Molecular interaction of NFκB and NICD in monocyte–macrophage differentiation is a target for intervention in atherosclerosis
Abstract
The activation of two transcription factors, NFκB and NICD (notch intracellular domain), plays a crucial role in different stages of atherosclerotic disease progression, from early endothelial activation by modified lipids like oxidized low-density lipoprotein (oxyLDL) to the imminent rupture of the atherosclerotic plaque. Inflammatory mediators and the notch pathway proteins were upregulated in atherogenic diet-induced rats and the same was confirmed by the differentiation of monocyte to macrophage on exposure to oxyLDL. The inflammatory transcription factor NFκB and the notch signaling transcription factor NICD were analysed for their molecular interaction in monocyte to macrophage differentiation. Inhibition of NFκB by dexamethasone in monocyte to macrophage differentiation resulted in a concomitant downregulation of NICD, whereas inhibition of NICD by N-(N-[3, 5-difluorophenacetyl])-l-alanyl)-S-phenylglycine t-butyl ester (DAPT), a γ–secretase inhibitor, did not significantly influence the expression of NFκB, but downregulated macrophage differentiation. These findings revealed that NFκB inhibition using dexamethasone regulated NICD, which turned down macrophage differentiation. Thus, inhibition of both NFκB–NICD is a potential target for intervention in atherosclerosis.
1 INTRODUCTION
Atherosclerosis is an eclectic process of inflammatory cells which involves multiple steps in disease progression. Inflammatory processes play a key role in different stages of atherosclerosis, from early endothelial activation by modified lipids like oxidized low-density lipoprotein (oxyLDL) to the imminent rupture of the atherosclerotic plaque (Libby, 2002; Lusis, 2000). Monocytes are the primary inflammatory cell types that infiltrate early atherosclerotic plaques. Their recruitment into plaques drives disease progression (Randolph, 2009). NFκB is a crucial transcription factor, which controls the transcription of many genes with established roles in atherosclerosis. NFκB may not only contribute to the different stages in atherosclerosis development, but also contributes to cell type-dependent regulation of various genes (e.g., macrophages vs. in endothelial cells).
Notch signaling is a highly conserved, complex, and juxtacrine pathway. The notch receptor (Notch1–4) and its ligands (Jagged: Jag1 and Jag2, and Delta-like: DLL1, DLL3, and DLL4) are transmembrane proteins. Binding of ligand to notch receptor results in two proteolytic cleavage events in the notch receptor, first by the ADAM-family metalloproteases and the second by γ-secretase, an enzyme complex that contains presenilin, nicastrin, PEN2, and APH1. The second cleavage releases the notch intracellular domain (NICD) into the cytoplasm. NICD then translocates to the nucleus and results in the expression of target genes HES1-HES7 (hairy/enhancer of split) and HES-related (Hey1–2 and HeyL) genes (Kratsios et al., 2010; Quillard & Charreau, 2013).
Many studies suggest that various signaling pathways including AKT, JNK, p38 MAPK, ERK, JAK/STAT, and Notch pathway individually contribute to monocyte–macrophage differentiation, but more recent studies suggest that interaction of different cell signaling pathways are important for disease progression. Fewer studies revealed the interaction of different pathways in monocyte–macrophage differentiation, which forms the basis of this study, where interaction between NFκB (which is an inflammatory mediator) and NICD (which is a cell differentiation factor) was investigated.
2 MATERIALS AND METHODS
2.1 Chemicals and antibodies
Cholesterol (C75209, Sigma, Bangalore, India), cholic acid (C1129, Sigma), Dexamethasone (D4902, Sigma), N-(N-[3, 5-difluorophenacetyl])-l-alanyl)-S-phenylglycine t-butyl ester (DAPT; D5942, Sigma), anti-CD14, anti-NICD (Abcam, Cambridge, UK), anti-MAC387, anti-NFκBp65, anti-Notch1, anti-DLL1, anti-Hes1, anti-Hey1 (SantaCruz Biotechnology, CA), anti-NICD, FITC and TRITC-conjugated secondary antibodies (Abcam, Cambridge, UK), GAPDH (Cell signaling technology, MA), RPMI-1640 (AL028A, Hi Media, PA), fetal bovine serum (FBS; 10270, Gibco, India), anti-anti solution, TRIzol reagent, random hexamer, deoxynucleoside triphosphates (dNTPs), moloney murine leukemia virus (M-MLV) reverse transcriptase enzyme and its buffer, PCR ready master mix (2×), DNA 100 bp ladder, agarose, and bovine serum albumin (BSA) were purchased from GeNeiTM (Bangalore, India).
