LINC00641 regulates autophagy and intervertebral disc degeneration by acting as a competitive endogenous RNA of miR-153-3p under nutrition deprivation stress
Abstract
Emerging evidence supports the involvement of autophagy in the pathogenesis of intervertebral disc degeneration (IDD). MicroRNAs (miRNAs) and long noncoding RNAs (lncRNAs) play fundamental roles in various cellular processes, including autophagy. However, it remains largely unknown as to how autophagy is regulated by miRNAs and lncRNAs in IDD. Biological functions of miR-153-3p and long intergenic nonprotein coding RNA 641 (LINC00641) were investigated. Luciferase reporter assays was done to validate miR-153-3p targets. To induce nutritional stress, nucleus pulposus (NP) cells were cultured in the normal nutritional condition and the low nutritional condition. Quantitative reverse-transcription polymerase chain reaction (RT-qPCR) was used to analyze miR-153-3p and LINC00641 in response to nutrient deprivation. Autophagic activity was assessed by transmission electron microscopy, western blot analysis and green fluorescent protein-light chain 3 puncta. Pull-down assay and RNA fluorescent in situ hybridization were performed to validate LINC00641 target and the location. MiR-153-3p is downregulated in NP tissues from IDD patients. Further, LINC00641 can affect collagen II and matrix metalloproteinase-3 expressions. Upregulation of LINC00641 and downregulation of miR-153-3p are detected in NP cells under nutritional stress. LINC00641 can regulate autophagic cell death by targeting miR-153-3p and autophagy-related gene 5 (ATG5). MiR-153-3p inhibits autophagy and IDD by targeting ATG5. More important, LINC00641 targets miR-153-3p, and thus affects ATG5 expression, autophagic cell death and IDD. These findings uncover a novel regulatory pathway that is composed of LINC00641, miR-153-3p, and ATG5 in IDD. This mechanism may stimulate to a more understanding of IDD pathogenesis and provide new sights for the treatment of this disorder.
1 INTRODUCTION
Intervertebral disc degeneration (IDD) underlies musculoskeletal disorders of the spine that lead to economic loss and incalculable individual distress due to associated pain, morbidity, and physical disability (Brox et al., 2010; Global Burden of Disease Study Collaborators, 2015). However, many people with degenerate discs may present no symptom (Brinjikji et al., 2015). The pathogenesis of IDD is a complex process and has a number of poorly understood biological determinants (Dudek et al., 2017; Phillips et al., 2013; M. Wang et al., 2012). Autophagy is an evolutionarily conserved process that sequesters nonessential intracellular components for lysosomal degradation in response to a variety of stress stimuli (Green & Levine, 2014). It has been suggested that abnormal autophagy plays a critical role in the onset of IDD (Chen et al., 2016; Gruber et al., 2015; Ma et al., 2013; Miyazaki et al., 2015). Several autophagy-related genes (ATG) control the autophagy process. Ablation of ATG5 impairs autophagy and can diminish cell survival capacity (Conway et al., 2013; J. H. Kim et al., 2015; Maskey et al., 2013; Simon & Friis, 2014). Further, ATG5 is involved in numerous diseases, including rheumatic diseases, cardiovascular, neurological diseases, and cancer (Bouderlique et al., 2016; Brisson et al., 2016; Byrne et al., 2016; Rockel & Kapoor, 2016; Shirakabe et al., 2016). Although ATG5 plays an important role in cell survival/death determination and disease progression, the molecular regulation of ATG5 during stress response in nucleus pulposus (NP) cell remains to be elucidated.
MicroRNAs (miRNAs) are short noncoding RNAs (~22 nucleotides [nts]) that mediate posttranscriptional regulation through interactions of their seed region with complementary sequences in the 3′-untranslated region (3′-UTR) of target messenger RNA (mRNA; Bartel, 2009; V. N. Kim, 2005). MiRNAs play a key role in the regulation of various biological processes. Notably, several miRNAs have been reported to be associated with autophagy. MiR-155 suppresses autophagy in chondrocytes (D’Adamo et al., 2016). MiR-30a regulates autophagy by targeting to beclin1 in cancer (Zhu et al., 2009). MiR-188-3p regulates autophagy by targeting to ATG7 in cardiomyocytes (K. Wang et al., 2015). However, it remains largely unknown as to how autophagy is regulated by miRNAs in NP cell.
Long noncoding RNAs (lncRNAs) are historically defined as RNA transcripts >200 nts in length with lack of protein-coding capability (Bär et al., 2016). LncRNAs participate in a wide range of biological and cellular processes. In line with the wide range of biological roles lncRNAs may have, a handful of studies have implicated abnormal expression and function of lncRNAs in many human diseases, including human IDD (Lan et al., 2016; Li et al., 2017; Mendell, 2016; Wan et al., 2014; Zheng et al., 2016). The study of lncRNAs function in IDD has only just begun. Two previous studies have indicated that lncRNA expression profile was dysregulated in NP cells (Lan et al., 2016; Wan et al., 2014). However, whether lncRNA participates in the regulation of NP cell autophagy remains unknown.
Our present work reveals that ATG5 is a target of miR-153-3p. MiR-153-3p inhibits autophagy and promotes cell death through targeting ATG5. Moreover, our study further suggests that long intergenic nonprotein coding RNA 641 (LINC00641) directly binds to miR-153-3p and acts as an endogenous miR-153-3p sponge to inhibit its activity, which results in the increase of ATG5 expression. Our data shed new light on the understanding of the interaction between lncRNA and miRNA in molecular regulation of autophagic programme and IDD.
