Estrogen deprivation aggravates intracellular calcium dyshomeostasis in the heart of obese-insulin resistant rats
Abstract
The incidence of cardiovascular disease and metabolic syndrome increases after the onset of menopause, giving evidence for the vital role of estrogen. Intracellular calcium [Ca2+]i regulation plays an important role in the maintenance of left ventricular (LV) contractile function. Although either estrogen deprivation or obesity has been shown to strongly affect the metabolic status and LV function, the effects of estrogen deprivation on the cardiometabolic status and cardiac [Ca 2+]i regulation in the obese-insulin resistant condition have never been investigated. Our hypothesis was that estrogen deprivation aggravates LV dysfunction through the increased impairment of [Ca 2+]i homeostasis in obese-insulin resistant rats. Female rats were fed on either a high-fat (HFD, 59.28% fat) or normal (ND, 19.77% fat) diet for 13 weeks. Then, rats were divided into sham (HFS and NDS) operated or ovariectomized (HFO and NDO) groups. Six weeks after surgery, metabolic status, LV function and incidence of [Ca 2+]i transients were determined. NDO, HFS, and HFO rats had evidence of obese-insulin resistance indicated by increased body weight with hyperinsulinemia and euglycemia. Although NDO, HFS, and HFO rats had markedly reduced %LV fractional shortening, E/A ratio and decreased [Ca 2+]i transient amplitude and decay rate, HFO rats had the most severe impairments. These findings indicate that estrogen deprivation had a strong impact on abnormal LV function through [Ca 2+]i regulation. In addition, evidence was found that in obese-insulin resistant rats, estrogen deprivation severely aggravates LV dysfunction via increased impairment of [Ca 2+]i homeostasis.
Abbreviations
-
- BP
-
- blood pressure
-
- [Ca2+]i
-
- intracellular calcium
-
- EF
-
- ejection fraction
-
- FS
-
- fraction shortening
-
- HDL
-
- high density lipoprotein
-
- HFD
-
- high-fat diet
-
- HFO
-
- high-fat-diet fed ovariectomized rats
-
- HFS
-
- high-fat-diet fed sham-operated rats
-
- HOMA
-
- homeostasis model assessment
-
- LDL
-
- low density lipoprotein
-
- LV
-
- left ventricle
-
- MDA
-
- malondialdehyde
-
- NCX
-
- sodium-calcium exchanger
-
- ND
-
- normal diet
-
- NDS
-
- normal-diet fed sham-operated rats
-
- OVX
-
- ovariectomized
-
- ROS
-
- reactive oxygen species
-
- RyR
-
- ryanodine receptors
-
- SERCA
-
- sarcoplasmic reticulum calcium-ATPase
-
- SR
-
- sarcoplasmic reticulum
1 INTRODUCTION
Cardiovascular diseases (CVDs) have been a major cause of death worldwide for many decades (Ginsberg, 2000; Maas et al., 2011; Mathers & Loncar, 2006; Rivera et al., 2009; Sivasinprasasn et al., 2015; Vitale, Mendelsohn, & Rosano, 2009). There is also evidence of the incidence of an increase in CVDs in women after the onset of menopause (Vitale et al., 2009). The evidence from both clinical (Pines et al., 1993) and animal studies (Scheuer, Malhotra, Schaible, & Capasso, 1987; Sivasinprasasn et al., 2015) suggests there is an important cardioregulatory role of estrogen in cardiac contractile function. Investigations into the impact of the lack of estrogen exhibited an impairment of cardiac contractility (Pines et al., 1993), and a decreased stroke volume and fractional shortening (Scheuer et al., 1987; Sivasinprasasn et al., 2015). In addition, obesity is also known to be a major risk factor in the development of cardiac contractile dysfunction (Ginsberg, 2000; Maas et al., 2011; Sivasinprasasn et al., 2015). Currently, the growing number of obese people and also an aging population stresses an increasing threat of both estrogen deprivation and obesity on the development of left ventricular (LV) dysfunction worldwide.
