; 92011) according to manufacturer’s instruction. The average telomere lengths of samples designed were directly compared by telomere primer set that recognized and amplified telomere sequences.
2.6 Flow cytometric analysis
For immunophenotyping 2.5 × 104 cells underwent flow cytometric analysis according to the International Society for Cellular Therapy (ISCT) for identification of human mesenchymal stem cells (hMSCs; Dominici et al., 2006). hDPSCs were stained for 25 min at RT with anti-CD90, CD105, CD34, CD73, and HLA-DR (all from Immuno Tools, Friesoythe, Germany) for 30 min at 4°C. For isotype controls, the primary antibodies were used instead of FITC- or PE-coupled nonspecific mouse immunoglobulin G. Cell fluorescence was evaluated in a FACSCalibur flow cytometry system (BD Italia, Milan, Italy) and data were analysed using FCS Express 6 Plus software (De Novo Software, Glendale, CA). In addition, physical parameters such as cell size (forward scatter [FSC]) and granularity (side scatter [SSC]) were analysed.
2.7 In vitro cell differentiation
For adipogenic, osteogenic, and chondrogenic differentiation, commercial kits from Life Technologies Corporation (Carlsbad, CA) were used according to the manufacturer’s instruction. For adipogenic differentiation 104 cells/cm2 were seeded in two-well Lab-Tek® Chamber Slide and treated with the appropriate medium (STEMPRO® Adipogenesis Kit, Gibco life technologies, Grand Island, NY) for 14 days, changing the medium twice a week. Differentiation was assessed by Oil red staining. Briefly, cells fixed in 4% PFA were exposed to Oil red O solution (0.5% in 100% isopropyl alcohol) for 30 min at RT, cleared with isopropanol 60%, washed in dH2O, and counterstained with hematoxylin. Cells were immediately observed under a light microscope to avoid the stain decay and counted.
For osteogenesis, cells were seeded at a density of 1.5 × 103 cells/cm2 in two-well Lab-Tek® II Chamber Slide and treated with STEMPRO® osteogenesis medium for 3 weeks, changing the medium twice a week. Alkaline phosphatase (ALP) staining was performed after 1 week by incubating cells with a solution of 5-bromo-4-chloro-3-indolyl phosphate (BCIP) and nitroblue tetrazolium (NBT) alkaline-phosphate substrate (Sigma-Aldrich) in 100 mM Tris-HCl (pH 9.5), 100 mM NaCl, 10 mM MgCl2 buffer for 1 hr at RT, and rinsed in dH2O. After 3 weeks mineralization was assessed by Alizarin Red S (ARS) staining (Sigma-Aldrich). Briefly, cells were fixed with 4% PFA in PBS for 10 min, incubated with ARS for 30 min at RT and finally washed in dH2O. The reaction was observed under Nikon Eclipse 600 light microscope. To quantitatively determine calcium mineral deposits, ARS was extracted with 10% cetylpyridinium chloride in 10 mM sodium phosphate for 60 min at RT. Concentration was quantified spectrophotometrically at 540 nm (Secoman, Anthelie light, version 3.8; Contardi, Cesano Maderno, Italy).
For chondrogenesis, hDPSCs were cultured in pellet culture system. For the preparation of each pellet, aliquots of 106 cells in 1 ml of STEMPRO® Chondrogenesis Kit (STEMPRO® Chondrogenesis Kit. Gibco life technologies, Grand Island, NY) were spun down at 1,200 rpm for 5 min. Pellets were cultured for 14 days, changing the medium twice a week. Pellets were then fixed in 4% PFA, paraffin embedded, and sectioned. Sections were exposed to a solution of Alcian Blue (pH 1; Bio-Optica, Milano, Italy) for 30 min at RT and observed under Nikon Eclipse 600 light microscope.
Odontogenic differentiation was obtained by seeding 2.5 × 103 cells/cm2 into six-well plates (VWR®) and cultured for 3 weeks in α-minimum essential medium (α-MEM) supplemented with 10% FBS, 1% penicillin/streptomycin, 10 mM/L β-glycerophosphate, 10 nM/L dexamethasone and 50 µg/ml ascorbic acid (all from Sigma-Aldrich). Differentiation was assessed by the detection of dentin matrix acidic phosphoprotein 1 (DMP1) and dentin sialophosphoprotein (DSPP). For cytoplasmic DMP1 detection, cell cultures underwent digestion with type I collagenase.
