Active vitamin D regulates macrophage M1/M2 phenotypes via the STAT-1-TREM-1 pathway in diabetic nephropathy
Abstract
Aim
Imbalance of M1/M2 macrophages phenotype activation is a key point in diabetic nephropathy (DN). This study aimed to investigate whether active vitamin D (VD) suppresses macrophage transition to the M1 phenotype via inhibiting the high glucose-induced STAT-1 phosphorylation to reduce TREM-1 expression.
Methods
In vivo, pathological changes in kidney tissue were detected and the expression of CD68 TREM-1, STAT-1, M1 makers, and M2 makers were acquired in renal tissue of patients with DN and 18w DN rats. In vitro, RAW 264.7 cells were incubated in the presence of high glucose with or without VD. Silencing and overexpression of TREM-1 and silencing and activate of STAT-1 were explored to elucidate the underlying mechanism. The expression of TREM-1 and STAT-1 and the changes of macrophage phenotype were examined separately by western blot and immunofluorescence staining.
Results
(a) Expression of TREM-1, p-STAT-1, and M1 markers (iNOS and TNF-α) were increased and positively correlated in kidneys from patients with DN. (b) In DN rats, the enlargement of glomerular surface area, expansion of glomerular mesangial matrix, the expression of CD68, TREM-1, p-STAT-1, and M1 marker (iNOS) were significantly increased in comparison with the normal control group, whereas above changes were markedly decreased in the diabetic group treated with the VD group. (c) In vitro, VD significantly decreased high glucose-induced CD68, TREM-1, p-STAT-1, and M1 marker (iNOS) expression. However, above-mentioned effects of VD are abolished when TREM-1 is overexpressed or STAT-1 is activated. Reductions in STAT-1 expression decreased the TREM-1 expression.
Conclusion
VD can inhibit macrophage transition to the M1 phenotype through the STAT-1/TREM-1 pathway.
1 INTRODUCTION
Macrophage activation is a key point in the progression of diabetic nephropathy (DN; Lee et al., 2011). Increasing evidence suggests that the heterogeneity of macrophage phenotype and function ultimately determine the outcome of kidney disease (Cao et al., 2010, Kluth et al., 2001, Kushiyama et al., 2011, Martinez, Sica, Mantovani, & Locati, 2008, Wang et al., 2007, Zhang, Guo, Song, & Zhou, 2014, Zheng et al., 2011). Classically activated macrophages (M1) promote tissue inflammation and fibrosis by expressing iNOS and proinflammatory cytokines (including tumor necrosis factor-α (TNF-α) and interleukin-12 (IL-12)), whereas alternatively activated macrophages (M2) mediate tissue repair. Typical markers of M2 macrophages include CD163, mannose receptor (MR), and arginase-1 (Arg-1; Liu & Yang, 2013, Rigamonti, Zordan, Sciorati, Rovere-Querini, & Brunelli, 2014).
Active vitamin D3 (VD) is the active form of vitamin D3 that is converted from vitamin D through the liver and kidney. Recent studies have demonstrated that VD has potential renoprotective effects and significantly ameliorates renal dysfunction and fibrotic lesions in patients with DN; however, the mechanism of action remains unclear (Nakayama, Ueda, & Okuda, 2014). Our previous studies have shown that VD can promote the switching of macrophage phenotypes from M1 to M2 and consequently inhibit podocyte injury and glomerular impairment, therefore protecting renal function (Zhang et al., 2014).
Triggering receptor expressed on myeloid cells (TREM-1), expressed on macrophage, is implicated in the amplification of inflammatory responses. It appears to be a novel biomarker for the diagnosis and treatment of DN (Carla bosco, Raggi, & Varesio, 2016). Using antibody microarray technology, Zhao (2013) found that TREM-1 was significantly increased in patients with DN compared with normal controls. Recently, evidence has shown that TREM-1 is involved in the regulatory functions of macrophages (Lo et al., 2014, Yuan et al., 2014). Our previous study showed that VD suppresses macrophage infiltration by downregulation of TREM-1 in rats with DN (Zhao, Guo, Jiang, Zhu, & Zhang, 2018). However, the possible role of TREM-1 in regulating macrophage phenotypes in the progression of DN has not been elucidated.