Cardiac contractile function is mainly regulated by intracellular calcium (Ca2+) homeostasis which relies on cyclical changes in intracellular Ca2+ concentration ([Ca2+]i transient (Dunay et al., 2015). Three major cardiac Ca2+ handling proteins including sarcoplasmic reticulum Ca2+-ATPase (SERCA), ryanodine receptor Ca2+ release channel proteins (RYR) and Na+-Ca2+ exchanger proteins (NCX) regulate [Ca2+]i (Bupha-Intr & Wattanapermpool, 2006). Previous studies have demonstrated the disturbances of cardiac [Ca2+]i regulation in conditions of estrogen deprivation (Bupha-Intr & Wattanapermpool, 2006; Dunay et al., 2015). In the hearts of ovariectomized rats, the expression of SERCA protein (Bupha-Intr & Wattanapermpool, 2006; Dunay et al., 2015) and Ca2+ uptake activities were decreased (Bupha-Intr & Wattanapermpool, 2006). Moreover, disturbances in cardiac [Ca2+]i regulation were found in obese-insulin resistant rats as indicated by an increase in [Ca2+]i diastolic level and a decrease in [Ca2+]i transient amplitude and [Ca2+]i transient decay rate (Apaijai, Chinda, Palee, Chattipakorn, & Chattipakorn, 2014).
Despite the findings from previous reports, the effects of estrogen deprivation on cardiac [Ca2+]i transient homeostasis in obese-insulin resistant rats have never been investigated. Therefore, we tested the hypothesis that estrogen deprivation aggravates LV dysfunction in obese-insulin resistant rats through the impairment of [Ca2+]i transient homeostasis and Ca2+ handling proteins.
2 MATERIALS AND METHODS
2.1 Animal preparation and ethical approval
This study was approved by the Institutional Animal Care and Use Committee of the Faculty of Medicine, Chiang Mai University (approval no. 5/2559 on May 5, 2016). All rat experiments conformed to the ARRIVE and the United State NIH guidelines. Thirty-two female Wistar rats (6 weeks of age, weighing 200–220 g) were obtained from the National animal center (Salaya campus, Mahidol University, Bangkok, Thailand). The rats were housed in a temperature-controlled room (25°C) with a 12-hr dark/light cycle setting and were given 1 week to acclimatize for.
2.2 Experimental protocol
Rats were randomly divided into two dietary groups: a normal diet group (ND, a diet containing 19.77% energy from fat), and a high-fat diet group (HFD, a diet containing 59.28% energy from fat; Pratchayasakul, Chattipakorn, & Chattipakorn, 2011). Thirteen weeks after the specific feeding, rats in each dietary group were divided into two subgroups to undergo either a sham operation (NDS or HFS) or a bilateral ovariectomy (NDO or HFO). Body weight and food intake of all rats were recorded throughout the experimental period. At the end of the sixth week after surgery, blood samples were collected from the tail vein for determination of metabolic parameters and estrogen levels. An oral glucose tolerance test (OGTT) and echocardiography were carried out. At the end of the study protocol, rats were deeply anesthetized with isoflurane, and the hearts were rapidly removed allowing single ventricular myocyte isolation (Palee, Apaijai, Shinlapawittayatorn, Chattipakorn, & Chattipakorn, 2016). The isolated cardiomyocytes were used for the measurement of intracellular Ca2+ transients. Cardiac Ca2+ regulatory proteins were investigated and biochemical studies were carried out.
2.3 Ovariectomy
In the ovariectomized group, rats were anesthetized using a rodent anesthesia machine (2% isoflurane vaporizer and 2 L/min oxygen flow) and then placed lying on the left and right lateral decubitus for left and right ovariectomy, respectively. After hair shaving and skin cleaning, a bilateral ovariectomy was carried out by initially making a midline dorsal skin incision. The incision was centered between the inferior crest of the rib cage and superior base of the thigh. The abdominal-pelvic cavity was accessed then the uterine tubes and ovaries were identified. Both ovaries were removed, and uterine horns were returned into the cavity. In the sham group, all rats underwent the same anesthesia and surgical preparation procedures as the OVX rats. Bilateral ovaries of sham rats were identified and exposed; the ovaries were not excised in these cases (Minta et al., 2018; Sivasinprasasn et al., 2017).