Neurogenic differentiation was generated by seeding hDPSCs at a density of 2.5 × 103 cells/cm2 into six-well plates (VWR®) in Neurobasal Medium (Gibco®, Life Technologies, Carlsbad, CA) supplemented with 1% penicillin/streptomycin, 1% B27 (Gibco®, Life Technologies), 40 ng/ml fibroblast growth factor 2, and 20 ng/ml epidermal growth factor (both from Immuno Tools). A medium was changed every 3–4 days. Differentiation was assessed by the detection of nestin and β-tubulin III after 2 weeks.
Control cultures were represented by cells maintained in an appropriate medium without differentiating factors for the same time.
2.8 qRT-PCR
qRT-PCR reactions were carried out in triplicate. Threshold cycle values were quantified, and expression of each gene was normalized relative to that of references genes.
Real-time assays were performed by the Mastercycler RealPlex2 (Eppendorf GmbH) using SsoFast™ EvaGreen Supermix 1×, in a final volume of 10 ml. All PCRs contained 1 μl of complementary DNA. Each PCR assay was performed in the white plate and comprised at 95°C for 30 s for enzyme activation and 40 cycles of denaturation at 95°C for 5 s, annealing, and extension at 60°C for 20 s. Every primer was used at 10 μM final concentration. Primer sequences were designed by Primer 3 web (Primer3Web version 4.1.0, http://primer3.ut.ee/) (and their specificity was tested by BLAST to avoid any appreciable homology to pseudogenes or other unexpected targets). In each assay, the messenger RNA (mRNA) of both reference genes and each gene of interest were measured simultaneously under identical conditions. Primers showed the same amplification efficiency. The specificity of the PCR reactions was confirmed by melt curve analysis: for each amplicon, the detected melting temperature was the expected one.
Each assay was performed in triplicate and threshold Cycle (Ct) values for reference genes were used to normalize cellular mRNA data. In this instance, normalization involved the ratio of mRNA concentrations for specific genes of interest (as mentioned above) to that corresponding to Ct medium values for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and β-glucuronidase (GUSB; Ragni et al., 2013). The amount of each mRNA was calculated using the comparative Ct method with ΔCt = Ct (mRNA) − Ct (GAPDH/GUSB), and data were expressed as gene relative expression (). Moreover, to highlight the effect of aging on cell behavior, ΔΔCt method for the evaluation of fold-change () was used comparing values obtained in cells of groups B and C with those of group A (Livak & Schmittgen, 2001). The qPCR efficiency in all our experiments was more than 90%. The difference between the actual and theoretical (100%) efficiencies would result in an underestimation of the mRNA concentration of all the analysed samples.
Oligonucleotide sequences for target genes are reported in the Supporting Information Table S1.
2.9 Western blot (WB) analysis
The expression of DMP1, nestin, and β-tubulin III in hDPSC after odontogenic and neurogenic differentiation was quantified by WB. hDPSC total proteins were extracted at the fourth passage of subculture in basal cultures (i.e., cells grown in DMEM/F12) and after cell differentiation toward odontoblast and neuron phenotypes. Moreover, to ascertain the possible natural hDPSC differentiation a further control was represented by cells cultured in appropriate media without differentiating cues. Radioimmunoprecipitation assay buffer (RIPA Lysis Buffer System; Santa Cruz Biotechnology) was used for protein extraction. Protein concentration was determined using Bradford reagent (Sigma-Aldrich). Electroblotting was performed using Wet Blotting System (XCell II Blot Module; Invitrogen™). Membranes were incubated overnight with primary antibodies anti-DMP1 (1:200, Santa Cruz Biotechnology), anti-nestin and β-tubulin III (1:500; Santa Cruz Biotechnology) followed by incubation with a secondary antibody conjugated to horseradish peroxidase (1:1,000; Santa Cruz Biotechnology). The signals were captured using an Alliance Mini system (UVITEC, Cambridge, UK); the DMP1, nestin, and β-tubulin III bands were quantified with UVITEC software and their intensity was normalized by comparison to the GAPDH housekeeping, used as a loading control. The intensity of each band was then compared with the negative controls and any change was expressed as ratio.