Signal transducer and activator of transcription (STAT) is a key factor that regulates the activation of macrophage M1/M2 phenotypes (Li & Watowich, 2014, Miklossy, Hilliard, & Turkson, 2013, Szanto et al., 2010, Yue et al., 2014, Zhou et al., 2014). The activation of macrophages is related to STAT signaling, and VD plays a biological role via TREM-1; however, whether there is an association between these two factors has not been elucidated. This study aimed to investigate the molecular mechanisms of VD regulation of macrophage M1/M2 activation in diabetic nephropathy and elucidate the related intracellular signaling pathway. The overall study was divided into three parts, including clinical research, an animal study, and cell cultures. The results of the study are expected to highlight novel information regarding therapeutic targets for DN.
2 MATERIALS AND METHODS
2.1 Source of human kidney tissue
We retrospectively evaluated six patients with DN who had a confirmed diagnosis based on renal biopsy performed at Zhong Da Hospital. The other four normal renal tissue specimens that were used as the control group were obtained from patients with renal trauma or renal tumors. Zhong Da Hospital Ethical Committee approved the study with clinical research.
2.2 Animal experiments
All the animal care and experimental protocols are in compliance with the Animal Management Rules of the Ministry of Health of the People's Republic of China. The experimental protocol was approved by the Ethical Committee of Southeast University.
Six-week-old healthy male Sprague–Dawley (SD) rats weighing 200–220 g were obtained from Shanghai Slac Laboratory Animal (Shanghai, China). After one week of acclimation, the rats were randomly divided into the following four groups: (a) NC (normal control group, n = 8), (b) VD (NC treated with VD, n = 8), (c) DN (DN rats, n = 10), and (d) DN + VD (DN rats treated with VD, n = 10). Diabetes was induced with a single intraperitoneal injection of STZ (Sigma) dissolved in a 0.1 M citrate buffer (pH 4.5) at 58 mg/kg, and the control rats received only the 0.1 M citrate buffer solution. Three days later, the diabetic state was confirmed by measuring the tail blood glucose (BG) level. Rats with BG levels >16.7 mmol/l were considered diabetes. VD was purchased from the Shanghai Roche Company (Shanghai, China). After diabetes model built, VD was administered orally at a daily dose of 0.1 µg/kg every day. During the treatment period, the body weights were measured weekly. BG and 24 hr albuminuria were monitored biweekly. The rats were killed at 18th week. The kidneys were collected for histological examination and molecular assays after sacrifice.
2.3 Cellular experiments
The mouse macrophage cell line RAW264.7 was purchased from Shanghai Bogoo Biotechnology Company (Shanghai, China) and was routinely cultured in Roswell Park Memorial Institute (RPMI) 1640 media (containing 11.1 mM glucose) supplemented with 10% fetal bovine serum (ScienCell, Santiago) and incubated at 37°C in 5% CO2. First, the effects of VD on the expression of TREM-1, STAT-1, and M1/M2 macrophage markers were examined when the RAW264.7 cells were incubated with 30 mM glucose for 48 hr with or without 10−8 mol/l of 1,25-dihydroxyvitamin D3 (VD) (Sigma, St. Louis; Zhang, Song, Guo, & Zhou et al., 2015). Then, RAW264.7 cells were transfected with different plasmids to silence TREM-1 and STAT-1 expression, or overexpress TREM-1, and the expression of M1/M2 macrophage markers, TREM-1 and STAT-1, was re-examined. The cells were randomly divided into different groups as shown in Table 1.