2 MATERIALS AND METHODS
2.1 Patient samples
A total of 62 lumbar NP specimens were obtained from patients with degenerative disc disease undergoing discectomy (59.3 ± 7.2 years) between May 2014 and December 2015. The surgical indications were as follows: (a) failed conservative treatment and (b) progressive neurologic deficits such as progressive motor weakness or cauda equine syndrome. Patients with spondylolisthesis, ankylosing spondylitis, or diffuse idiopathic skeletal hyperostosis were excluded. The control samples (NP) were taken from 59 patients with fresh traumatic lumbar fracture (26.2 ± 6.8 years) who underwent decompressive surgery due to neurological deficits. Routine magnetic resonance imaging scans of the lumbar spine were taken of these patients before surgery and degree of disc degeneration was graded from T2-weighted images, using Pfirrmann classification (Pfirrmann et al., 2001). This study protocol was approved by the Ethics Committee of our institution, and written informed consent was obtained from each participant.
2.2 Isolation, primary culture of human NP cells, and treatment
The tissue specimens were first washed twice with phosphate-buffered saline (PBS), and the NP was separated from the annulus fibrosus (AF) using a stereotaxic microscope, cut into pieces (2–3 mm3), and the NP cells were released from the NP tissues by incubation with 0.25 mg/ml type II collagenase (Invitrogen, Carlsbad, CA) for 12 hr at 37°C in Dulbecco’s modified Eagle medium (DMEM; Gibco, Grand Island, NY). After isolation, the NP cells were resuspended in DMEM containing 10% fetal bovine srum (FBS; Gibco), 100 μg/ml streptomycin, 100 U/ml penicillin, and 1% l-glutamine and then incubated at 37°C in a humidified atmosphere with 95% (vol/vol) air and 5% (vol/vol) CO2. The confluent cells were detached by trypsinization, seeded into 35-mm tissue-culture dishes in complete culture medium (DMEM supplemented with 10% FBS, 100 μg/ml streptomycin, and 100 U/ml penicillin), and incubated in a 37°C, 5% CO2 (vol/vol) environment. The medium was changed every 2 days. The second passage was used for subsequent experiments.
To induce nutritional stress, NP cells were cultured in DMEM supplemented with 1% (vol/vol) FBS, defined as the low nutritional (LN) condition, compared with 10% (vol/vol) FBS defined as the normal nutritional (N) condition (Miyazaki et al., 2015).
2.3 RNA isolation
Total RNA from NP tissues and cultured NP cells was isolated using TRIzol reagent (Life Technologies), further purified (RNeasy column; Qiagen), and the RNA integrity number (RIN) was assessed (Agilent Bioanalyzer). All RIN values were greater than seven, and 260:280 ratios (measured by NanoDrop ND-1000 Spectrophotometer) were greater than 1.7. Subsequently, RNA was eluted in 50 μl of nuclease-free water, and stored at −80°C for further analysis.
2.4 Reverse-transcription (RT) and real-time quantitative polymerase chain reaction (qPCR)
Complementary DNA (cDNA) was produced using the ExScript RT Reagent Kit (Takara) or the TaqMan MicroRNA Reverse Transcript Kit (Applied Biosystems). TaqMan probes were obtained from Applied Biosystems. The cDNA samples were subjected to real-time PCR using SYBR Premix Ex Taq (Takara). TaqMan miRNA and LncRNA Assays were used for RT-qPCR analyses (Applied Biosystems). Reactions were performed on the 7900 Real-Time PCR System (Applied Biosystems). The miRNA quantification data were normalized to U6 small nuclear RNA (snRNA) expression and lncRNA quantification data were normalized to glyceraldehyde 3-phosphate dehydrogenase. The cycle threshold (Ct) values were collected and normalized to the level of the housekeeping gene. Relative expression was calculated using the comparative threshold cycle method.
2.5 Cell death assay
Cell death was determined by trypan blue exclusion, and the numbers of trypan blue-positive and trypan blue-negative cells were counted on a haemocytometer.
2.6 Transmission electron microscopy
NP cells were fixed with 2.5% glutaraldehyde and then postfixed with 1% osmium tetraoxide, dehydrated in a graded series of ethanol concentrations and embedded in EMbed812 resin. The ultrathin sections were mounted on copper grids and then double-stained with uranyl acetate and lead citrate. The number of autophagic vacuoles was determined for a minimum of 100 cells. The samples were examined and photographed with a FEI Tecnai spirit transmission electron microscope.
2.7 Adenoviral constructions and infection
To produce mutated LINC00641 (LINC00641-mut) and mutated ATG5 (ATG5-mut), the mutations were generated using QuikChange II XL Site-Directed Mutagenesis Kit (Stratagene). The adenoviruses harboring the LINC00641, LINC00641-mut, ATG5-mut, and β-galactosidase (β-gal) were constructed using the Adeno-XTM expression system (Clontech). The LINC00641 RNA interference (siRNA) target sequence is 5′-GATTCTGACTACGTCAATC-3′. A scramble form was used as a control, 5′-ATGTACTCTATCTAGCACT-3′. The ATG5 RNA interference (siRNA) target sequence is 5′-CTGTCACAATGGTGGTTGA-3′. A scramble form was used as a control, 5′-TTGACTCAGATCAGGTTAG-3′. The adenoviruses harboring LINC00641 siRNA, ATG5 siRNA, and their scramble forms were constructed using the pSilencer adeno 1.0-CMV System (Ambion) according to the kit’s instructions. All constructs were amplified in HEK293 cells.