2.4 Cardiac function assessment
Echocardiography was used to determine LV function as a non-invasive method. Animals were given light anesthesia using 2% isoflurane with oxygen (2 L/min). An echocardiography probe (S12, GE Healthcare, CT) was placed on the chest at the parasternal short axis and connected to an echocardiography machine (GE vivid-i, GE healthcare). An M-mode echocardiogram at LV papillary muscle level was recorded, and %fractional shortening (%FS) was determined. A pulsed-wave Doppler spectrum of mitral flow was recorded from the apical four-chamber view using a color Doppler for guidance. The mitral E/A ratio was measured in each rat.
2.5 Determination of oxidative stress
Malondialdehyde (MDA) concentrations in cardiac tissues were measured using a high-performance liquid chromatography (HPLC) system (Thermo Scientific, Bangkok, Thailand) as described previously (Apaijai et al., 2014). Proteins from cardiac tissues were mixed with 10% trichloroacetic acid (TCA) containing BHT then heated at 90°C for 30 min and cooled to room temperature. The mixture was centrifuged, and the supernatant was mixed with 0.44 M H3PO4 and 0.6% thiobarbituric acid solution to generate thiobarbituric acid reactive substances (TBARS). The solution was filtered through a syringe filter (polysulfone type membrane, pore size 0.45 μm, Whatman International, Maidstone, UK) and analyzed using the HPLC system. Data were analyzed using BDS software (BarSpec Ltd., Rehovot, Israel), and plasma TBARS concentration was determined directly from a standard curve generated from a standard reagent for MDA at different concentrations and reported as MDA equivalent concentration.
2.6 Cardiomyocyte isolation and calcium transient measurement
Cardiomyocytes were isolated from the hearts of rats using a method described previously (Palee et al., 2016). In brief, under deep anesthesia, the heart was removed immediately and placed into a Langendroff apparatus. The hearts were perfused with modified Krebs solution (130 mM NaCl, 4.5 mM KCl, 1.4 mM MgCl2, 0.4 mM NaH2PO4, 0.75 mM CaCl2, 4.2 mM HEPES, 20 mM taurine, 10 mM creatine and 10 mM glucose, pH 7.3 at 37°C) for 5 min, followed by a Ca2+-free solution (100 μM EGTA) for 4 min, and a modified Krebs solution containing 100 μM CaCl2 and 1 mg/ml Type II collagenase for another 8 min. The ventricles were removed from the cannula, cut into small pieces and incubated in 10 ml of collagenase solution gassed with 100% oxygen for 7 min at 37°C, with regular triturating. The cardiomyocytes were separated from undigested ventricular tissues by filtering through a cell strainer and were allowed to settle into a loose pellet. Then, the supernatant was removed and replaced with modified Krebs solution containing 1% BSA and 500 μM CaCl2. This process was repeated with modified Krebs solution containing 1 mM CaCl2. After this procedure, the cardiomyocytes were ready for the recording process.
The isolated cardiomyocytes were used for Ca2+ transient measurement using the CELLR imaging software (Olympus Soft Imaging Solutions GmbH, Germany). Isolated cardiomyocytes were loaded with Fura-2/AM, and fluorescent intensity was recorded during electrical pacing (1 Hz, 10-ms duration, 15 V). The ratio of the emissions wavelengths is directly related to the amount of intracellular Ca2+. When Ca2+ binds to a ratiometric indicator, it changes the optimum excitation or emission wavelength of the indicator. An elevation of Ca2+ concentration induces an increase in Fura-2 emission fluorescence when the indicator is excited at 340 nm, with a corresponding decrease in fluorescence at 380 nm excitation (Bootman, Rietdorf, Collins, Walker, & Sanderson, 2013; Waldeck-Weiermair et al., 2015). The experiments were performed in a temperature-controlled chamber system at 37°C. Intracellular Ca2+ transient decay rate which approximates the rate of Ca2+ uptake into the SR by SERCA, intracellular Ca2+ transient amplitude which approximates the intracellular Ca2+ level during the systolic period, and diastolic Ca2+ level which approximates the intracellular Ca2+ level during the diastolic period were all calculated.