| Pretreatment | Group | |||||
|---|---|---|---|---|---|---|
| – | NC | HG | HG + VD | VD | Mannitol | – |
| TREM-1 overexpression | NC | HG | HG + VD | VD | Mannitol | NTC |
| TREM-1 siRNA | NC | HG | HG + VD | VD | Mannitol | NTC |
| STAT-1 siRNA | NC | HG | HG + VD | VD | Mannitol | NTC |
| STAT-1 siRNA + TREM-1 overexpression | NC | HG | HG + VD | VD | Mannitol | NTC |
- Note. NC: 11.1 mM glucose; HG: 30 mM glucose; HG + VD: 30 mM glucose + 10−8 mol/L 1,25(OH)2D3; VD: 10−8 mol/L of 1,25(OH)2D3; Mannitol: 11.1 mM glucose + 19 mM mannitol; HG, high glucose; NC, normal control; NTC: nontarget control; siRNA: small interfering RNA; STAT: signal transducer and activator of transcription; VD: vitamin D.
2.4 Serum and urine chemistry analyses
Blood urea nitrogen (BUN) and creatinine (Scr) were analyzed with an automatic biochemistry analyzer (Hitachi, Tokyo, Japan). Urinary proteinuria was measured using an ELISA kit (Jiancheng, Nanjing, China) according to the manufacturer's instructions.
2.5 Renal histology analyses
Kidney sections were stained with periodic acid-Schiff (PAS) trichrome staining and were then examined by light microscopy (magnification, ×400) in a blinded manner. A semi-quantitative analysis of mesangial hyperplasia was performed in 20 randomly selected areas using the Image-Pro Plus image analysis system. The percentage of mesangial hyperplasia was calculated as the ratio of the pink mesangial area and the total glomerular area in each glomerulus.
2.6 Western blot
Proteins from renal tissues or RAW264.7 cells were separated with sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a nitrocellulose membrane. After blocking, the membranes were incubated at 4°C overnight with the following primary antibodies: After blocking, the membranes were incubated at 4°C overnight with the following primary antibodies: anti-CD68 (SC59103), anti-TNF-α (SC-1350), and anti-Arg-1 (SC20150) from Santa Cruz Biotechnology; anti-p-STAT-1 (SAB4300133) from Sigma; anti-iNOS (ab15323) and anti-MR (ab64693) from Abcam, UK; and anti-β-actin (YM2189) from Immunoway Biotechnology. Different antibodies were used to detect TREM-1 in kidney (bs-4886R, Bioss, China) or RAW264.7 (ab106153, Abcam, UK) proteins. After three washes with PBST for 5 min, the nitrocellulose membranes were incubated with horseradish peroxidase-conjugated secondary antibody for 1–2 hr. Finally, the membranes were evaluated with an enhanced chemiluminescence advanced system (GE Healthcare, Buckinghamshire, UK) and captured on X-ray film. Immunoreactive bands were quantified with densitometry using the ImageJ software (NIH, Bethesda).
2.7 Immunohistochemistry
Immunohistochemistry was performed on paraffin sections using a microwave-based antigen retrieval technique which involved heat-induced antigen retrieval (HIAR) with sections incubated in ethylenediaminetetraacetic acid (Trise-EDTA) buffer (pH 9.0). The sections were incubated with the following primary antibodies: anti-CD68 (ab201340) from Abcam, UK; anti-p-STAT-1 (SAB4300133) from Sigma; different antibodies were used to detect TREM-1 in patients (HPA005563, Sigma) or rats (bs-4886R, Bioss, China) proteins. Then, the appropriate secondary antibody was chosen. The immunostaining was visualized using diaminobenzidine tetrahydrochloride, and the slides were counterstained with hematoxylin.
2.8 Immunofluorescence staining
For immunofluorescence, the tissues and cells were fixed by 4% paraformaldehyde and then incubated with the following primary antibodies as follows: anti-CD68 (ab201340) and anti-MR (ab64693) from Abcam, UK; anti-p-STAT-1 (SAB4300133) from Sigma; different antibodies were used to detect TREM-1 in patients (HPA005563, Sigma) or rats (bs-4886R, Bioss, China) proteins; iNOS in patients (NB300–605, Novus, UK) or rats (ab15323, Abcam, UK) proteins. Then, the tissues and cells were washed and incubated with secondary antibody. After staining the nuclei with DAPI, the tissues and cells were visualized.