2.8 Luciferase reporter assay
The primers were used as followed: wt-ATG5, forward, 5′-GTCGCTCTAGAACTCGCACATTCACAGT-3′ and reverse, 5′-GTAGCGGCCGCATCATTAGCAGTTACGG-3′. To produce mutated 3′-UTR, the mutations were generated using QuikChange II XL Site-Directed Mutagenesis Kit (Stratagene). Wild type and mutated 3′-UTRs were subcloned into the pGL3 vector (Promega) immediately downstream the coding region of luciferase gene. A 3′-UTR of ATG5 construct with a mutant seed sequence of miR-153-3p was also generated using the primers: mut-ATG5, forward, 5′-ATCCGAGGTTCCTGAGAATAGAATC-3′, and reverse, 5′-TGTGTTGTAGCTCAGTGTGCATACG-3′. LINC00641 wild-type (LINC00641-wt) and the mutant derivative devoid of miR-153-3p-binding site (LINC00641-mut) were cloned downstream the coding region of luciferase gene. The forward primer was 5′-GTAACTCTATGTACAACGTTAA-3′; the reverse primer was 5′-TAGAAGTCAACTCATTATGCTGCTG-3′. All constructs were verified by DNA sequencing. HEK293 cells or NP cells were seeded in 48-well plates (2 × 103 cells per well). After 24-hr incubation, Lipofectamine 2000 (Invitrogen) were used to transfected indicated cells according to the manufacturer’s protocol. Forty-eight hours later, the Dual Luciferase Reporter Assay System (Promega) Luciferase activity was used to measure firefly luciferase activity. All experiments were performed in triplicate and repeated three times independently.
2.9 Western blot analysis
Total protein was extracted from frozen NP tissues stored at −80°C and cultured cells. Samples were homogenized in triton lysis buffer supplemented with protease inhibitors (Roche) and centrifuged at 13,000 r.c.f. for 15 min. The supernatants containing protein extracts were used in subsequent biochemical analysis. Protein concentration was measured by Pierce BCA Protein Assay Kit (Thermo Fisher Scientific, Waltham, MA). Equal amount of total protein lysates were separated by 8 or 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred onto a polyvinylidene difluoride membrane (Millipore, Darmstadt, Germany). Blots were probed using the primary antibodies. The anti-ATG5 antibody (1:500; Abcam), anti-β-actin (1:500; Abcam) and anti-LC3 antibody (1:500; Abcam) were used in this study. Horseradish peroxidase-conjugated anti-rabbit (Amersham ECL; GE Healthcare, Milwaukee, WI) was used as secondary antibody. Protein bands were detected using an EzWestLumi ECL solution (ATTO Corporation, Tokyo, Japan) as per the manufacturer’s specifications (Ez-Capture II; ATTO Corporation). Densities of protein bands were measured using CS Analyzer software (Version 3.00.1011; ATTO & Rise Corporation).
2.10 Immunofluorescence
Coverslips were placed into 24-well plate, where NP cells were plated for 48 hr. Medium was removed and the cells were washed twice with PBS and fixed with 3.5% formaldehyde for 30 min at 37°C. The cells were rinsed with PBS for three times, permeabilized with 0.1% (vol/vol) Triton X-100 in PBS for 20 min and blocked with 3% (wt/vol) bovine serum albumin (BSA) and 0.05% (vol/vol) Tween 20 in PBS for 30 min at room temperature. After blocking, cells were incubated overnight at 4°C with primary antibody (PBS used as control), rabbit monoclonal anti-type II collagen (1:5,000; Abcam) and rabbit anti-matrix metalloproteinase-3 (anti-MMP3) antibody (1:3,000; Abcam). The cells were then treated with fluorescent anti-rabbit secondary antibody (1:500; Abcam) for 2 hr at room temperature. Nuclei were stained with 4,6-diamidino-2-phenylindole (DAPI). Fluorescence images were acquired with a Leica TCS SP2 confocal microscopy (Leica, Mannheim, Germany) using the Leica Confocal Software.
For ATG5 immunostaining, 4.5 × 104 cells were plated on coverslips placed in a 12-well plate and cultured for a total of 48 hr before harvesting. Cells were washed in PBS, fixed with 4% paraformaldehyde at 4°C, blocked and incubated overnight with rabbit anti-ATG5 antibody at 4°C (1:50). Cells were then incubated 2 hr with Alexa Fluor 546 goat anti-rabbit IgG antibody (1:200). For LC3 immunostaining, in the shorter lysosomal inhibition experiments, cells were plated on coverslips as above for ATG5 and exposed to the indicated inhibitors for the last 4 hr before harvesting; in the longer lysosomal inhibition experiments, cells were plated on 60-mm Petri dishes, exposed to the indicated inhibitors for the last 8 hr, and then trypsinized and cytospun onto glass slides (2 × 104 cells per slide). Cells on coverslips or on slides were then washed in PBS, fixed with cold methanol, blocked and incubated overnight with rabbit anti-LC3 antibody at 4°C (1:50). Cells were then incubated 2 hr with Alexa Fluor 546 goat anti-rabbit IgG antibody (1:200).
2.11 Transfection of inhibitor and mimic
MiR-153-3p inhibitor, inhibitor control, miR-153-3p mimic, and mimic control were purchased from GenePharma Co. Ltd. Cells were transfected with the mimic or inhibitor at 50 nM. The transfection was performed using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instruction.
2.12 In situ hybridization
Tissue microarray for NP tissues were used for miR-153-3p quantification. The formalin-fixed paraffin-embedded tissue sections were dewaxed in xylenes, and rehydrated through an ethanol dilution series. The tissue sections were digested with 15 μg/ml proteinase K for 20 min at room temperature, then loaded onto Ventana Discovery Ultra for in situ hybridization analysis. The tissue slides were incubated with double-DIG-labeled mercury LNA miR-153-3p probe (Exiqon) for 2 hr at 52°C. The double-digoxigenin (DIG)-labeled control U6 snRNA probe (Exiqon) was used as a positive control. The DIGs were then detected with a polyclonal anti-DIG antibody and alkaline phosphatase-conjugated second antibody (Ventana) using nitro blue tetrazolium-5-bromo-4-chloro-3-indolyl-phosphate (NBT-BCIP) as the substrate. Representative light field images were obtained using a Nikon Microphot-FXA microscope and Leica DFC320 digital camera. The expression levels of miR-153-3p were determined using CellProfiler 2.0 software to establish the staining intensity threshold levels and to quantify the number of positively stained cells per high-power field.