2.7 Determination of the expression of cardiac calcium regulatory proteins
To determine cardiac Ca2+ regulatory proteins, western blot analysis was used to examine the expression of the SERCA, RYR and NCX proteins. Briefly, rat myocardial tissues were homogenized in a lysis buffer (containing 1% Nonidet P-40, 0.5% sodium deoxycholate, and 0.1% sodium dodecyl sulfate [SDS] in 1× PBS) to extract the proteins. Then, the homogenate was centrifuged at 13,000 rpm for 10 min. Total protein (50–80 mg) was mixed with the loading buffer consisting of 5% mercaptoethanol, 0.05% bromophenol blue, 75 nM Tris, 2% SDS, and 10% glycerol with pH 6.8, and the mixture was boiled for 5 min and loaded into 10% gradient SDS-polyacrylamide gel. Proteins were then transferred to a nitrocellulose membrane with the presence of a glycine/methanol transfer buffer (containing 20 mM Tris, 0.15 M glycine, and 20% methanol) in a transfer system (Bio-Rad). The membranes were incubated in 5% skim milk in 1× TBS-T buffer (containing 20 mM Tris [pH 7.6], 137 nM NaCl, and 0.05% Tween-20) for 1 hr at room temperature and then exposed to anti-SERCA, RYR and NCX (Cell Signaling Technology, Danvers, MA) and anti-actin (Sigma-Aldrich, St. Louis, MO) for 12 hr. Bound antibody was detected by the conjugation of horseradish peroxidase with anti-rabbit IgG. Enhanced chemiluminescence detection reagents were administered to visualize peroxidase reaction products.
2.8 Determination of metabolic parameters and hormone levels
Plasma was prepared from fasted blood samples and was kept frozen at −80°C until analysis of glucose, cholesterol, triglyceride, insulin, and estradiol levels could be completed. Plasma estrogen concentration was measured by using a competitive enzyme immunoassay kit (Cayman Chemical Company, MI). Plasma insulin level was detected using a sandwich ELISA kit (Millipore, MI). Plasma glucose and triglyceride levels were determined by colorimetric assay from a commercially available kit (Biotech, Bangkok, Thailand). Fasting plasma HDL and LDL were determined using commercially available kits (ERBA Diagnostic, Mannheim, Germany) (Sivasinprasasn et al., 2015).
2.9 Statistical analysis
Data were expressed as mean ± standard error of the mean (SEM). Comparisons of variables were performed using the one-way analysis of variance followed by an LSD post-hoc test. The comparison of the % fractional shortening was performed using the Kruskal-Wallis test followed by Dunn's test. p < 0.05 was considered statistically significant.
3 RESULTS
3.1 Estrogen deprivation aggravated adverse changes of metabolic profiles and oxidative stress in obese-insulin resistant rats
After consumption of an HFD for 13 weeks, HFD rats developed insulin resistance as indicated by markedly increased body weight (318.20 ± 5.48 vs. 272.08 ± 2.69 g) and an increased HOMA index (14.28 ± 2.69 vs. 7.56 ± 1.34), when compared with ND rats. However, the fasting blood glucose level (143.54 ± 3.47 vs. 144.92 ± 4.27 mg/dl) and the amount of daily energy intake (58.85 ± 1.63 vs. 62.13 ± 2.21 kcal/day) was not significantly different between the HFD and ND rats.
Six weeks after ovariectomy (i.e., 19 weeks after HFD or ND consumption), both estradiol level and uterus weight showed a significant decrease in NDO and HFO groups, compared with NDS rats, confirming the endogenous estrogen-deprived condition resulting from the removal of ovaries. Moreover, the body weight and visceral fat deposition were increased in NDO, HFS, and HFO when compared with NDS rats (Table 1). However, there was an observable marked increase in both body weight and visceral fat deposition in HFO rats when compared with NDS, NDO and HFS rats (Table 1). Furthermore, plasma cholesterol levels in NDO, HFS, and HFO rats were significantly increased when compared with NDS rats (Table 1). Insulin sensitivity was decreased in NDO, HFS, and HFO groups when compared with NDS rats (Table 1) indicated by an increased area under the curve following an OGTT. However, HFO rats had the most severe impairment of insulin sensitivity (Table 1). Moreover, the cardiac oxidative stress indicated by cardiac tissue MDA and plasma MDA was markedly increased in NDO, HFS, and HFO rats when compared with NDS (Table 1). However, HFO rats had the most severe cardiac oxidative stress (Table 1).