2.9 Statistical analysis
All the experiments were repeated at least three times. The data are expressed as the mean and the standard deviation (SD) and were analyzed with SPSS 19.0. One-way analysis of variance (ANOVA) was used to evaluate multiple groups; the SNK test was adopted for two comparisons; and the correlation analysis between the two variables was analyzed by Pearson correlation. A difference was considered significant if the P value was <0.05.
3 RESULTS
3.1 Association of TREM-1 expression, iNOS expression, and renal injury in the kidneys of DN patients
As shown in Figure 1a,b, the kidneys of patients with DN exhibited severe morphological lesions, such as enlargement of the glomerular surface area, expansion of the glomerular mesangial matrix, and increases in the glomerular basement membrane. CD68 and M1 macrophage infiltration were apparent in the interstitium. The number of CD68 and M1 macrophages in the DN group was increased compared with the NC group (p < 0.05, Figure 1c,d,k,l). MR, which is an M2 macrophage marker, did not differ between the two groups (Figure 1g,h). TREM1-positive cells were also apparent in the interstitium of the DN group, and expression level was strongly positively correlated with iNOS, which is an M1 macrophage maker (r = 0.757, p < 0.05, Figure 1e,f,m). Similarly, p-STAT-1 expression was increased in the DN group compared with the NC group and was positively correlated with iNOS (r = 0.808, p < 0.05, Figure 1i,j,n). In addition, p-STAT-1 expression was positively correlated with TREM-1 (r = 0.625, p < 0.05, Figure 1o).
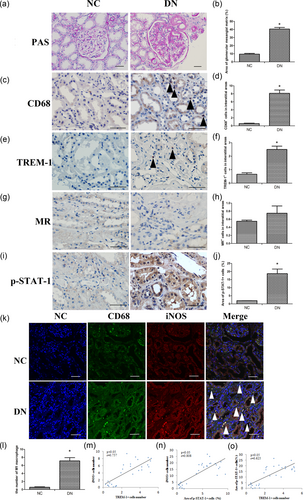
The histopathological features and the expression of CD68, TREM-1, iNOS, and MR in the kidneys of patients with diabetic nephropathy. (a) PAS stains of patients' renal tissues (×200, bar = 50 μm). (b) Semi-quantitative analysis of the glomerular mesangial matrix. (c) Immunohistochemical staining of CD68 macrophages (black arrows) in the interstitial areas (×400, bar = 50 μm). (d) Quantification of the number of CD68-positive macrophages per interstitial area. (e) Immunohistochemical staining of TREM-1 (black arrows) in the interstitial areas (×400, bar = 50 μm). (f) Quantification of the number of TREM-1 macrophages per interstitial area. (g) Immunohistochemical staining of MR (black arrows) in the interstitial areas (×400, bar = 50 μm). (h) Quantification of the number of MR macrophages per interstitial area. (i) Immunohistochemical staining of p-STAT-1 (black arrows) in the interstitial areas (×400, bar = 50 μm). (j) Quantification of the number of p-STAT-1 macrophages per interstitial area. (k) Correlation between CD68 and iNOS expression. CD68 (green) and iNOS (red) were observed by immunolabeling and confocal microscopy imaging detection in rat kidney tissue (white arrows) (×400, bar = 50 μm). (l) M1 macrophages = CD68 macrophages and M2 macrophages (MR positive cells). (m) Correlations between TREM-1 and iNOS (r = 0.0.757, p < 0.05). (n) Correlations between p-STAT-1 and iNOS (r = 0.808, p < 0.05). (o) Correlations between TREM-1 and iNOS (r = 0.625, p < 0.05). The data are presented as the mean ± SD (n = 6 DN group, n = 4 NC group). *p < 0.05 vs. NC group. DN: diabetic nephropathy; PAS: periodic acid-Schiff; MR: mannose receptor; NC: normal control; STAT: signal transducer and activator of transcription; TREM-1: triggering receptor expressed on myeloid cells [Color figure can be viewed at wileyonlinelibrary.com]
3.2 VD ameliorates renal histopathological changes and regulates M1/M2 macrophage phenotype in STZ-induced rats
To verify the protective effect of VD on DN kidneys, a DN rat model was established by intraperitoneal injection with streptozocin (STZ). We found that both BG and body weight did not differ among the four groups at the beginning of the study. After the injection of STZ, the DN rats developed overt diabetes with higher BG levels and lower body weights than the NC and VD rats beginning at the 2nd week after injection until the end of the experiment (p < 0.05, Figure 2a, Table 2). As expected, the DN rats exhibited increased proteinuria from the 2nd to the 18th week, which was attenuated after the 6th week of calcitriol treatment (p < 0.05, Figure 2b). The results from our renal histological analyses showed enlargement of the glomerular surface area and expansion of the glomerular mesangial matrix (Figure 2c,g). The immunohistochemical data showed that the number of CD68-positive macrophages (Figure 2d,h), the expression of TREM-1 (Figure 2e,i), and p-STAT-1 (Figure 2f,j) were significantly increased in the DN group compared with the NC group (p < 0.05); however, the above-mentioned changes were markedly decreased in the DN + VD group (p < 0.05).