2.2 Experimental animals and treatments
Male Wistar rats weighing between 180 and 200 g were given standard pellet diet (Hindustan Lever, Bangalore) and water ad libitum for a period of 15 weeks. The animals were housed under adequate temperature (22 ± 1°C) and humidity (55 ± 5%) with a 12 hr light/12 hr dark cycle. The atherogenic diet contained 10% dairy butter, 20% sucrose, 2% cholesterol, and a 0.5% cholic acid. Animal experiments were conducted according to the guidelines of the Institutional Animal Ethics Committee (IAEC No: 39/03/2014).
The rats were divided randomly into two groups of six each. Group I: Normal control rats and Group II: Atherogenic diet (AD)-induced rats. At the end of the experimental period, after overnight fasting, rats were weighed and blood samples were collected by retro orbital bleeding and serum was separated. Then, the rats were euthanized by an excessive dose of ketamine and xylazine, the aorta and heart were dissected out and used for further molecular analysis.
2.3 Assessment of activities of LDH, CK, LPO, and NO
The marker enzymes, lactate dehydrogenase (LDH; Nieland, 1955) and creatine kinase (CK; Hall & DeLuca, 1976) were assayed in serum and in heart homogenate. The malondialdehyde (an end product of fatty acid peroxidation (LPO)) formed was measured as thiobarbituric acid reactive substances (Ohkawa et al., 1979). Nitrite concentration (an indirect measurement of nitric oxide (NO) synthesis) in heart was assayed using a Griess reagent (sulphanilamide and N-(1-Naphthyl) ethylenediamine dihydrochloride) in acidic medium (Marcocci, Maguire, Droylefaix, & Packer, 1994).
2.4 Reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was extracted using a TRIzol reagent and cDNA of each sample was synthesized by the reverse transcription method (Binesh, Devaraj, & Halagowder, 2018). Polymerase chain reaction (PCR) was carried out using the primers listed in the supplementary materials.
2.5 Protein extraction and western blot analysis
Protein extraction from THP-1 cells/aorta was performed by homogenizing in lysis buffer. The homogenate was centrifuged at 8,000 rpm at 4°C for 30 min and the supernatant was collected. Cells/aorta were lysed and the protein content was measured using the Bradford method (Bradford, 1976). Protein extracts (40–50 µg) were analyzed by standard sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to a polyvinylidene fluoride (PVDF) membrane (Amersham Biosciences, GE Healthcare, Bangalore, India) and probed with primary antibodies (details in supplementary materials) in 5% non-fat dry milk powder in Tris-buffered saline 0.01% Tween20), overnight at 4°C. Peroxidase-conjugated corresponding secondary antibodies were used and developed with the enhanced chemiluminescence reagent kit (RPN2135-Amersham, ECL advance, Western blot analysis Detection Kit, Loughborough, UK) as per manufacturer's protocol. Quantification of the western blots was performed using the ImageJ software.
2.6 Isolation of lipoproteins
LDL + VLDL were isolated by the method of Burstein, Scholnick, and Morfin (1970).
2.7 Protein estimation and in vitro oxidation
The Bradford method was used to measure the protein concentration using the Biorad protein assay kit. The estimated lipoproteins (LDL + VLDL) were subjected to in vitro oxidation by adopting the method described by Maggi et al. (1994). Electrophoresis was done in 0.5% agarose gel to determine the electrophoretic mobility of oxidized LDL + VLDL (oxyLDL).