2.13 RNA fluorescent in situ hybridization (FISH)
Sequential protein staining and RNA detection were performed as previously described (H. Wang et al., 2017). In brief, RNA-FISH was performed by using a nick-translated probe (Roche) followed by a tyramide signal amplification kit (Life Technologies, Woburn, MA). After RNA-FISH, the cells were treated by RNase A and denatured. Nick-translated BAC containing LINC00641 was labeled as a DNA probe.
2.14 Target protector preparation and transfection
Target protector was designed to disrupt the interaction of specific miRNA–mRNA pairs. Target protector sequence is complementary to miR-153-3p-binding site in target ATG5. In brief, ATG5-TPmiR-153-3p sequence is 5′-TCACGCTACTTCGGAGACATTCATG-3′. ATG5-TPcontrol sequence is 5′-ATACGGAGCACAGGCACTTGCTAAC-3′. They were synthesized by Gene Tools, and transfected into the cells using the EndoPorter Kit (Gene Tools) according to the kit’s instructions.
2.15 Northern blot analysis
The cell samples were collected and run on a 15% polyacrylamide-urea gel, transferred to positively charged nylon membranes (Millipore) followed by cross-linking through UV irradiation. The membranes were subjected to hybridization with 100 pmol 3′-DIG-labeled probes overnight at 42 °C. Probes were labeled with DIG using a 3′-End DIG Labelling Kit (Roche). The detection was performed using a DIG luminescent detection kit (MyLab) according to the manufacturer’s instructions. The probe sequence for miR-153-3p was 5′-CTCATACGCTGTACGTGAGAT-3′. U6 was used as an internal control, and its probe sequence was 5′-AGTACTCTTCTCTGTATCGATCG-3′.
2.16 Pull-down assay with biotinylated DNA probe
The biotinylated DNA probe complementary to LINC00641 was synthesized and dissolved in 500 ml of wash/binding buffer (0.5 M NaCl, 20 mM Tris-HCl, pH 7.5, and 1 mM ethylenediaminetetraacetic acid [EDTA]). The probes were incubated with streptavidin-coated magnetic beads (Sigma) at 25°C for 2 hr to generate probe-coated magnetic beads. NP cells lysates were incubated with probe-coated beads, and after washing with the wash/binding buffer, the RNA complexes bound to the beads were eluted and extracted for northern blot analysis; LINC00641 pull-down probe, 5′-CATGTAGTATAGCTGAACTCTAGACAG-3′; and random pull-down probe, 5′-CTATGTCGAGCGCCTGTGCTATA-3′.
2.17 Pull-down assay with biotinylated miRNA
NP cells were transfected with biotinylated miRNA (50 nM), harvested 48 hr after transfection. The cells were washed with PBS followed by brief vortex, and incubated in a lysis buffer on ice for 10 min. The lysates were precleared by centrifugation, and 50 ml of the sample was aliquoted for input. The remaining lysates were incubated with M-280 streptavidin magnetic beads (Sigma). To prevent nonspecific binding of RNA and protein complexes, the beads were coated with RNase-free BSA and yeast tRNA (both from Sigma). The beads were incubated at 4°C for 3 hr, washed twice with ice-cold lysis buffer, three times with the low salt buffer (0.1% SDS, 1%Trition X-100, 2 mM EDTA, 20 mM Tris-HCl, pH 8.0, and 150 mM NaCl) and once with the high salt buffer (0.1% SDS, 1% Trition X-100, 2 mM EDTA, 20 mM Tris-HCl, pH 8.0, and 500 mM NaCl). The bound RNAs were purified by TRIzol for the analysis.
2.18 Statistical analysis
Data are expressed as the mean ± SEM of at least three independent experiments for each cellular experimental group. All statistical analysis was performed using the Student’s t test, analysis of variance test or Mann–Whitney test U test, as indicated in figure legends. Analyses were performed with GraphPad Prism (version 5; GraphPad Software, Inc., La Jolla, CA). A p < 0.05 was considered statistically significant.
3 RESULTS
3.1 Downregulation of miR-153-3p in NP tissues
In previous studies using Solexa sequencing and locked nucleic acid (LNA)-based miRNA microarrays (Ji, Lu, et al., 2016; Ji, Zhang, et al., 2016), we found that miR-153-3p was significantly downregulated (fold change <4) in NP tissues from IDD patients compared with controls (Figure 1a–c). To validate this finding, RT-qPCR with additional NP tissues (62 IDD vs. 59 controls) was performed. In agreement with the sequencing and array data, the level of miR-153-3p is downregulated in degenerative NP tissues (Figure 1d), which is further confirmed by ISH staining (Figure 1e). The disc degeneration grade was positively correlated with miR-153-3p level (r = 0.892; p < 0.001; Figure 1f).