2.10 Immunofluorescence (IF)
hDPSCs were seeded at the density of 2.5 × 104 cells per well on two-well Lab-Tek® Chamber Slides and treated with neurogenic differentiating media for 2 weeks. For the detection of nestin and β-tubulin III localization IF was performed. After differentiation cells were fixed in 4% PFA and then permeabilised in 0.1% Triton X-100 in PBS. For immunofluorescence morphological analysis, samples were blocked with a 2% bovine serum albumin (BSA) solution in PBS for 30 min. Cells were then incubated overnight at 4°C with mouse anti-human–nestin or anti-human–β tubulin III (1:500; Santa Cruz Biotechnology). After overnight incubation, cells were stained with a goat anti-mouse FITC-conjugated secondary antibody (Bethyl Laboratories Inc., Montgomery, Alabama; dilution, 1:100). Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) and samples were mounted using Vectashield® mounting medium (Vector Laboratories Inc, Burlingame, CA). For negative controls, the primary antibody was replaced with nonimmune serum. Cells were observed with a Nikon E600 Fluorescence microscope.
2.11 Statistical analysis
Statistical analysis was performed by Prisma 4 Software (GraphPad software, La Jolla, CA). Mean and SD of three different experiments was reported. Data were analysed by analysis of variance and Bonferroni’s T test. Statistical significance was tested at p < 0.05.
3 RESULTS
3.1 Senescent features in hDPSCs
To ascertain the presence of age-related changes in cells derived from the three groups, we analysed the expression of SA-β-Gal and p16ink4a, SSC, and FSC cytofluorimetric parameters, as well as changes in telomere length. The positive rate of SA-β-Gal and p16ink4a was remarkably higher in hDPSCs of group C in comparison with cells harvested from younger subjects (Figure 1a,b). As far as p16ink4a is concerned we detected a significantly (p < 0.05) higher number of positive cells in groups B (81 ± 4%) and C (97 ± 5%) in comparison to group A (56 ± 3%). Flow cytometry analysis confirmed SA-β-Gal observations, evidencing an increase of SSC values with aging. On the contrary, no changes in cell size were observed (Figure 1c). PDT showed no differences until the passage 13 of subculture. After then, cells from groups B and C started to slow and at P17 hDPSCs of group C stopped their proliferation (Figure 1d).
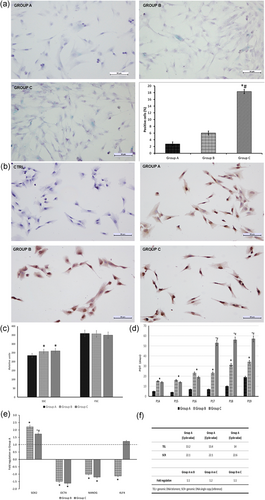
(a) Detection of SA-β-Gal in hDPSCs isolated from three different age groups. (b) Immunocytochemical analysis of p16ink4a in the differently aged hDPSCs. (c) Histogram of cytofluorimetric detection of changes in cell internal complexity (SSC) and size (FSC). (d) Histogram of the evaluation of PDT during subculturing of hDPSCs isolated from three different age groups. (e) Histogram depicts changes in stemness gene mRNA expression in the differently aged hDPSCs. Data are expressed as fold-regulation (; see Section 2), the dotted black line indicates the mRNA expression of group A; *p < 0.05 versus group A, #p < 0.05 versus group B. (f) Table showing the qPCR quantification of telomere length in the three different age groups. FSC: forward scatter; hDPSCs: human dental pulp stem cells; mRNA: messenger RNA; PDT: population doubling time; qPCR: quantitative polymerase chain reaction; SA-β-Gal: senescence-associated-β galactosidase assay; SSC: side scatter [Color figure can be viewed at wileyonlinelibrary.com]
qRT-PCR analysis displayed changes in the expression of stemness genes in relation to age (Table 1). Comparing gene expression of hDPSCs from groups B and C with those of group A, a slight upregulation of sex-determining region Y (SRY)-Box 2 (Sox2) mRNA expression and a downregulation of both octamer-binding transcription factor 4 and Nanog were showed. On the contrary, the expression of Kruppel-like factor 4 (Klf4) appeared downregulated in cells from group B and almost unchanged in hDPSCs from group C (Figure 1e).