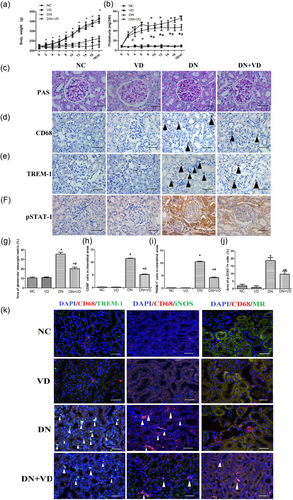
The effect of calcitriol on renal histopathological changes and M1/M2 macrophage phenotypes in DN rats at 18 weeks. Body weight (a); Proteinuria (b); PAS stains of rats' renal tissues at 18 weeks as indicated (c, ×400, bar = 50 μm); Immunohistochemical staining of CD68 macrophages (d), TREM-1 (e), and p-STAT-1 (f) in the interstitial areas ( ×400, bar = 50 μm). Semi-quantitative analysis of glomerular mesangial matrix (g); Quantification of the number of CD68-positive macrophages per interstitial area (h); Quantification of the number of TREM-1 positive macrophages per interstitial area (i); Quantification of the number of p-STAT-1 macrophages per interstitial area (j). The data are presented as the mean ± SD (n = 6 per group). *p < 0.05 vs. NC and VD group; #p < 0.05 vs. DN group; The immunofluorescence of CD68 and TREM-1, iNOS, and MR in the kidney of DN rats (k). CD68 and TREM-1, CD68 and Inos, and CD68 and MR were observed by immunolabeling and confocal microscopy imaging detection in rat kidney tissue ( ×400, bar = 50 μm). DN: diabetic nephropathy; PAS: periodic acid-Schiff; MR: mannose receptor; NC: normal control; STAT: signal transducer and activator of transcription; TREM-1: triggering receptor expressed on myeloid cells; VD: vitamin D [Color figure can be viewed at wileyonlinelibrary.com]
| NC | VD | DN | DN + VD | |
|---|---|---|---|---|
| BG (mmol/l) | 6.19 ± 0.18 | 6.23 ± 0.56 | 31.37 ± 3.13* | 30.56 ± 8.2* |
| KW/BW (mg/g) | 3.09 ± 0.45 | 3.16 ± 0.28 | 5.61 ± 0.61* | 3.69 ± 0.59*, ** |
| Scr (µmol/l) | 31.17 ± 6.78 | 32.09 ± 4.46 | 50.13 ± 3.42* | 43.42 ± 3.23*, ** |
| BUN (mmol/l) | 5.89 ± 0.96 | 5.83 ± 0.93 | 15.78 ± 3.16* | 11.43 ± 1.98*, ** |
- Note. BG: blood glucose; BUN: blood urea nitrogen; KW/BW: kidney weight/body weight; NC: normal control; Scr: serum creatinine; VD: vitamin D. Data are presented as the mean ± SD (n = 8 per group).