2.8 Grouping of cells
THP-1 cells were obtained from National Centre for Cell Science (NCCS), Pune, India. The cells were categorized into two groups (Control and Induced). The “control” contained THP -1 cells alone and “induced” contained cells that were induced with oxyLDL at 15 μg/ml. All the experimentally grouped cells were maintained for 7 days under 5% CO2 atmosphere, 37°C and 70% humidity.
2.9 Immunofluorescence analysis
Immunofluorescence analysis was used to detect the localization of NFκBp65, NICD, the expression of monocyte cell surface marker CD14 and macrophage surface marker MAC387. The cells were incubated with required primary antibody (1:200) and with FITC (490/525 nm) conjugated secondary antibodies (1:400). The cells/tissues were counter stained with propidium iodide (PI) or 4′,6-diamidino-2-phenylindole, dihydrochloride (DAPI) to visualize nucleus. Images were observed and visualized under an Axioskope 2d microscope (Carl Zeiss, Germany).
2.10 Inhibition of NFκB activation and NICD translocation
Macrophage (monocytes differentiated using oxyLDL) was treated with different concentrations (1, 2, 4, 8, and 16 µM) of dexamethasone (DEX; Inoue & Tanabe, 1998) and DAPT (Ambika, Devaraj S. N., & Devaraj H., 2018) and the expression levels of NFκB, NICD, and MAC387 were analyzed. Briefly, monocytes were treated with oxyLDL for 7 days to differentiate into macrophage. Inhibitors DEX and DAPT were added on 4th day of the experimental period. Macrophage differentiation on 4th day was confirmed by coimmunostaining of CD14 and MAC387 markers.
2.11 Fluorescence - activated cell sorting analysis
Cells were harvested and gently dissociated using trypsin to get a single cell population of 1 × 106 cells/ml. Detailed protocol is provided in supplementary materials.
2.12 Statistical analysis
Data obtained from this study were expressed as the mean ± SD (n = 3). Statistical analysis was performed by using SPSS software package version 21. Multiple-group comparisons were performed by using one-way analysis of variance (ANOVA) followed by Bonferroni post hoc analysis. Statistical significance was achieved when p < 0.05 at 95% confidence interval.
3 RESULTS
3.1 Activities of LDH, CK, and the levels of LPO and NO
AD-induced rat showed a significant increase in the activities of LDH and CK in serum (Supporting Information SM.1a,b) and a concomitant decrease in heart tissue (Supporting Information SM.1c,d). The AD-induced group showed a significant increase in LPO and NO levels when compared with that of control (Supporting Information SM.1e,f). Supporting Information SM.1g represents the electrophoretic mobility pattern of serum lipoproteins. The oxidized lipoproteins showed more electrophoretic mobility than native lipoproteins.
3.2 mRNA and protein expression of inflammatory mediators in aorta
The messenger RNA (mRNA) expressions of MCP-1, TNF–α, IL-6, NFκBp65, and internal control GAPDH are represented in Figure 1a. Increased expression of the inflammatory mediators was observed in AD-induced group when compared with control. Figure 1b exhibits the protein expression of macrophage marker MAC387, inflammatory mediator NFκBp65 and GAPDH as internal standard. Increased expressions of MAC387 and NFκBp65 were observed in AD-induced group versus the control. The intense nuclear translocation of NFκBp65 was observed in aorta of AD-induced group when compared with control rat (Figure 1c).
