Slug mediates myofibroblastic differentiation to promote fibrogenesis in buccal mucosa
Abstract
Epithelial–mesenchymal transition (EMT) has been implicated in fibrogenesis and carcinogenesis; however, the exact role of EMT-inducer Slug in the progression of precancerous oral submucous fibrosis (OSF) has not been investigated. In the current study, we showed that the expression of Slug was upregulated in OSF tissues and associated with various myofibroblast markers. After silence of Slug in fibrotic buccal mucosal fibroblasts (fBMFs), the elevated myofibroblast activities and fibrosis markers were all downregulated. Our data revealed that arecoline, an areca nut alkaloid, increased the expression of Slug in normal BMFs, and inhibition of Slug successfully prevented the arecoline-induced myofibroblast activation. Additionally, overexpression of Slug in BMFs stimulated the activities of myofibroblasts, indicating that upregulation of Slug by arecoline contributes to the myofibroblast transdifferentiation. Most importantly, Slug was able to bind to the E-box of type I collagen, leading to increased expression of type I collagen. Altogether, this study demonstrated the abnormal elevation of Slug in OSF and its significance in arecoline-induced fibrogenesis. Moreover, downregulation of Slug could be a potential target for OSF remedy via suppression of myofibroblast activities and type I collagen.
1 INTRODUCTION
Oral submucous fibrosis (OSF) has been recognized as a precancerous condition characterized by the persistent inflammation and accumulation of connective tissue associated with the habit of areca nut chewing (Hsieh et al., 2015; Warnakulasuriya, Johnson, & van der Waal, 2007). These elevated inflammatory cytokines contributed to the imbalance of tissue repair and fibrogenesis in the oral cavity (J. Z. C. Chang, Hsieh, Lin, Chen, & Kuo, 2017). Patients often suffer from ulcerations, xerostomia, burning sensation, and limitation in mouth opening (Angadi & Rekha, 2011), which greatly affect their daily life and lead to other comorbidities.
Myofibroblasts are critical to the process of tissue repair as these contractile and secretory cells could help close the wound and produce the components of extracellular matrix (ECM) and various cytokines (Hinz et al., 2007). Nevertheless, persistent activation of myofibroblasts also results in pathological fibrosis if there is an excessive accumulation of ECM proteins, such as collagen. α-Smooth muscle actin (α-SMA) is one of the distinct markers of the differentiated myofibroblasts (Darby, Skalli, & Gabbiani, 1990), and the presence of α-SMA increases the cell contractile force (Hinz, Celetta, Tomasek, Gabbiani, & Chaponnier, 2001). Moreover, deposition of collagen further reinforces the contraction (Gabbiani, 2003). In OSF, it has been found that the expression of α-SMA was upregulated (Y. C. Chang et al., 2014) and myofibroblasts produced a higher amount of type I collagen compared with normal fibroblasts (Kuo, Chen, Hahn, Hsieh, & Chiang, 1995). Another filament vimentin was also increased in OSF tissues (Nayak, Singh, Desai, & Vanaki, 2013) and suggested to stabilize collagen (Challa & Stefanovic, 2011). Given that the increased myofibroblasts in OSF may be associated with the dysregulation of ECM, elucidation of the factor that regulates myofibroblasts transdifferentiation became extremely crucial.
Among various sources of myofibroblasts, cells undergo epithelial–mesenchymal transition (EMT) have been considered to engage in fibrosis and carcinogenesis (Kalluri & Weinberg, 2009). Several EMT-inducing transcription factors, such as Snail and Slug, have been discovered to initiate and facilitate the acquisition of a mesenchymal phenotype via repression of E-cadherin (Nieto, 2002). Numerous studies have proven that EMT activators are related to the areca nut-associated OSF (Y. C. Chang et al., 2014; Lee et al., 2016). Our previous work has identified that Snail was able to bind to the E-box in the α-SMA promoter after arecoline treatment, thereby stimulating the transdifferentiation of buccal mucosa fibroblasts (BMFs) into myofibroblasts (Yang et al., 2018). Nevertheless, limited evidence has shown the significance of Slug in the development of precancerous OSF. As such, we sought to investigate the role of Slug in areca nut-associated fibrogenesis.
2 MATERIALS AND METHODS
2.1 Chemicals, OSF tissue, and cell culture
Arecoline (an alkaloid from areca nut) and collagen solution from bovine skin were purchased from Sigma-Aldrich (St. Louis, MO). Ingredients in areca nut have been shown to be critical in the pathogenesis of OSF and oral cancer via regulation of inflammatory factors and oxidative stress (M. C. Chang et al., 2001, 2004; Jeng et al., 2000). Hence, we utilized arecoline to induce the myofibroblast transdifferentiation (M. C. Chang et al., 2013).