| Groups | ||||
|---|---|---|---|---|
| Parameters | NDS | NDO | HFS | HFO |
| Body weight (g) | 279.23 ± 3.34 | 317.46 ± 4.50* | 324.64 ± 4.87* | 373.02 ± 11.56*-*** |
| Visceral fat (g) | 6.71 ± 1.02 | 12.09 ± 0.94* | 24.79 + 1.30*, ** | 34.61 ± 1.77*-*** |
| Uterus weight (g) | 0.46 ± 0.05 | 0.14 ± 0.01* | 0.34 ± 0.02** | 0.14 ± 0.02*, *** |
| Estradiol level (pg/ml) | 113.38 ± 9.10 | 31.55 ± 3.92* | 48.49 ± 2.98* | 23.97 ± 2.05* |
| Glucose (mg/dl) | 128.56 ± 1.78 | 136.33 ± 4.86 | 138.66 ± 4.38 | 146.12 ± 5.97* |
| Insulin (ng/ml) | 0.88 ± 0.13 | 2.40 ± 0.33* | 2.45 ± 0.33* | 3.20 ± 0.31* |
| HOMA index | 6.70 ± 0.02 | 19.35 ± 4.55* | 20.08 ± 8.67* | 25.76.48 ± 5.44* |
| Plasma glucose AUC (AUCg) (mg/dl × min × 104) | 1.63 ± 0.07 | 1.89 ± 0.05* | 2.36 ± 0.11*, ** | 2.87 ± 0.14*-*** |
| Cholesterol (mg/dl) | 75.43 ± 4.64 | 96.59 ± 7.43* | 108.53 ± 7.50* | 117.67 ± 9.50* |
| HDL (mg/dl) | 7.62 ± 0.18 | 8.46 ± 0.20 | 8.44 ± 0.35 | 8.46 ± 0.31 |
| LDL (mg/dl) | 65.89 ± 4.44 | 77.59 ± 2.43 | 84.30 ± 3.63* | 85.37 ± 5.68* |
| Triglyceride (mg/dl) | 67.33 ± 5.24 | 70.38 ± 4.49 | 89.31 ± 8.21 | 90.45 ± 3.78 |
| Cardiac MDA (µMol/g) | 0.33 ± 0.01 | 0.77 ± 0.03* | 0.90 ± 0.23* | 1.24 ± 0.08*-*** |
| Plasma MDA (µM) | 3.48 ± 0.21 | 4.11 ± 0.09* | 4.43 ± 0.16* | 5.31 ± 0.20*-*** |
| Energy intake (kcal/day) | 58.85 ± 1.70 | 58.20 ± 1.73 | 63.88 ± 2.31*, ** | 63.78 ± 2.44*, ** |
- Note. Values are mean ± standard error of the mean (SEM).
- AUC: area under the curve; HFO: high-fat-diet fed ovariectomized rats; HFS: high-fat-diet fed sham-operated rats ; HOMA: homeostasis model assessment; MDA: Malondialdehyde; NDO: normal-diet fed ovariectomized rats; NDS: normal-diet fed sham-operated rats.
- * p < 0.05 versus NDS.
- ** p < 0.05 versus NDO.
- *** p < 0.05 versus HFS.