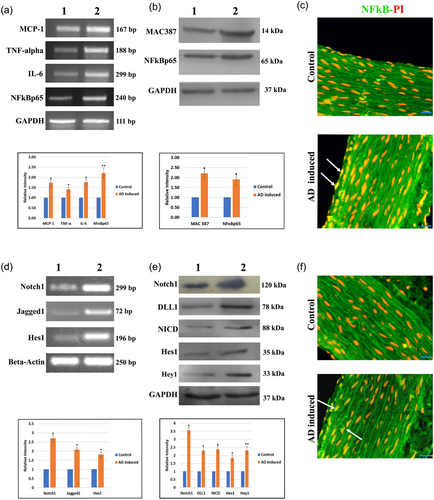
(a) mRNA expression of the inflammatory mediators MCP-1, TNF-α, IL-6, and NF-Bp65 were observed. GAPDH was used as internal control. (b) Protein expressions of the macrophage marker MAC387 and inflammatory mediator NFκBp65 of control and experimental group. GAPDH was used as internal control. (c) Representative photomicrographs of aorta in all groups stained with NF-Bp65 (FITC-green), and PI as nuclear stain. Arrowhead represents the nuclear localization of the NF-Bp65. Scale bar: 50 μm. (d) mRNA expressions of Notch1, Jagged1, and Hes1 were observed in control and experimental rat. β–actin was used as a loading control. (e) Protein expressions of Notch1, DLL1, NICD, Hes1, and Hey1. GAPDH was used as internal control. Lane 1: Normal control rats, Lane 2: Atherogenic diet-induced rats. Relative densities were measured in mean ± SD total pixel % by the ImageJ software. Hypothesis testing method included one-way ANOVA followed by bonferroni post hoc correction. Statistical significance (n = 3) *p < 0.05, **p < 0.01 when compared with control. (f) Representative photomicrographs of aorta in all groups stained with NICD (FITC-green), and PI as nuclear stain. Arrowhead represents the nuclear localization of the NICD. Scale bar: 50 μm. ANOVA, analysis of variance; mRNA, messenger RNA; NICD, notch intracellular domain; PI, propidium iodide [Color figure can be viewed at wileyonlinelibrary.com]
3.3 mRNA and protein expressions of notch pathway molecules in aorta
Increased mRNA expressions of Notch1, Jagged1, and Hes1 were observed in AD-induced rats compared with that of control rats (Figure 1d). GAPDH was used as a loading control. Notch pathway proteins Notch1, DLL1, NICD, Hes1, and Hey1 were upregulated in AD-induced rats when compared with control group (Figure 1e). GAPDH was used as internal control. Figure 1f. represents the immunostaining of NICD. The intensity of nuclear translocation of NICD was more prominent in AD-induced group than that of control group.
3.4 mRNA and protein expressions of inflammatory mediators in THP-1 cells
Figure 2a depicts the mRNA expressions of the macrophage markers CD36 and CD68, and the inflammatory mediators like COX-2 and NFκBp65, with β-actin as internal standard. The macrophage markers and the inflammatory mediators were markedly increase in oxyLDL-induced group when compared with the control group. The protein expressions of monocyte marker (CD14) and the inflammatory mediator (NFκBp65) are represented in Figure 2b, with GAPDH as internal control. CD14 showed increased expression in control group compared with induced group. Monocyte to macrophage differentiation was noted in induced cells, associated with an over expression of NFκBp65. Marked increase in the nuclear expression and translocation of NFκBp65 (Figure 2c) were observed in induced cells.
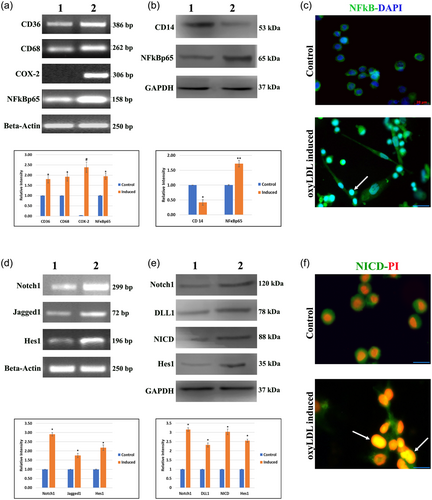
(a) RT-PCR expression of cell differentiation markers CD36 and CD68, and the inflammatory mediators COX-2 and NFκBp65. β–actin was used as internal control. (b) Protein expressions of monocyte marker CD14 and the inflammatory mediator NFκBp65. GAPDH was used as internal control. (c) Representative photomicrographs of cells in all groups immuno stained with NFκBp65 (FITC-green) and DAPI as nuclear stain. Arrowhead represents the nuclear localization of the NFκBp65. Scale bar: 20 μm. (d) mRNA expressions of Notch1, Jagged1, and Hes1 were observed in control and experimental cells. β-actin was used as a loading control. (E) Protein expressions of Notch1, DLL1, NICD, and Hes1. GAPDH was used as internal control. Lane 1: Control cells, Lane 2: OxyLDL-induced