Dysregulation of miRNA in human NP tissues. (a) Heat map diagram generated by unsupervised clustering analysis with 23 significantly dysregulated miRNAs in human NP tissues. Yellow represents higher and blue represents lower expression relative to the mean intensity value (black) across all samples (paired t test). Hierarchical clustering was performed with average linkage and uncentered correlation. miRNA expression profile effectively segregated human NP samples from controls. (b) Volcano plot illustrating the biological and statistical significances of differential miRNA expressions between human NP tissues and controls. miRNAs that were significantly underexpressed in IDD are highlighted in blue. In contrast, miRNAs that were significantly upregulated in IDD are marked in red (Benjamini–Hochberg-corrected p). (c) Scatter-plot of miRNA expression in IDD. (d) Compared with controls, miR-153-3p expression level is lower in IDD patients. **p < 0.01 by the Mann–Whitney U test, purple, IDD; green, NC. (e) ISH analysis further show that miR-153-3p is downregulated in NP tissues from IDD patients. (f) The disc degeneration grade was positively correlated with miR-153-3p level (r = 0.892, p < 0.001). IDD: intervertebral disc degeneration; ISH: in situ hybridization; miRNA: microRNA; NC, normal controls; NP: nucleus pulposus [Color figure can be viewed at wileyonlinelibrary.com]
3.2 Identification of ATG5 as a target gene for miR-153-3p
Based on the miRanda, TargetScan, PicTar, and PITA, ATG5 was identified as a putative target of miR-153-3p, which contains one putative target seed sequence (Figure 2a,b). To identify whether miR-153-3p is involved in the regulation of ATG5, we used immunoblot to detect ATG5 levels and the result indicated that miR-153-3p significantly reduced the levels of ATG5 (Figure 2c). We then investigated whether knockdown of miR-153-3p could alter the expression of endogenous ATG5. Our results shows that miR-153-3p knockdown induced an increase in ATG5 expression (Figure 2d). N/LN, which had been reported to induce autophagy (Miyazaki et al., 2015), resulted in an increase in ATG5 expression, while enforced expression of miR-153-3p inhibited the increase of ATG5 expression level on N/LN (Figure 2e). To understand whether the effect of miR-153-3p on ATG5 was specific, we then used the target protector technology. The inhibitory effect of miR-153-3p on ATG5 expression was reduced in the presence of the target protector (Figure 2f). ATG5 upregulation was attenuated by the target protector in response to N/LN (Figure 2g). Further, the luciferase assay system was used to determine whether N/LN could influence the translation of ATG5. As shown in Figure 2h, the luciferase reporter assay revealed that the wild type 3′-UTR of ATG5 exhibited a low translation level in the presence of miR-153-3p, whereas the mutated 3′-UTR did not show a significant response to miR-153-3p. The disc degeneration grade was negatively correlated with ATG5 expression level (r = −0.903; p < 0.001; Figure 2i). Taken together, these data suggest that ATG5 is a specific target of miR-153-3p.
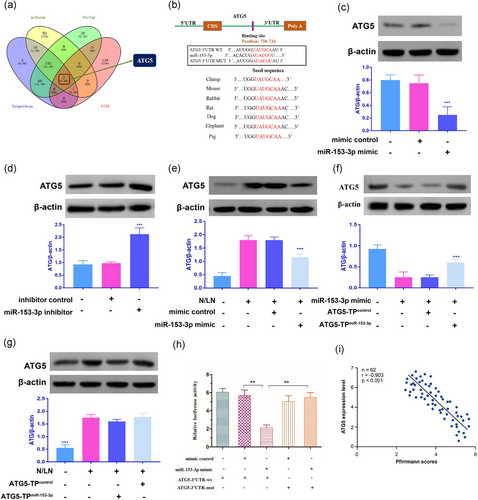
Identification of ATG5 as a target of miR-153-3p. (a) Venn diagram displaying miR-153-3p computationally predicted to target ATG5 by different algorithms. (b) Putative miR-153-3p-binding site in the 3′-UTR region of ATG5. (c) miR-153-3p suppresses the expression of ATG5. NP cells were transfected with miR-153-3p mimic or mimic control (n = 3). (d) Knockdown of miR-153-3p increases the expression levels of ATG5. NP cells were transfected with miR-153-3p inhibitor or inhibitor control (n = 3). (e) miR-153-3p decreases ATG5 expression levels on N/LN. NP cells were transfected with miR-153-3p mimic or the mimic control, and then exposed to N/LN (n = 3). (f) ATG5 target protector attenuates the reduction of ATG5 induced by miR-153-3p (n = 3). (g) ATG5 target protector inhibits N/LN-induced ATG5 upregulation. NP cells were transfected with the ATG5-TPmiR-153-3p or ATG5-TPcontrol, and then exposed to N/LN (n = 3). (h) MiR-153-3p suppresses ATG5 translation. HEK293 cells were transfected with miR-153-3p mimic, mimic control, the luciferase constructs of the wild-type ATG5-3′-UTR (ATG5-3′-UTR-wt) or a mutated ATG5-3′-UTR (ATG5-3′-UTR-mut). NP cells were exposed first to N and then to LN conditions. NP cells were exposed to N for 3 days, and then were exposed to LN for 3 days. Data are shown as mean ± SEM of three independent experiments. **p < 0.01 in one-way analysis of variance. (i) The disc degeneration grade was negatively correlated with ATG5 expression level (r = −0.903, p < 0.001). ATG5: autophagy-related gene 5; HEK293: human embryonic kidney 293; miR: microRNA; N/LN: normal/low nutritional; NP: nucleus pulposus; 3′-UTR: 3′-untranslated region [Color figure can be viewed at wileyonlinelibrary.com]
3.3 MiR-153-3p inhibits autophagy and cell death
We observed that N/LN reduced the levels of miR-153-3p (Figure 3a). LC3-II relocalizes to the autophagosomal membranes during autophagy. Thus, the accumulation of green fluorescent protein-light chain 3 (GFP-LC3) puncta provides an effective way to detect autophagosomes. We found that N/LN induced an increased GFP-LC3 punctuate structures, whereas enforced expression of miR-153-3p significantly reduced punctate accumulations of GFP-LC3 (Figure 3b). In addition, miR-153-3p also induced a reduction in LC3-II expression levels on N/LN treatment (Figure 3c). Also, enforced expression of miR-153-3p significantly increased cell death induced by N/LN (Figure 3d). Taken together, these results suggest that miR-153-3p inhibits autophagy and promotes cell death induced by N/LN in NP cells. Additionally, serum starvation may be a normal condition for the intervertebral disc (IVD).