| Group A | Group B | Group C | |||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Bonferroni’s T test | |
| Sox2 | 9.66E−06 | 4.83E−07 | 2.13E−05 | 2.13E−05 | 1.66E−05 | 1.66E−05 | Group C versus groups A and B Group B versus Group A |
| Oct4 | 4.69E−04 | 2.34E−05 | 3.15E−04 | 1.58E−05 | 2.90E−04 | 1.45E−05 | n.s. |
| Nanog | 8.93E−05 | 4.46E−06 | 8.83E−05 | 4.42E−06 | 1.66E−05 | 8.30E−07 | Group C versus groups A and B |
| Klf4 | 3.43E−02 | 1.71E−03 | 3.04E−02 | 1.52E−03 | 4.21E−02 | 2.11E−03 | Group C versus groups A and B |
| Bmp2 | 3.48E−04 | 1.74E−05 | 2.98E−03 | 1.49E−04 | 1.81E−03 | 9.04E−05 | Group C versus groups A and B |
| Runx2 | 6.96E−03 | 3.48E−04 | 2.09E−03 | 1.05E−04 | 3.08E−03 | 1.54E−04 | Group C versus groups A and B Group B versus group A |
| β-Catenin | 2.37E−02 | 1.19E−03 | 1.51E−02 | 7.57E−04 | 2.50E−02 | 1.25E−03 | Group B versus groups A and C |
| BGLAP | 1.41E−04 | 7.03E−06 | 1.14E−05 | 5.70E−07 | 3.30E−05 | 1.65E−06 | Group C versus groups A and B |
| DMP1 | 4.69E−05 | 2.35E−06 | 6.79E−05 | 3.40E−06 | 5.73E−05 | 2.87E−06 | Group C versus group A and B Group B versus group A |
| DSPP | 2.16E−05 | 1.08E−06 | 3.30E−05 | 1.65E−06 | 2.59E−05 | 1.30E−06 | Group C versus group A and B |
| Nestin | 1.01E−02 | 5.04E−04 | 4.52E−03 | 2.26E−04 | 8.75E−03 | 4.38E−04 | Group C versus group A and B Group B versus group A |
| β-tubulin | 1.−03 | 6.78E−05 | 1.71E−03 | 8.55E−05 | 1.71E−03 | 8.57E−05 | n.s. |
- Note. BGLAP: bone γ-carboxyglutamic acid-containing protein; Bmp2: bone morphogenetic protein 2; DMP1: dentin matrix acidic phosphoprotein 1; DSPP: dentin sialophosphoprotein; Klf4: Kruppel like factor 4; n.s: not significant; Oct4: octamer-binding transcription factor 4; Runx2: runt-related transcription factor 2; Sox2: sex-determining region Y (SRY)-box 2.
At last, the length average of telomere of group A was 1.1- and 1.2-fold longer than that of groups B and C, respectively, strengthening the features of age-related changes in our hDPSCs (Figure 1f).
3.2 hDPSCs characterization and mesenchymal tissues differentiation
hDPSCs isolated from the three groups showed no differences in their ability to adhere to plastic, in morphology (Figure 2a) and in their phenotypic profile according to ISCT criteria: Our cells were positive for CD73, CD90, and CD105, and negative for CD45, HLA-DR, and CD14 (Figure 2b). The analysis of their differentiation capability exhibited a reduction of the adipogenic and osteogenic potential in cells from group C in comparison with both groups A and B, as evidenced by Oil red, ALP and ARS stainings. On the contrary, no changes in chondrogenic potential were observed (Figure 2c). Age-related modifications of genes involved in osteogenic differentiation were detected, with a decrease in runt-related transcription factor 2 (Runx2), β-catenin, and bone γ-carboxyglutamic acid-containing protein (BGLAP) expression (Table 1). It was interesting to observe that, after 3 weeks in odontogenic media, the expression of mRNA for Bmp2, markedly upregulated before differentiation, appeared downregulated in groups B and C in comparison with group A. On the contrary, no significant changes were detected for the other osteogenic genes except for BGLAP (Figure 2d and Supporting Information Table S2).