- * p < 0.05 versus NC and VD group.
- ** p < 0.05 versus DN group.
Furthermore, CD68 and TREM-1 and CD68 and iNOS were observed by immunolabeling and confocal microscopy imaging detection in rat kidney tissue. The results showed that immunofluorescence colocalization was also reduced in rats after the calcitriol treatment compared with untreated DN rats. In contrast, the immunofluorescence colocalization of CD68 and MR was increased in rats after the calcitriol treatment compared with untreated DN rats (Figure 2k).
3.3 The effect of p-STAT-1/TREM-1 pathway in the VD regulates M1/ M2 macrophage phenotype
3.3.1 VD effects on the STAT-1/TREM-1 pathway regulating the M1/M2 macrophage phenotype
To clarify whether VD regulates high glucose-induced macrophage phenotype via the STAT-1/TREM-1 pathway. First, the most appropriate VD intervention concentration was selected by the methods described in our previously published article (Zhao et al., 2018); we used a 30-mM glucose concentration and a 48-hr time period in the experiments.
Then, the effects of VD on M1/M2 macrophage phenotype and the expression of TREM-1 and p-STAT-1 were observed by western blot, and the results showed VD normalizes upregulation of pSTAT-1, TREM-1, and iNOS and downregulation of MR induced by high glucose (Supporting Information Figure S2).
3.3.2 Regulating TREM-1 does not affect p-STAT-1 expression but can regulate M1/M2 macrophage phenotype
To clarify the effect of TREM-1 on p-STAT-1, small interfering RNA (siRNA)-targeting TREM-1 was transfected into RAW264.7 cells. A nontarget control (NTC) siRNA was used to eliminate the nonspecific effects of the transfection reagents. The inhibition ratios of TREM-1 siRNA-1, 2, and 3 were 69.1%, 51.3%, and 37.8%, therefore, we used TREM-1 siRNA-1 as the final intervention siRNA. NTC siRNA clearly showed no effect on TREM-1 expression (Figure 3a).

The expression of p-STAT-1, TREM-1, iNOS, and MR protein observed under different conditions. The effect of TREM-1 siRNA and TREM-1 plasmid on TREM-1 expression. RAW264.7 cells were transfected with three specific TREM-1 siRNAs or a nontarget control (NTC) siRNA. The TREM-1 protein levels were measured and a quantification analysis was conducted (a). RAW264.7 cells were transfected with TREM-1 plasmid or a nontarget control (NTC) plasmid. The TREM-1 protein levels were measured and a quantification analysis was conducted (b). The effect of STAT-1 siRNA on STAT-1 expression. RAW264.7 cells were transfected with three specific STAT-1 siRNAs or a nontarget control (NTC) siRNA. The STAT-1 protein levels were measured (a) and a quantification analysis was conducted (c). RAW264.7 cells were treated with different reagents. The cells were collected for western blot analysis, quantification analysis of p-STAT-1 (d), TREM-1 (e). The protein expression of iNOS and MR were observed under different conditions. The cells were collected for western blot analysis analysis, quantification of iNOS (g) and MR (h). β-actin was used as an internal control. The data are presented as the mean ± SD (n = 3 per group). *p < 0.05 vs. NC group; #p < 0.05 vs. HG group. DN: diabetic nephropathy; HG: high glucose; PAS: periodic acid-Schiff; MR: mannose receptor; NC: normal control; siRNA: small interfering RNA; STAT: signal transducer and activator of transcription; TREM-1: triggering receptor expressed on myeloid cells; VD: vitamin D [Color figure can be viewed at wileyonlinelibrary.com]
First, the effect of TREM-1 siRNA on p-STAT-1, iNOS, and MR was observed by western blot and immunofluorescence, and the results showed that the suppression of TREM-1 expression decreased high glucose-mediated iNOS expression and increased MR expression but caused no obvious changes in p-STAT-1 expression (Figures 3d).