MiR-153-3p inhibits autophagy under the pathological condition. (a) NP cells were exposed to N/LN and miR-153-3p levels were analyzed by RT-qPCR. Data are shown as mean ± SEM of four independent experiments. **p < 0.01 versus control in the Student’s t test. (b) miR-153-3p inhibits punctate accumulations of GFP-LC3 induced by N/LN. The percentage of cells with GFP-LC3 puncta was quantified. Data are shown as mean ± SEM of three independent experiments. **p < 0.01 versus N/LN alone in one-way analysis of variance (scale bar = 20 μm). (c) Representative immunoblot for conversion of LC3-I to LC3-II. The densitometric analysis of western blot analysis of LC3-II is shown (**p < 0.01). (d) Quantfication of cell death is shown. Data are shown as mean ± SEM of three independent experiments. **p < 0.01 versus N/LN alone in one-way analysis of variance. GFP: green fluorescent protein; LC3: light chain 3; miR: microRNA; N/LN: normal/low nutritional; NP: nucleus pulposus; RT-qPCR: quantitative reverse-transcription polymerase chain reaction [Color figure can be viewed at wileyonlinelibrary.com]
3.4 LINC00641 is able to directly bind to miR-153-3p
We analyzed the underlying mechanism responsible for miR-153-3p downregulation in response to N/LN treatment. Recent studies have shown that lncRNAs may act as competing endogenous RNA (ceRNA) sponge to interact with miRNAs and influence the expression of miRNA (Cesana et al., 2004). This encourages us to explored whether lncRNAs can also function as miR-153-3p sponge to regulate IDD. We screened lncRNAs profile in NP tissues from a result of lncRNA array performed by Affymetrix Company. We then carried out RT-qPCR to detect lncRNAs levels on N/LN treatment. Among those lncRNAs, only LINC00641 was significantly upregulated on N/LN treatment (Figure 4a). The full-length of LINC00641 is poorly conserved across species (Figure 4b), but it is conserved between species in the binding site of miR-153-3p. Using RNA hybrid, we noticed that LINC00641 contained a binding site of miR-153-3p (Figure 4c). Next, we produced a luciferase construct of LINC00641 (Luc-LINC00641-wt) and a mutated form (Luc-LINC00641-mut). Luciferase assay showed that miR-153-3p could suppress the luciferase activity of LINC00641, but it had less effect on the mutated form of LINC00641 (Figure 4d), indicating that LINC00641 may interact with miR-153-3p by this putative binding site. Further, we investigated whether LINC00641 could directly bind to miR-153-3p in vivo. We applied a biotin–avidin pull-down system to test whether miR-153-3p could pull-down LINC00641. NP cells were transfected with biotinylated miR-153-3p, then harvested for biotin-based pull-down assay. LINC00641 was pulled down and analysed by RT-qPCR. The introduction of mutations that disrupt base pairing between LINC00641 and miR-153-3p led to the inability of miR-153-3p to pull-down LINC00641 (Figure 4e), indicating that miR-153-3p recognizes LINC00641 in a sequence-specific manner. We also used inverse pull-down assay to test whether LINC00641 could pull-down miR-153-3p using biotin-labeled-specific LINC00641 probe. MiR-153-3p was coprecipitated and analysed by northern blot (Figure 4f). Further, we detected the subcellular location of LINC00641 and miR-153-3p. FISH revealed that there is a colocalization between LINC00641 and miR-153-3p in cytoplasm (Figure 4g). Finally, we tested whether LINC00641 could affect the activity of miR-153-3p. The luciferase activity analysis showed that enforced expression of LINC00641 (Figure 4h) counteracted the inhibitory effect of miR-153-3p (Figure 4i), whereas the mutant LINC00641 had no effect on miR-153-3p (Supporting information Figure 1), suggesting that LINC00641 inhibited the activity of miR-153-3p. Taken together, these data suggest that LINC00641 is able to directly bind to miR-153-3p and regulate its activity.
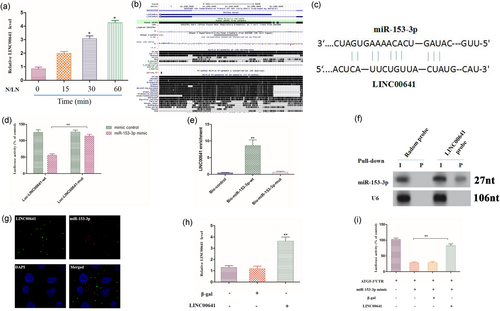
The interaction between LINC00641 and miR-153-3p. (a) LINC00641 expression levels on treatment with N/LN, which was analyzed by RT-qPCR. Data are shown as mean ± SEM of three independent experiments. *p < 0.05 versus control in Student’s t test. (b) LINC00641 is a poorly conserved lncRNA. Graphical views showing multi-species comparisons of LINC00641 using UCSC genome browser. (c) LINC00641 contains a site complementary to miR-153-3p. (d) HEK293 cells were transfected with miR-153-3p mimic or mimic control, then transfected with the luciferase constructs of Luc-LINC00641-wt or Luc-LINC00641-mut. The luciferase activity was analyzed. Data are shown as mean ± SEM of three independent experiments. **p < 0.01 in one-way analysis of variance. (e) miR-153-3p can bind directly to LINC00641 in vivo. Forty-eight hours after transfection, cells were harvested for biotin-based pull-down assay. Data are shown as mean ± SEM of four independent experiments. **p < 0.01 versus Bio-NC in one-way analysis of variance. (f) LINC00641 can bind to miR-153-3p in vivo. NP cells were incubated with LINC00641 probe or random probe-coated magnetic bead. RNA was analyzed by northern blot analysis. I: input (10% samples were loaded); P: pellet (100% samples were loaded). (g) FISH show that there is a colocalization between LINC00641 and miR-153-3p in cytoplasm. (h) Enforced expression of LINC00641 increases the expression levels of LINC00641. Data are shown as mean ± SEM of three independent experiments. **p < 0.01 versus control in one-way analysis of variance. (i) LINC00641 inhibits miR-153-3p activity. Data are shown as mean ± SEM of four independent experiments. **p < 0.01 in one-way analysis of variance. FISH: fluorescent in situ hybridization; HEK293: human embryonic kidney 293; LINC00641: long intergenic nonprotein coding RNA 641; lncRNA: long noncoding RNA; miR: microRNA; N/LN: normal/low nutritional; NC, normal controls; NP: nucleus pulposus; RT-qPCR: quantitative reverse-transcription polymerase chain reaction [Color figure can be viewed at wileyonlinelibrary.com]
3.5 LINC00641 conveys the autophagic signal in NP cells
We investigated whether LINC00641 played a functional role in autophagy. In NP cells knockdown of LINC00641 by an siRNA technology effectively reduced the expression of LINC00641 (Figure 5a). Further, knockdown of LINC00641 reduced LC3-II levels (Figure 5b) and LC3-II punctate accumulations of GFP-LC3 (Figure 5c–e) on N/LN treatment. Also, knockdown of LINC00641 reduced autophagic vesicles (Figure 5f) and increased cell death (Figure 5g) on N/LN treatment. Additionally, LINC00641 promotes collagen II expression, while inhibits MMP3 (Figure 5h,i). These results suggest that LINC00641 is involved in the regulation of autophagic pathway in NP cells and IDD.