cells. Relative densities were measured in mean ± SD total pixel % by the ImageJ software. Hypothesis testing method included one-way ANOVA followed by bonferroni post hoc correction. Statistical significance (n = 3) *p < 0.05, **p < 0.01, and #p < 0.001 when compared with control. (f) Representative photomicrographs of cell in all groups stained with NICD (FITC-green) and PI as nuclear stain. Arrowhead represents the nuclear localization of the NFκBp65. Scale bar: 10 μm. ANOVA, analysis of variance; DAPI, 4′,6-diamidino-2-phenylindole, dihydrochloride; NICD, notch intracellular domain; OxyLDL, oxidized low-density lipoprotein; PI, propidium iodide; RT-PCR, real-time polymerase chain reaction [Color figure can be viewed at wileyonlinelibrary.com]
3.5 mRNA and protein expression of notch pathway proteins in THP-1 cells
Increased mRNA expression of Notch1, Jagged1, and Hes1 were observed in induced cells compared with control cells (Figure 2d). β-actin was used as a loading control. Notch pathway proteins like Notch1, DLL1, NICD, and Hes1 were upregulated in oxyLDL-induced group when compared with control (Figure 3e). GAPDH was used as internal control. Increased nuclear expression of NICD (Figure 2f) was observed in oxyLDL-induced cells with minimal expression in control cells.
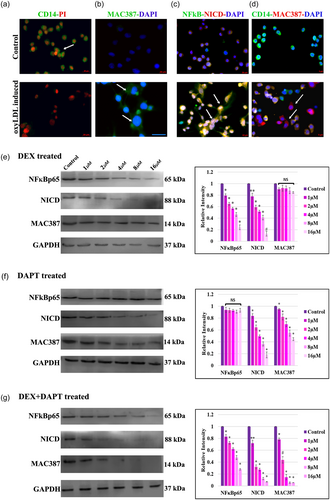
(a) Representative photomicrographs of cell in all groups stained with monocyte marker CD14 (FITC-green) and PI as nuclear stain. Scale bar: 20 μm. (b) Representative photomicrographs of cell in all groups stained with macrophage marker MAC387 (FITC-green) and DAPI as nuclear stain. (c) Representative photomicrographs of cell in all groups coimmuno stained with NFκBp65 (FITC-green), NICD (TRITC-Red), and DAPI as nuclear stain. Arrowhead represents the colocalization of NFκBp65 and NICD. (d) Representative photomicrographs of cells in all groups coimmuno stained with monocyte marker CD14 (FITC-green), macrophage marker MAC387 (TRITC-Red), and DAPI as nuclear stain. Scale bar: 5 μm. (e) OxyLDL-induced differentiating macrophage cells as control group. NFκBp65 activation was inhibited by DEX with different concentrations and the expression of NICD and MAC387 were observed. (f) NICD was inhibited by adding DAPT with various concentrations and the expression of NFκB and MAC387 were observed. (G) Both DEX + DAPT were added to differentiating macrophage cells, the expression of NFκB, NICD, and MAC387 were observed. Relative densities were measured in mean ± SD total pixel % by the ImageJ software. Hypothesis testing method included one-way ANOVA followed by bonferroni post hoc correction. Statistical significance (n = 3) *p < 0.05, **p < 0.01, and #p < 0.001 and NS, non-significant when compared with control. ANOVA, analysis of variance; DAPI, 4′,6-diamidino-2-phenylindole, dihydrochloride; DAPT, N-(N-[3, 5-difluorophenacetyl])-l-alanyl)-S-phenylglycine t-butyl ester; DEX, dexamethasone; NICD, notch intracellular domain; OxyLDL, oxidized low-density lipoprotein [Color figure can be viewed at wileyonlinelibrary.com]
3.6 Immunofluorescence analysis in THP-1 cells
Immunofluorescence analysis of CD14 (Figure 3a) showed prominent cell surface expression in control cells compared with induced cells. Immunostaining with MAC387 (Figure 3b) showed prominent cell surface expression in induced cells compared with control cells. Marked increase in the nuclear expression and colocalization of NFκBp65 and NICD (Figure 3c) were observed in oxyLDL-induced cells. In coimmunostaining of Day 4 of differentiation, expression of CD14 was observed in control group, whereas MAC387 and CD14 (Figure 3d) were observed in induced cells.