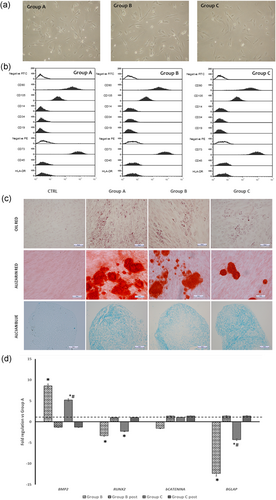
(a) Representative phase-contrast images of hDPSCs isolated from the three different age group. (b) Cytofluorimetric analysis of the detection of MSC surface markers in hDPSCs isolated from the three groups (white plots indicate FITC and PE negative controls). (c) Differentiation of hDPSCs isolated from the differently aged groups toward adipocytes (Oil red staining; scale bars = 10 µm); osteoblasts (ARS and ALP stainings; scale bars = 50 µm), and chondrocytes (Alcian blue staining; scale bars = 50 µm). (d) Histogram depicts changes in mRNA expression of genes involved in osteogenic differentiation in the differently aged DPSCs before and after (post) odontogenic differentiation. Data are expressed as fold-regulation (; see Section 2), the dotted black line indicates the mRNA expression of group A; *p < 0.05 versus group A, #p < 0.05 versus group B. ALP: alkaline phosphatase; ARS: Alizarin red S; hDPSCs: dental pulp stem cells; human dental pulp stem cells; mRNA: messenger RNA; MSC: mesenchymal stem cells; PDT: population doubling time; qPCR: quantitative polymerase chain reaction [Color figure can be viewed at wileyonlinelibrary.com]
As odontogenic differentiation is concerned, even if no significant morphological age-related changes were observed, ARS showed a marked increase in cells of groups B and C in comparison with hDPSCs of group A (Figure 3a). This was sustained by the changes of odontogenic gene expression and DMP1 protein. After differentiation, a decrease in the expression of DMP1 and DSPP mRNAs was observed in hDPSCs of groups B and C in comparison to cells of group A (Figure 3b and Appendix Table 2). WB analysis showed a faint expression of DMP1 fragments in undifferentiated cells, except for the N-terminal one in cells from group B. The expression of cytoplasmic DMP1 increased in cells undergoing odontogenic differentiation as well as in hDPSCs cultured without an odontogenic media (Figure 3c). In hDPSCs treated with odontogenic medium, the majority of DMP1 was in the form of 105 kDa, while in cells cultured, without odontogenic cues, it was in the size of 37 kDa fragments. Protein aggregates (120 kDa), accumulated on top of the sodium dodecyl sulfate–polyacrylamide gel electrophoresis gel, were also present, especially in cells of group C cultured for 3 weeks without odontogenic induction. Mineralization nodules were detected only in cells treated with differentiating media, indicating that DMP1 favored mineralization (Figures 3a,d). Moreover, ARS quantization evidenced age-related variations in the mineralization capability of hDPSCs after osteogenic or odontogenic stimuli (Figure 3e).
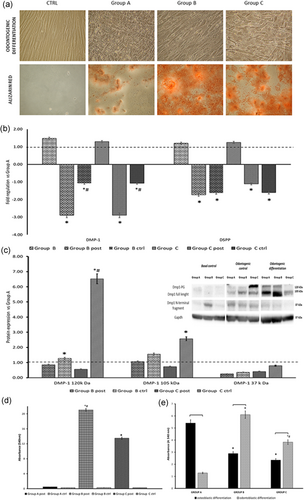
(a) Representative images displaying the different morphology and the formation of mineralized nodules in hDPSCs after 3-weeks culture in odontogenic media. (b) Histogram depicts changes in the expression of DMP1 and DSPP mRNAs in the differently aged hDPSCs before and after (post) odontogenic differentiation and in DPSCs maintained for 3 weeks in ctrl. Data are expressed as fold-regulation (; see Section 2), the dotted black line indicates the mRNA expression of group A. (c) Western blot analysis of DMP1 protein expression; histogram depicts changes in differently aged hDPSCs (post) odontogenic differentiation and in DPSCs maintained for 3 weeks in ctrl in comparison to group A (dotted black line). (d) Histogram of Alizarin red quantization in differently aged hDPSCs (post) odontogenic differentiation and in hDPSCs maintained for 3 weeks in ctrl. (e) Histogram of Alizarin red quantization in hDPSCs after osteogenic or odontogenic differentiation. *p < 0.05 versus group A, #p < 0.05 versus group B. ctrl: control media; DMP1: dentin matrix acidic phosphoprotein 1; DPSCs: dental pulp stem cells; DSPP: dentin sialophosphoprotein; hDPSCs: human dental pulp stem cells; mRNA: messenger RNA [Color figure can be viewed at wileyonlinelibrary.com]
3.3 Neurogenic differentiation
Following 2 weeks of induction with neurogenic medium, hDPSCs decreased in proliferation and acquired a stellate morphology with no differences among the groups (Figure 4a). qRT-PCR analysis indicated that nestin and β-tubulin III mRNA expression profile decreased with aging (Figure 4b). WB protein expression patterns of early (nestin) and intermediate (β-tubulin III) neuronal markers showed an increase of nestin 90 kDa in hDPSCs with age, whilst no changes were detected for nestin 120 kDa and β-tubulin III (Figure 4c). Nestin and β-tubulin III IF detection evidenced age-related differences in their intensity and localization (Figure 4a).