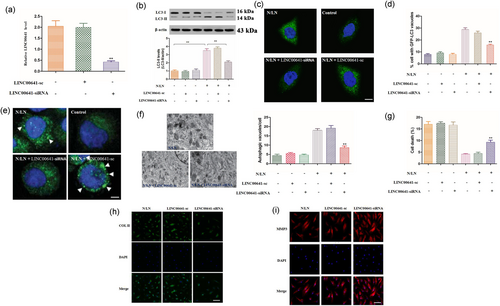
LINC00641 conveys the autophagic signal in NP cells. (a) Knockdown of LINC00641 by an siRNA technique decreases the expression levels of LINC00641. Data are shown as mean ± SEM of three independent experiments. **p < 0.01 versus control in one-way analysis of variance. (b) Knockdown of LINC00641 inhibits the conversion of LC3-I to LC3-II in response to N/LN. The densitometric analysis of western blot analysis of LC3-II is shown (**p < 0.01). (c, d) Knockdown of LINC00641 inhibits punctate accumulations of GFP-LC3. Representative images show GFP-LC3 staining (c; scale bar=20 μm). The percentage of cells with GFP-LC3 puncta was quantified in (d). Data are shown as mean ± SEM of four independent experiments. **p < 0.01 versus N/LN alone in one-way analysis of variance. (e) ATG5 puncta reflect the autophagic flux in three dimensional. (f, g) Knockdown of LINC00641 reduces autophagic vacuoles and promotes cell death. Quantification of autophagic vacuoles was shown in (f). Quantification of cell death was shown in (g; scale bar = 500 nm). Data are shown as mean ± SEM of four independent experiments. **p < 0.01 versus N/LN alone in one-way analysis of variance. (h, i) LINC00641 can affect collagen II and MMP3 expressions. The representative collagen II and MMP3 were detected by the immunofluorescence (collagen II, scale bar = 50μm; MMP3, scale bar = 25 μm). ATG5: autophagy-related gene 5; DAPI: 4,6-diamidino-2-phenylindole; GFP: green fluorescent protein; LC3: light chain 3; LINC00641: long intergenic nonprotein coding RNA 641; MP3: matrix metalloproteinase-3; N/LN: normal/low nutritional; NP: nucleus pulposus; siRNA: small interfering RNA [Color figure can be viewed at wileyonlinelibrary.com]
3.6 LINC00641 regulates autophagy through miR-153-3p and ATG5
Since LINC00641 interacted with miR-153-3p, we then tested whether LINC00641 was able to regulate ATG5, which was the downstream target of miR-153-3p. Knockdown of LINC00641 reduced ATG5 expression (Figure 6a). Overexpression of LINC00641 resulted in an increase in ATG5 expression levels (Figure 6b). LINC00641 but not LINC00641-mut counteracted the inhibitory effect of miR-153-3p on ATG5 expression (Figure 6c and Supporting information Figure 2A). These results indicate that LINC00641 regulates ATG5 expression through miR-153-3p. Then, we further tested whether ATG5 is a mediator of LINC00641. To confirm the relationship between LINC00641 and ATG5 in autophagic machinery, we knocked down the endogenous LINC00641 by LINC00641-siRNA construct (Supporting Information Figure 2B), and observed that the inhibitory effect of LINC00641 knockdown on ATG5 levels and autophagy (Figure 6d) was decreased in the presence of ATG5 target protector. The disc degeneration grade was negatively correlated with LINC00641 (r = −0.871; p < 0.001; Figure 6e). Taken together, these data suggest that LINC00641 targets miR-153-3p/ATG5 axis in the autophagic cascades (Figure 6f).