3.7 Assessment of effect of inhibitors
Differential expression of NICD and MAC387 were noted in the DEX-treated group (Figure 3e). Upon the DAPT treatment, expression of NFκB was sustained and the expression of MAC387 was gradually diminished (Figure 3f). Expression levels of NFκB, NICD, and MAC387 were decreased on an even plane in DEX and DAPT-treated cells (Figure 3g).
3.8 FACS analysis for monocyte to macrophage differentiation
Flow cytometry data showed the gating of the monocyte and macrophage differentiation using CD14 as monocyte marker and MAC387 as macrophage marker. The control group with THP-1 cells alone showed 72.66% of monocytes and the oxyLDL-induced group showed 29.22% of monocytes and 52.64% of differentiated macrophages. Treatment with DEX, at a concentration of 1 μM, showed 37.18% of monocytes and 44.75% of differentiated macrophages, whereas DEX, at a concentration of 16 μM expressed 48.35% of monocytes and 31.62% of differentiated macrophages. Treatment with DAPT at a concentration of 1 μM, showed 44.49% of monocytes and 34.90% of differentiated macrophages, whereas DAPT at a concentration of 16 μM expressed 57.49% of monocytes and 17.89% of differentiated macrophages (Figure 4).
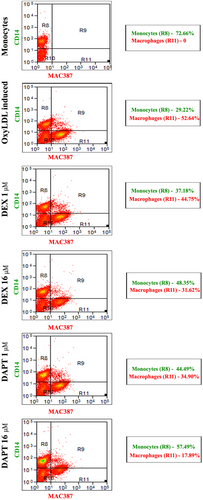
FACS analysis for monocyte to macrophage differentiation: Flow cytometry data show the monocytes and macrophages in oxyLDL-induced, DEX-treated, and DAPT-treated cells. CD14 (FITC-Green) as monocyte marker and MAC387 (APC-red) as macrophage marker. First two columns represent histogram of CD14 and MAC387 and the third column is scatter plot of both CD14 and MAC387. Scatter percentage of gating R9-CD14 and MAC387 positive cells, R10-debris. DAPI, 4′,6-diamidino-2-phenylindole, dihydrochloride; DAPT, N-(N-[3, 5-difluorophenacetyl])-l-alanyl)-S-phenylglycine t-butyl ester; DEX, dexamethasone; OxyLDL, oxidized low-density lipoprotein; NC, negative control [Color figure can be viewed at wileyonlinelibrary.com]
4 DISCUSSION
The current study showed the upregulation of transcription factors NFκBp65 and NICD in different stages of atherosclerosis such as monocyte to macrophage differentiation (early stage) using oxyLDL in THP-1 cells, endothelial dysfunction (EC), and lesion formation in rat fed on AD. This study provides the information that NFκB mediate NICD, in turn, NICD regulated macrophage differentiation and the inhibition of both NFκB–NICD is a potential therapeutic target in the early stage of atherosclerosis.
LDH and CK are indicators of tissue damage. Elevation of these two enzymes in AD-induced rats is the result of increased leakage of these enzymes from tissue into serum. Entrapment of LDL in the subendothelium, where it is subjected to oxidative modification, involves the process of LPO. Excessive leakage of NO from inflammatory cells results in cellular disturbance leading to heart failure. Elevated levels of LPO and NO in AD-induced rats indicated the generation of oxidative stress which led to the cellular disturbance in heart.