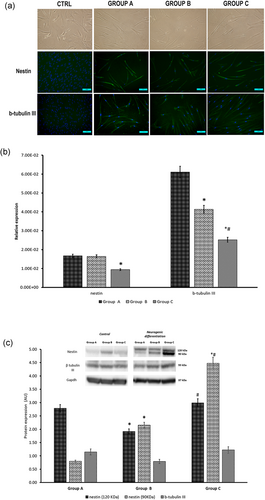
(a) Representative images displaying the morphology of hDPSCs after culturing in neurogenic media and the immunofluorescence detection of nestin and β-tubulin III (scale bars = 50 µm). (b) Histogram depicts changes in the expression of nestin and β-tubulin III mRNAs in the differently aged hDPSCs after neurogenic differentiation. Data are expressed as relative expression (; see Section 2). (c) Western blot analysis of nestin and β-tubulin III protein expression; *p < 0.05 versus group A, #p < 0.05 versus group B. hDPSCs: human dental pulp stem cells; mRNA: messenger RNA [Color figure can be viewed at wileyonlinelibrary.com]
4 DISCUSSION
Although great progress has been made in dentistry, the treatment of pulpitis and periodontitis continues to have postoperative complications. Nowadays regenerative approaches, as well as biomaterials commonly used in dentistry, have been considered for patients without a specific age target. The world’s population is aging, and it has been appraised that by 2050 the number of people 65 years of age and older will reach 1.5 billion (Richard & John, 2011). Hence, the concept of customized therapies must meet this contingency. Like others, DP tissue undergoes age-depended changes and the decline of its regenerative capacity might be related to stem cell senescence (Chu, 2013). Up to now, few and conflicting data are available on possible age-related changes in the regenerative potential of DPSCs (Chu, 2013; Yi et al., 2017). These cells possess a great self-expansion and differentiation capabilities toward osteogenic (Teti et al., 2015), odontogenic (Gronthos et al., 2002) and neurogenic (Gervois et al., 2015) phenotypes, making them an interesting cell source for regenerating pulp and periodontal tissues, and in the treatment of neurodegenerative diseases.
Based on these considerations, we investigated changes in morphology, proliferation, and in vitro differentiation potential in hDPSCs isolated from differently aged donors. Cells were split into juvenile (group A), middle age (group B), and aged (group C) clusters based on the donors’ age (Bernick & Nedelman, 1975). No significant age-related morphological differences were detected. On the contrary, we noticed a significant increase of SA-β-Gal and p16ink4a nuclear expression in group C compared with both groups A and B. SA-β-Gal is a lysosomal enzyme highly correlated with cellular senescence in cultured cells and in tissues from various animals (Dimri et al., 1995). P16ink4a is a protein involved in cell cycle progression (X. Feng et al., 2014), and along with SA-β-Gal activity, is an established biomarker of senescent cells (Dimri et al., 1995). These changes were in accordance with the cytofluorimetric detection of an augmented internal complexity (i.e., SSC values) with aging.
We additionally investigated if hDPSCs progressively lose their self-renewal potential during aging, undergoing a dysregulation of proliferative activities and declining functional capacity as described for other MSCs (Oh, Lee, & Wagers, 2014). The evaluation of the gene expression profile of Sox2, Oct4, Nanog, and Klf4 transcription factors experienced changes in all age groups. These molecules are critical to preserve stem cell phenotype and, therefore, their impairment could be responsible for the loss of stemness properties and pluripotency commitment of DPSCs (Gronthos et al., 2000; Huang et al., 2014). The different regulation of Klf4 detected during aging could be at least in part due to its role in the odontoblastic differentiation and inhibition of hDPSC proliferation (Lin et al., 2011). A further confirmation of age-related changes in our hDPSCs was represented by the shortening of telomere length in both groups B and C in comparison with cells of group A (Mokry et al., 2010).