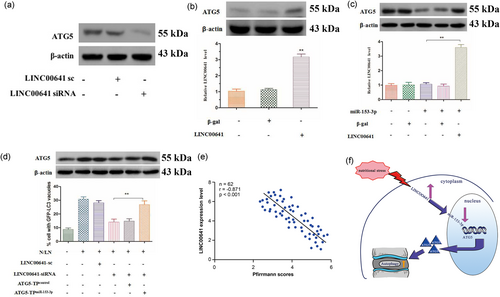
LINC00641 exerts its autophagic effect through miR-153-3p and ATG5. (a) Knockdown of LINC00641 reduces the expression levels of ATG5 (n = 3). (b) Enforced expression of LINC00641 induces the increase of ATG5 levels (n = 3). Data are shown as mean ± SEM of three independent experiments. **p < 0.01 versus control in one-way analysis of variance. (c) LINC00641 reduces the inhibitory effect of miR-153-3p on ATG5 expression. ATG5 expression levels were analyzed by immunoblot (n = 4). LINC00641 levels were analyzed by RT-qPCR. Data are shown as mean ± SEM of four independent experiments. *p < 0.01 in one-way analysis of variance. (d) ATG5 target protector attenuates the inhibitory effect of LINC00641 knockdown on autophagy induced by N/LN. ATG5 levels were analyzed by immunoblot (n = 3). Autophagy was assessed by GFP-LC3 staining. The percentage of cells with GFP-LC3 puncta was quantified. Data are shown as mean ± SEM of three independent experiments. **p < 0.01 in one-way analysis of variance. (e) The disc degeneration grade was negatively correlated with LINC00641 (r = −0.871; p < 0.001). (f) Schematic summary of the major findings of this study. LINC00641 regulates autophagy and IDD by targeting miR-153-3p. ATG5: autophagy-related gene 5; GFP: green fluorescent protein; LC3: light chain 3; LINC00641: long intergenic nonprotein coding RNA 641; miR: microRNA; N/LN: normal/low nutritional; RT-qPCR: quantitative reverse-transcription polymerase chain reaction [Color figure can be viewed at wileyonlinelibrary.com]
4 DISCUSSION
Increased level of autophagy has been observed in IVD of aged rats and NP cells from degenerative human discs (Chen et al., 2016; Gruber et al., 2015; Ma et al., 2013; Miyazaki et al., 2015). Various stresses such as high glucose, aberrant mechanical compression, lactate overload, glucosamine, reactive oxygen species (ROS), and nutrient deprivation, activate autophagy in NP cells, further implicating autophagy modulation as a possible contributor to disc pathologies (Chen et al., 2016). The IVD is the largest avascular tissue in the body, and maintenance of an adequate nutrient supply has long been regarded as essential for preventing disc degeneration (Urban et al., 2006). With aging and degeneration, NP tissue undergoes structural changes including gradual loss and degradation of proteoglycan-rich extracellular matrix, and ingrowth of neural and vascular structure along fissures in the outer AF, which in conjunction with sclerosis of the subchondral bone or endplate calcification can block transport from blood supply to the disc (Grunhagen et al., 2016). Given its physiological and pathological characteristics, NP cells were exposed to N/LN to mimic nutritional stress in this study. Our data support this idea and provide the direct evidence that autophagy prevents IDD in physiological setting. It is principally thought that autophagy plays an adaptive role in protecting NP cells from N/LN injury. Upregulation of autophagy in NP cells is also considered to be an adaptive response to stress, while suppressing the process accelerates IDD.
A growing body of evidence indicates that miRNAs-mediated regulation of gene expression represents an integral part of the autophagy regulatory network and may have a substantial effect on autophagy-related physiological and pathological conditions (D’Adamo et al., 2016; Frankel et al., 2014; Ouimet et al., 2014; K. Wang et al., 2015; Zhu et al., 2009). However, no studies have identified the regulation mechanism of these miRNAs in NP cells autophagy. Our findings suggest that miR-153-3p is involved in the development of IDD. Further, miR-153-3p can inhibit autophagic programme through targeting ATG5. MiR-153-3p represents a novel mechanism for regulating ATG5 expression and therefore autophagy. Accordingly, knowledge gained from the miR-153-3p study could be translatable to humans, providing a potential clinical relevance.
Several lncRNAs from different transcriptional elements of the genome, for example, the 3′-UTR and coding region, are involved in the regulation of the autolysosome pathway (Ge et al., 2002; K. Wang et al., 2015). Our study showed that LINC00641 is an autophagy-related lncRNA derived from intergenic regions in the genome, implying that autophagy associated lncRNAs could be derived from 3′-UTR, coding region of genes and also long intergenic. However, lncRNAs from other regions, introns or promoter, remain to be investigated. Further studies focusing on this aspect are required. Although a set of lncRNAs have been reported to be ceRNAs, none were reported to have a role in IDD through autophagic pathways. This study is the first to show that LINC00641, a natural sponge for miR-153-3p, that targets ATG5 by an miRNA-mediated silencing pathway and controlled the level of autophagy.
Unfortunately, we were unable to investigate the role of LINC00641 in autophagy and IDD development in vivo. In our preliminary study, we established nicotine-induced IDD (Iwahashi et al., 2015) and sub-endplate microcirculation disturbance (intravenous injection of endotoxin and corticosteroid) rabbit models (Ou-Yang & Lu, 2008) to mimic human IDD induced by nutrient stress. However, our results (unpublished) show that the patterns of disc degeneration in these two models are significantly different from that of human disc degeneration, and the degree of IDD is very low. We speculate that the IDD models by the two methods may have minimal effect on disc microenviroment. Future studies focusing on IDD model established by local blood vessel block may be more helpful in analyzing IDD induced by nutrient loss. In the study by Miyazaki et al. (2015), the authors defined NP cells cultured in DMEM supplemented with 1% (vol/vol) FBS as LN condition and 10% (vol/vol) FBS as the N condition. The culture conditions may not reflect the in vivo situation. In addition, a low serum environment of 1% (vol/vol) FBS supplementation as a condition of nutrient deprivation because serum interacts with other nutrients and plays a major role in NP cells (Johnson et al., 2008). It remains to be elucidated whether this serum concentration is an acceptably low nutritional condition for NP cells. These should be confirmed in vivo.
In conclusion, we demonstrated that LINC00641 acts as endogenous sponge RNA and inhibits miR-153-3p expression and its biological function. Therefore, modulation of LINC00641 may represent a novel treatment paradigm in human IDD. These findings shed new light on the importance of the orchestrated interactions between lncRNAs, miRNAs and autophagy in maintaining cellular homeostasis and suggest that a disturbance in these networks might lead to diseases. Nutrition intervention for malnourished IVD should be a right strategy to prevent IDD.
Acknowledgements
We thank all donors enrolled in the current study. This study has received support from the fund of Jiangsu Provincial Medical Youth Talent (QNRC2016801), China Postdoctoral Science Foundation funded project (2016M591929, 2017T100408), and Jiangsu Postdoctoral Science Foundation funded project (1601193C).
CONFLICTS OF INTEREST
The authors declare that there are no conflicts of interest.