The chemokine monocyte chemotactic protein-1 (MCP-1) plays a key role in the development of fatty streak lesion (Gerszten et al., 1999). Macrophages differentiated by oxyLDL and high-fat diet secrete tumor necrosis factor alpha (TNF-α) and its elevation leads to the production of reactive oxygen species (ROS), resulting in endothelial cell dysfunction (Zhang et al., 2009). Interleukin-6 (IL-6) is significantly upregulated in aortic lesions and copious IL-6 expression is detected in cells of all stages of atherosclerosis, such as macrophages, T cells, endothelial cells, and vascular smooth muscle cells (Voloshyna, Littlefield, & Reiss, 2014). Elevated mRNA expression of MCP-1, TNF-α, IL-6, and NFκBp65 indicated the generation of ROS, endothelial dysfunction, and progression of lesion in AD-induced rats (Binesh et al., 2018). The expression of the inflammatory hub, nuclear factor-κB (NFκB), was triggered by the endothelial dysfunction. Generation of ROS also contributes to NFκB activation (Kim, Seo, Kim, & Bae, 2016), which induced a cascade of inflammatory responses (Akira & Takeda, 2004) in this study. MAC387 (Stoneman et al., 2007) was used as a differentiated macrophage marker to confirm the disease progression which was upregulated in induced group. Immunostaining of NFκBp65 in aorta indicated the intense nuclear translocation in the induced group when compared with control.
Within blood vessels, notch signaling integrates with multiple pathways by mechanisms like coregulation of transcriptional targets (Rostama, Peterson, Vary, & Liaw, 2014). Upregulation of multiple notch receptors, including Notch1, Notch3, and Notch4, were noted in atherosclerotic lesions (Liu et al., 2012). Delta-like 4 (DLL4)-induced notch activations has been observed in macrophages within atherosclerotic plaques (Fung et al., 2007). Dysfunction of ECs was regulated by inhibition of Delta 1 Like (DLL1) homolog, an inhibitor of Notch-1 and thereby prevents lesion formation (Schober et al., 2014). In this study, increased mRNA expression of Jagged 1 was observed in induced rats which indicates EC dysfunction. To restitute the homeostasis of EC, the expression of Jagged 1 was increased (Wu et al., 2011). Hairy and enhancer of split (hes1, 5, and 7) and the Hes-related proteins (hey1 and 2) were significantly higher in atherosclerotic lesions. In AD-induced rats the interactions of notch ligand (Jagged1 and DLL1) with notch receptor (Notch1) were increased, so more NICD was translocated to nucleus which thereby increased the expression of the target genes Hes1 and Hey1. Immunofluorescence analysis of NICD in aorta also indicated the intense nuclear translocation in the induced group when compared with control.
Lipoproteins have been classified by their electrophoretic mobility. In this study, increased electrophoretic mobility of oxidized LDL indicated the process of oxidation.
CD36, an 88-kDa transmembrane glycoprotein receptor, is expressed on different types of cells, including monocytes and macrophages, and is responsible for the uptake of oxyLDL in human macrophages (Park, 2014). Increased levels of CD36 mRNA were observed in oxyLDL-induced group compared with normal monocyte. Macrophage marker CD68 (Rosenfeld, 2015), also called myeloid specific surface marker, was expressed prominently on oxyLDL-induced cells. Inducible enzyme COX-2, which is linked to inflammatory responses (Binesh et al., 2018), was expressed prominently in oxyLDL-induced and CD68-positive macrophages (Gu et al., 2015). Ample evidence indicates that NFκB is responsible for the transcription of the genes encoding myriad of proinflammatory cytokines and chemokines (Lawrence, Bebien, Liu, Nizet, & IKKalpha, 2005), and it was upregulated in induced cells versus control. To confirm the monocyte to macrophage differentiation and the linkage between inflammation (caused by macrophage differentiation), immunostaining was done with monocyte marker CD14, macrophage marker MAC387, and inflammatory marker NFκB. Expression of CD14 was observed in control and the expression of MAC387 was detected in oxyLDL-induced group. The intense and prominent nuclear translocation of NFκB was found in induced versus control cells.