3.2 Estrogen deprivation aggravated cardiac dysfunction in obese-insulin resistant rats
Six weeks after ovariectomy (i.e., 19 weeks after HFD or ND consumption); NDO, HFS, and HFO rats had increased SBP, DBP, MAP and heart rate, compared with NDS rats (Figure 1a–d). In all groups, HFO rats had the most severe impairment of BP and heart rate regulation (Figure 1a–d). Echocardiograms demonstrated that HFS, HFO and NDO also developed LV systolic dysfunction as indicated by decreased %FS and also LV diastolic dysfunction as indicated by a decreased mitral E/A ratio when compared with NDS rats (Figure 2a,b). Similar to observed BP impairment, HFO rats had the most severe LV dysfunction (Figure 2a,b). The representative images of cardiac M-Mode and cardiac doppler are shown in Figure 2c,d respectively.
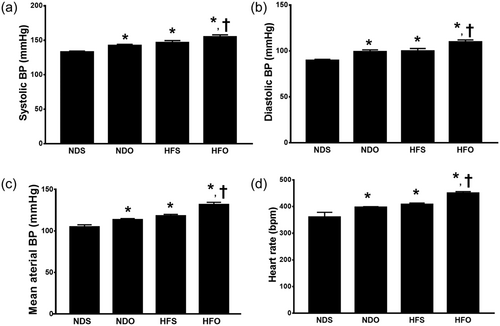
The effects of estrogen deprivation on blood pressure and heart rate in obese-insulin resistant rats. Estrogen deprivation aggravates evidenced by increases in: (a) systolic pressure; (b) diastolic pressure; (c) mean arterial blood pressure, and (d) heart rate. *p < 0.05 vs. NDS, †p < 0.05 vs. HFS. HFO: high-fat-diet fed ovariectomized rats; HFS: high-fat-diet fed sham-operated rats; NDO: normal-diet fed ovariectomized rats; NDS: normal-diet fed sham-operated rats
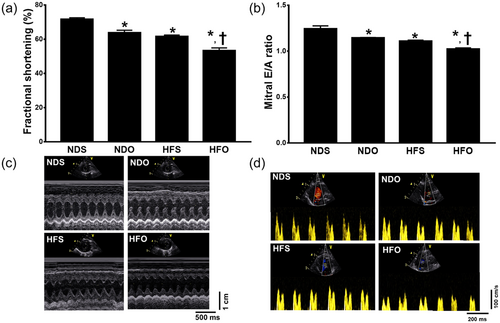
The effects of estrogen deprivation on left ventricular contractile function and oxidative stress in obese-insulin resistant rats. Estrogen deprivation aggravates ventricular contractile dysfunction evidenced by (a) decreased %fractional shortening and (b) a decreased mitral E/A ratio. Representative images of (d) cardiac M-Mode, and (e) cardiac doppler. *p < 0.05 vs. NDS, †p < 0.05 vs. HFS. HFO: high-fat-diet fed ovariectomized rats; HFS: high-fat-diet fed sham-operated rats; NDO: normal-diet fed ovariectomized rats; NDS: normal-diet fed sham-operated rats [Color figure can be viewed at wileyonlinelibrary.com]
3.3 Estrogen deprivation aggravated intracellular calcium dyshomeostasis in obese-insulin resistant rats
In this study, the intracellular Ca2+ transients were determined to assess intracellular Ca2+ homeostasis. Six weeks after ovariectomy (i.e., 19 weeks after HFD or ND consumption), intracellular Ca2+ transient amplitude, intracellular Ca2+ transient rising rate, and intracellular Ca2+ transient decay rate were markedly decreased in NDO, HFS, and HFO rats, compared with NDS rats (Figure 3a–c). In addition, the diastolic Ca2+ level was increased in NDO, HFS, and HFO rats, compared with NDS rats (Figure 3d). In all groups, HFO rats had the most severe impairment of intracellular Ca2+ transient amplitude, intracellular Ca2+ transient rising rate, intracellular Ca2+ transient decay and diastolic Ca2+ level (Figure 3a–d). The representative intracellular Ca2+ transient tracings are shown in Figure 3e.