Then, TREM-1 plasmid overexpression efficiency (198.8%) was evaluated by western blot (Figure 3b). The effects of TREM-1 overexpression on p-STAT-1, iNOS, and MR were observed by western blot and immunofluorescence, and the results showed that the overexpression of TREM-1 increased MR expression and decreased iNOS expression but caused no obvious changes in p-STAT-1 expression (Figures 3d,f).
3.3.3 STAT-1 siRNA can not only affect TREM-1 expression but also regulate M1/M2 macrophage phenotype
To further clarify the effect of p-STAT-1 on TREM-1, siRNA-targeting STAT-1 was transfected into RAW264.7 cells. A NTC siRNA was used to eliminate the nonspecific effects of the transfection reagents. The inhibition ratios of STAT-1 siRNA-1, 2, and 3 were 66.7%, 49.3%, and 24.8%; therefore, we used STAT-1 siRNA-1 as the final intervention siRNA. NTC siRNA clearly showed no effect on TREM-1 expression (Figure 3c).
Then, the effects of STAT-1 siRNA on TREM-1, iNOS, and MR were observed by western blot (Figure 3) and immunofluorescence (Figure 3s), the results showed that the suppression of p-STAT-1 expression decreased high glucose-mediated iNOS expression and TREM-1 expression and increased MR expression.
To further determine the regulatory effects of p-STAT-1 on TREM-1 and macrophage phenotypes, RAW264.7 cells were first stimulated with STAT-1 siRNA and then treated with the TREM-1 overexpression plasmid, and the resulted show that the expression of iNOS increased, and MR expression decreased.
4 DISCUSSION
Macrophage accumulation is a pathological feature of DN (Wilson, Walbaum, & Rees, 2004). These infiltrating cells are strongly associated with the severity of renal injury and progressive chronic renal failure (Eardley et al., 2006). Macrophages comprise a heterogeneous population of cells. Different macrophage phenotypes have different functions. M1 macrophages contribute to tissue inflammation and damage, whereas M2 macrophages display antiinflammatory and tissue repair properties. Wang et al. (2007) provided direct evidence that the adoptive transfer of macrophages primed ex vivo by exposure to IL-4 and IL-13 to induce an M2 macrophage phenotype reduced renal injury and facilitated repair in adriamycin-induced nephropathy in mice. However, the possible role and regulation of macrophage phenotypes in the progression of DN has not been elucidated.
VD has been reported as a novel immunomodulator for the prevention and treatment of DN through the regulation of immunocytes and cytokines (Thomas & Cooper, 2010). In recent years, previous studies have identified the effects of VD in the prevention of albuminuria and podocyte injury in animal models (Gonçalves et al., 2014, Gravellone et al., 2011). Our prior research also indicates that VD effectively decreases proteinuria and exerts a renoprotective effect in STZ-induced DN rats (Zhang, Zhou, Guo, Song, & Liu, 2015).
Our group has also been devoted to the study of macrophage phenotypes and their effects on the prognosis of renal diseases. We found that under high glucose conditions in vitro, RAW264.7 macrophages tended to switch to the M1 phenotype and express higher iNOS and higher amounts of proinflammatory cytokines. Although VD significantly inhibits M1 activation, it enhances M2 macrophage activation (Zhang et al., 2015). These findings led us to consider whether the transformation of the macrophage activation state induced by VD could improve renal tissue damage in DN rats. In our subsequent study, we found that VD therapy decreased the number of M1 macrophages, increased the number of M2 macrophages, reduced proteinuria, and alleviated podocyte impairments in STZ-induced DN rats. Furthermore, the protein expression of M1 macrophage markers were negatively correlated with the expression of either nephrin or podocin (Zhang et al., 2014). This finding suggests that the renoprotective effects of VD may be related to regulation of the activation state of macrophages, which provides a new theoretical clue for understanding the renoprotective mechanisms of VD. Therefore, further studies evaluating the signaling mechanism by which VD regulates M1/M2 macrophage activation status is important for applying VD for the clinical prevention and treatment of early diabetic nephropathy.