No changes in the expression pattern of the common ISCT markers (Dominici et al., 2006; Teti et al., 2015) were detected independently from the age of donors. On the contrary impaired osteogenic and adipogenic potentials were found in hDPSCs of groups B and C in comparison with group A, noticing a marked decline in group C, as suggested by previous work (Yi et al., 2017). Concerning odontoblastic differentiation, qRT-PCR showed a marked reduction of DMP1 and DSSP mRNAs in cells from aged subjects in comparison to young ones. The decrease in DMP1 and DSSP expression was less marked in cells cultured without odontogenic media in comparison to differentiated hDPSCs. DMP1 is an acidic no collagenous protein naturally present in bone and dentin extracellular matrix (ECM) as proteolytically processed fragments (Qin et al., 2003). DMP1 occurs predominantly as a C-terminal (57 kDa) and a N-terminal (37 kDa) fragments, both promoters of HA formation and growth. Further aggregates occur as DMP1 proteoglycan fragment (DMP1-PG; Qin, D’Souza, & Feng, 2007), an inhibitor of mineralization (Gericke et al., 2010). DMP1 is implicated in a complex biological process: It is initially localized in the nucleus, where it acts as transcriptional factor, during cell maturation DMP1 is in the cytoplasm and, further, Ca2+ is responsible for its export into the ECM, where it regulates the nucleation of HA and the formation of calcified tissue (Narayanan et al., 2003). The WB of cytoplasmic DMP1 expression, showed an increase in DMP1 full-length form (105 kDa) and N-terminal fragment in hDPSCs after three weeks of culture without differentiating medium, with a marked amount in cells from group C. This suggests that in vitro our cells possess a spontaneous odontogenic potential, even being unable to form mineralized nodules as suggested by ARS. After hDPSC odontoblastic differentiation, 37 kDa fragment of DMP1 decreased in the cytoplasm in support of ECM mineralization (Maciejewska et al., 2009).
The decrease of differentiation potential toward mineralized tissues (i.e., bone and dentin) with age was also sustained by the changes the expression of Bmp2, β-catenin, Runx2, and BGLAP (osteocalcin) after odontogenic differentiation (Ferretti et al., 2014). In hDPSCs, β-catenin is upregulated during in vitro odontogenic differentiation (Han et al., 2014) and its overexpression is linked to the suppression of mineralization (Scheller, Chang, & Wang, 2008). On the contrary, Runx2 expression must be downregulated in odontoblast to reach full differentiation for a correct dentinogenesis (Chen et al., 2005). In this regard, the increase of mineralized nodules in hDPSCs of group B treated with odontogenic medium may be connected to the diffusing calcific degeneration of pulp, a pathologic condition occurring as a response to aging (Piattelli & Trisi, 1993).
A vast body of evidence indicates that neural crest-derived DPSCs express specific markers of neuronal cell (Arthur, Rychkov, Shi, Koblar, & Gronthos, 2008; Chai et al., 2000). Consequently, they might provide a promising source of stem cells for therapeutic application in neurodegenerative diseases. The comparison of our biological and morphological analyses evidenced not only an age-related decrease of markers of neurogenic differentiation (X. Feng et al., 2013), but also an incorrect localization of β-tubulin III (Martens et al., 2012).
In conclusion our results confirmed that the differentiation potential of hDPSCs could be differently impaired by aging. These observations must be considered for the development of customized strategies based on the possibility to encourage osteogenesis, pulpogenesis, and dentinogenesis as well as for application in cell-therapy to treat neurological disorders. DPSCs of young donors may provide an ideal source of stem cells that could extend their therapeutic application in oral (i.e., alveolar bone, DP, and dentin regeneration) and neurodegenerative (i.e., Alzheimer and Parkinson) diseases. Aged DPSCs could be used as in vitro tool for the study of biomaterials solving the challenges of an aging population. Moreover, considering the immunomodulative properties of MSCs, further in vivo investigation also assessing this phenomenon in DPSCs during aging, will be hugely important, making them potential novel immunotherapeutic resources in inflammatory age-related diseases.
ACKNOWLEDGMENTS
The authors thank Dr. Alessandra Nori and Dr. Vittorio Zavaglia of the Odontostomatological and Special Surgery Unit, Ospedali Riuniti of Ancona, for their helpful patient selection and sample collection. The authors are grateful to Dr. Manuela Dicarlo for her valid collaboration in flow cytometry analysis.
CONFLICTS OF INTEREST
The authors declare that there are no conflicts of interest.