OxyLDL-activated macrophages selectively increase notch1 expression (Monsalve et al., 2006). Increased expression of Jagged 1 by differentiating macrophages promoted notch signaling leading to higher translocation of NICD into nucleus and ultimately leading to the expression of more amount of the target gene Hes1 (Boulter et al., 2012). In addition to immunofluorescence analysis of NFκB, NICD was also analyzed, and, like NFκB, it showed intense nuclear expression and translocation in activated macrophages. The deregulation of notch signaling has also been reported to be involved in various pathological processes (Andersson, Sandberg, & Lendahl, 2011).
In this study, an interesting observation was that, the two transcription factors NFκB and NICD were upregulated in AD-induced rats and the same was confirmed using THP-1 monocyte cells on exposure to oxyLDL to induce macrophage differentiation. As a next step, to confirm the dependency of NICD and NFκB, the NICD inhibitor DAPT was used to check the expression of NFκB and the NFκB inhibitor dexamethasone (Aghai et al., 2006) was used to examine the change in expression level of NICD. Macrophage marker MAC387 was used to confirm the macrophage differentiation. The inhibitors were added on 4th day of the experiment and the differentiation of macrophage was confirmed by coimmunostaining of CD14 and MAC387.
The current study goes along with Inoue and Tanabe (1998), who reported the induction of NFκB by lipopolysaccharide and suppression by DEX in U937 cells. In DEX-treated differentiating macrophage, the expression of NFκB showed that NFκB is gradually inhibited and the concomitant suppression of NICD showed that inhibition of NFκB activation downregulated NICD. The level of expression of MAC387 did not show significant changes with DEX treatment. Inhibition of NFκB subsequently downregulated the expression of NICD, but it neither enhanced nor diminished the viability of the differentiated macrophage.
A potent and specific inhibitor of γ-secretase enzyme, N-(N-[3, 5-difluorophenacetyl])-l-alanyl)-S-phenylglycine t-butyl ester (DAPT), was used to block the proteolytic processing and reduce the release of NICD (Ambika et al., 2018; Li et al., 2009). Differentiating macrophage treated with different concentrations of DAPT showed gradual decrease in the expression of NICD. Even though, the expression of NICD was declined placidly, the expression of NFκB remained parallel, but simultaneous downregulation of macrophage marker MAC387 was detected versus control. Our experimental results suggested that NFκB might interact with NICD in differentiating macrophage, but, NFκB was not dependent on NICD. As notch signaling is mainly involved in cell fate decision, inhibition of NICD affects the differentiation of macrophage. Data showed that NICD enhances NFκB nuclear translocation and DNA binding in LPS-activated macrophages (Li et al., 2009), but, whether the NICD inhibition affects NFκB expression in differentiating macrophage on exposure to oxyLDL is not clear.
Both DEX and DAPT were added to the differentiating macrophage to ascertain the NFκB and NICD expression. NFκB, NICD, and MAC387 expression were unvaryingly reduced. NFκB may regulate NICD in respect to inflammation and NICD independently influenced macrophage differentiation. It is also possible that both NF-kB and NICD might bind to independent promoter sites and cooperate to enhance transcriptional activity (Li et al., 2009). These findings have to be confirmed in an in vivo model system.
5 CONCLUSION
Activation of transcription factors, NFκB and NICD plays a crucial role in different stages of atherosclerotic disease progression. Inflammatory mediators and the notch pathway proteins were upregulated in AD-induced rats and the same was confirmed by using monocyte to macrophage differentiation on exposure to oxyLDL. Inhibition of NFκB regulates NICD, which, in turn, downturned macrophage differentiation. Inhibition of both NFκB–NICD is a potential target for intervention in early stage of atherosclerosis (monocyte–macrophage differentiation), to prevent the furtherance of disease.
ACKNOWLEDGEMENT
This study was supported by a grant from the University Grants Commission–University with Potential for Excellence (UGC–UPE, Phase II/Drug development - No. F 142/2008(NS/PE)), India.
CONFLICTS OF INTEREST
All authors disclose that there are no conflicts of interest that could inappropriately influence the outcome of the study.