Triggering receptor expressed on myeloid cells (TREM-1), which is a newly identified cell surface molecule expressed on macrophages, is implicated in the amplification of inflammatory responses (Carla bosco et al., 2016, Saravanan Subramanian, Sharma, Agrawal, & Nandipati, 2017, Wu et al., 2011, Yuan et al., 2014, Zhou et al., 2014). Our preliminary data have shown that macrophage infiltration and the expression of TREM-1 is increased in renal tissues of DN rats, but VD significantly inhibits this progress (Zhao et al., 2018). Researchers (Breyer & Susztak, 2016; Lo et al., 2014) have found that TREM-1 is crucial for modulating macrophage polarization and plays a pivotal role in the development of obstructive nephropathy. However, whether TREM-1 has a similar effect in DN has not yet been characterized. In this study, our data showed that there is a close association between TREM-1 expression and macrophage phenotypes in the kidneys of STZ-induced DN rats and patients with DN. In addition, in vitro TREM-1 expression is inhibited by TREM-1 siRNA, and the expression of high glucose-induced M1 macrophage markers were decreased, whereas M2 markers were increased. In contrast, TREM-1 was overexpressed by TREM-1 plasmid, and the expression of M1 markers was increased. The above-mentioned results indicate that TREM-1 is involved in a key pathway for regulating macrophage phenotypes. However, the mechanism by which TREM-1 regulates macrophages under high glucose conditions is still unknown and was explored for first time in this study.
Signal transducer and activator of transcription (STAT) regulates the differentiation and maturation of multiple immune cells (Gordon & Martinez, 2010, Szanto et al., 2010, Yue et al., 2014). Darnell, Kerr, and Stark (1994) found that IFN-γ and LPS promote the activation of M1 macrophages, which is mediated by STAT1. STAT1 knockout mice lack the ability to respond to interferon stimulants, leading to a significant reduction in immunity to pathogens, such as bacteria and viruses (Durbin, Hackenmiller, Simon, & Levy, 1996). These studies suggest that STAT plays an important role in cell activation and functional maturation. Yue et al. (2014) found that the STAT1 and STAT3/6 signal balance was closely related to the activation state of M1/M2 macrophage phenotypes in a mouse model of ischemic reperfusion liver injury. However, whether STAT-1 regulates macrophage phenotypes via TREM-1 remains unknown and needs further study. In our study, by the observation of M1/M2 macrophage phenotypes, we found that treatment with STAT-1 siRNA significantly inhibited M1 activation and enhanced M2 macrophage activation. However, cells that were treated with TREM-1 siRNA exhibited similar changes in macrophage phenotype to the above-mentioned results, but TREM-1 siRNA had no effect on STAT-1 expression. These results prove that STAT-1 regulates macrophage phenotypes by regulating TREM-1.
As a novel immunomodulator, VD could inhibit inflammatory factor and regulate immunity response. The current study demonstrates that VD can inhibit the high glucose-induced STAT-1 phosphorylation by reducing TREM-1 expression and inhibiting macrophage transition to the M1 phenotype. These results contribute to a new understanding of the renoprotective effects of VD in diabetic nephropathy. However, the mechanism of VD regulates STAT-1/TREM-1 pathway is still unknown. Previous studies have suggested the cross-talk between VDR (VD receptor) and STAT1. Not only did the interaction between VDR and STAT1 promote the activation of STAT1 but also promoted the synthesis of VD, which further enhanced the interaction between VDR and STAT1 and regulates the immune regulation function of macrophages (Vidal, Ramana, & Dusso, 2002). Therefore, we speculate that VD, as an activator of VDR, may regulate macrophage function and phenotype through the genomic or rapid nongenomic effect of VDR. However, the mechanism by which VD regulates the above pathways through VDR needs to be further studied.
ACKNOWLEDGMENTS
This study is supported by grants from the National Natural Science Foundation of China (No. 81570612) and the Clinical Medical Research Center Program of Jiangsu Province (BL2014080).
CONFLICTS OF INTEREST
Authors declare that there are no conflicts of interest.