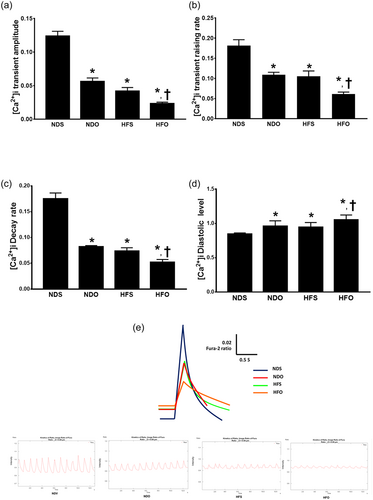
The effects of estrogen deprivation on intracellular Ca2+ transients in cardiomyocytes of obese-insulin resistant rats. Estrogen deprivation aggravates intracellular Ca2+ dyshomeostasis in obese-insulin resistant rats evidenced by (a) decreased intracellular Ca2+ transient amplitude, (b) decreased intracellular Ca2+ transient raising rate, (c) decreased intracellular Ca2+ transient decay rate, and (d) increased intracellular diastolic Ca2+ levels, when compared with the NDS group. (e) Representative images of Ca2+ transient tracing. *p < 0.05 vs. NDS, †p < 0.05 vs. HFS. HFO: high-fat-diet fed ovariectomized rats; HFS: high-fat-diet fed sham-operated rats; NDO: normal-diet fed ovariectomized rats; NDS: normal-diet fed sham-operated rats [Color figure can be viewed at wileyonlinelibrary.com]
3.4 Estrogen deprivation aggravated cardiac calcium handling protein dysregulation in obese-insulin resistant rats
In this study, the Ca2+ handling proteins were investigated to assess intracellular Ca2+ regulation. Six weeks after ovariectomy (i.e., 19 weeks after HFD or ND consumption), SERCA, NCX and RYR protein expression were markedly decreased in NDO, HFS, and HFO rats, compared with NDS rats (Figure 4a–c). In all groups, HFO rats had the most severe impairment of Ca2+ handling protein expression (Figure 4a–c) and representative Ca2+ handling protein expression is shown in Figure 4d.
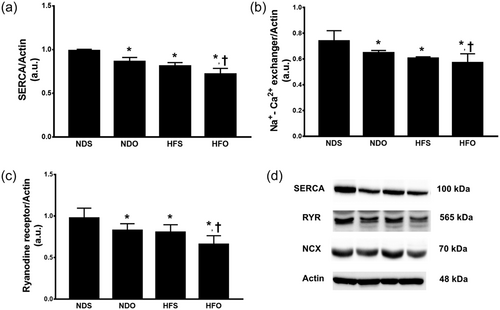
The effects of estrogen deprivation on Ca2+ regulatory protein in obese-insulin resistant rats. Estrogen deprivation aggravates Ca2+ regulatory protein dysregulation by decreases in: (a) SERCA, (b) NCX, and (c) RYR expression. *p < 0.05 vs. NDS, † p < 0.05 vs. HFS. HFO: high-fat-diet fed ovariectomized rats; HFS: high-fat-diet fed sham-operated rats; NDO: normal-diet fed ovariectomized rats; NDS: normal-diet fed sham-operated rats; NCX: sodium-calcium exchanger; NDO: normal-diet fed ovariectomized rats; NDS: normal-diet fed sham-operated rats; RYR: ryanodine receptors; SERCA: sarcoplasmic reticulum calcium-ATPase
4 DISCUSSION
The major findings from this study clearly demonstrate that both estrogen deprivation and obese-insulin resistance independently cause metabolic disturbance, oxidative stress, LV dysfunction, intracellular Ca2+ transient dyshomeostasis and impaired Ca2+ regulatory protein. However, the severity of these metabolic impairments and cardiac adverse effects were increased in obese-insulin resistant rats with estrogen deprivation, that is when both conditions were present.
In this study, both estrogen deprivation at the age of adolescence and obese-insulin resistance were associated with metabolic disturbance including obesity, insulin resistance, and dyslipidemia, compared with normal rats. Estrogen is known to promote energy homeostasis, improve body fat distribution, ameliorate insulin resistance (or enhance insulin sensitivity) and improve β-cell function (Mauvais-Jarvis, Clegg, & Hevener, 2013). The prevalence of metabolic syndrome has been shown to increase in women with low levels of estrogen such as those that are post-menopausal, and those who have undergone ovariectomy (Carr, 2003; Dorum et al., 2008). Although our previous study found that 8 weeks of estrogen deprivation in very young obese-insulin resistant rats did not aggravate metabolic disturbance (Sivasinprasasn et al., 2015), our findings in this study showed that 6 weeks of estrogen deprivation at the age of adolescence in obese-insulin resistant rats caused the worsening of metabolic disturbance. These findings suggest that an alteration of metabolic function in obese-insulin resistant rats with estrogen deprivation is initiated on the onset of estrogen deprivation.
Both oxidative stress and estrogen deprivation have been shown to influence sympathetic hyperactivity (Campese, Shaohua, & Huiquin, 2005; Ye, Zhong, Yanamadala, & Campese, 2006), leading to an increase of BP. Our previous studies showed that either estrogen deprivation or obese-insulin resistant rats had increased systemic, cardiac, and mitochondrial oxidative stress (Sivasinprasasn et al., 2015; Sivasinprasasn et al., 2017). Thus, a combination of oxidative stress and estrogen deprivation greatly provoked cardiac autonomic imbalance and increased both systolic and diastolic blood pressure and heart rate (Minta et al., 2018). We found this in obese-insulin resistant rats with estrogen deprivation in comparison to both NDO and HFS.
Estrogen plays a significant cardioregulatory role in cardiac contractile function (Scheuer et al., 1987; Sivasinprasasn et al., 2015). Our results clearly demonstrated that an impairment in cardiac contractile function, including decreased fractional shortening and mitral valve E/A ratio, was observed in estrogen-deprived rats and obese-insulin resistant rats, whereas the impairment of cardiac function was markedly aggravated in obese-insulin resistant rats with estrogen deprivation. The cardiac contractile function is mainly dependent on cardiac Ca2+ homeostasis which requires cyclical changes in intracellular Ca2+ concentration (9). Previous studies have demonstrated the impact of disturbances of cardiac [Ca2+]i regulation in either estrogen deprivation condition or obesity alone (Apaijai et al., 2014; Bupha-Intr & Wattanapermpool, 2006; Dunay et al., 2015). However, our current study demonstrates that these deleterious effects were aggravated in obese-insulin resistant rats with estrogen deprivation, that is when both conditions were present. This aggravation could be due to impairment in the expression of cardiac Ca2+ handling proteins in obese-insulin resistant rats with estrogen deprivation. Previous studies have shown that the expression of SERCA protein (Bupha-Intr & Wattanapermpool, 2006; Dunay et al., 2015) and Ca2+ uptake activities were decreased (Bupha-Intr & Wattanapermpool, 2006) in ovariectomized rats. Our results demonstrated that SERCA, NCX and RYR expression were decreased in estrogen-deprived rats and obese-insulin resistant rats. Interestingly, estrogen deprivation aggravated the expression of these cardiac Ca2+ handling proteins in obese-insulin resistant rats. All of these findings could be responsible for the impairment of cardiac [Ca2+]i regulation, leading to the impaired cardiac function found in this study.
In conclusion, our findings demonstrated that the loss of endogenous estrogen as a result of ovariectomy in obese-insulin resistant rats aggravated metabolic and cardiac dysfunction. This could be due to the worsening of cardiac [Ca2+]i dyshomeostasis. These findings suggest that prevention of obesity before menopause in aging females may help to attenuate cardiac dysfunction. Future studies are needed to investigate this preventive approach.
5 LIMITATIONS
In this study, although we measured SERCA expression and Ca2+ transient decay rate to assess the SERCA activity in the heart, no pharmacological interventions were done to see their effects on these parameters. Future studies are needed to evaluate the effects of the pharmacological interventions in these models.
ACKNOWLEDGMENTS
This study was supported by grants from the Thailand Research Fund: RTA6080003 (S.C. Chattipakorn), RSA6180056 (S. Palee) and RSA6180071 (W. Pratchayasakul), the NSTDA Research Chair grant from the National Science and Technology Development Agency Thailand (N. Chattipakorn), and the Chiang Mai University Center of Excellence Award (N. Chattipakorn).
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.