For tissue acquisition, all procedures have followed the tenets of the Declaration of Helsinki and were reviewed by the Institutional Review Committee at Chung Shan Medical University, Taichung, Taiwan. Specimens of histologically normal or fibrotic mucosa from 25 normal subjects and OSF patients were collected in Department of Dentistry, Chung Shan Medical University Hospital for quantitative real-time polymerase chain reaction (qRT-PCR), heat map, western blot analyses. Normal BMFs and fibrotic BMFs (fBMFs) were cultivated as previously described (Yu, Tsai, Hsu, & Chang, 2013). Cell cultures between the third and eighth passages were used in this study.
2.2 qRT-PCR
Trizol reagent was used for total RNA isolation and Superscript III first-strand synthesis system (Invitrogen Life Technologies, Carlsbad, CA) was used to reverse-transcribe the messenger RNAs (mRNAs) according to the manufacturer’s instruction. qRT-PCR reactions on resulting cDNAs were performed on an ABI StepOne Real-Time PCR Systems (Applied Biosystems, Foster City, CA; Y. C. Chang et al., 2015). Fifty nanograms of complimentary DNA (cDNA) sample were used in an SYBR Green-based qPCR reaction (Kapa Biosystems, Inc., Wilmington, MA); the cycling conditions were as follows: 50°C for 2 min, 95°C for 10 min, followed by 40 cycles of 95°C for 10 s and 60°C for 1 min. The end point used in the real-time quantification was calculated by the StepOne software (Applied Biosystems, Foster City, CA), and the threshold cycle number (Ct value) for each analyzed sample was calculated. The primer sequences were listed in Table 1.
| Gene | Primer Sequence (5′–3′) |
|---|---|
| SMA | F: AGCACATGGAAAAGATCTGGCACCR: TTTTCTCCCGGTTGGCCTTG |
| Vimentin | F: CAATGTTAAGATGGCCCTTGR: GGGTATCAACCAGAGGGAGT |
| Slug | F: GTGATTATTTCCCCGTATCTCTATR: CAATGGCATGGGGGTCTGAAAG |
| COL1A1 | F: GGGTGACCGTGGTGAGAR: CCAGGAGAGCCAGAGGTCC |
| GAPDH | F: CTCATGACCACAGTCCATGCR: TTCAGCTCTGGGATGACCTT |
- Note. COL1A1: collagen type I α1; GAPDH: glyceraldehyde 3-phosphate dehydrogenase; qRT-PCR: quantitative real-time polymerase chain reaction; SMA: smooth muscle actin.
2.3 Western blot analysis
Western blot analysis was carried out as previously described (Y. C. Chang et al., 2014). Briefly, cells were lysed with NP-40 lysis buffer, and protein concentration was determined by BCA protein assay reagent (Thermo Fisher Scientific Inc., Rockford, IL). A total of 25 μg of total protein was separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to a polyvinylidene fluoride membrane (Millipore, Billerica, MA). The membranes were then incubated with primary antibodies followed by corresponding secondary antibodies. The primary antibodies against Slug, α-SMA, collagen type I α1 (COL1A1), and vimentin were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Immunoreactive protein bands were detected by the ECL detection system (Amersham Biosciences Co., Piscataway, NJ).
2.4 Collagen contraction assay
fBMFs (2 × 105) were suspended in 0.5 ml of a 2 mg/ml collagen solution (Sigma-Aldrich) and cell per collagen mixture was added into 24-well plate. The plate was incubated at 37°C for 2 hr, which caused polymerization of the collagen gels. After detaching from well using a 200 μl pipet tip, the gels were further incubated in 0.5 ml MEMα medium for 48 hr. Contraction of the gels was photographed and contraction index was calculated using the ImageJ software (Yu, Tsai, Hsu, et al., 2013).
2.5 Migration and invasion assays
The Transwell system with a polycarbonate filter membrane of 8-μm pore size (Corning, NY) was utilized to assess the migration and invasion capacities. For invasion assay, the membrane was coated with Matrigel (BD Pharmingen, Franklin Lakes, NJ). In the upper chamber, cell were seeded at a cell density of 1×105 in serum-free medium followed by 24 hr of incubation. Serum-containing media (10% fetal bovine serum) was used as the chemoattractant in the lower chamber. Cells on the other side of the membrane were stained with 0.1% crystal violet (Sigma-Aldrich) after fixation. These cells were counted from five different visual areas of 100-fold magnification under a microscope (Chung et al., 2017).
2.6 Wound healing assay
Cells were seeded into a six-well culture plate, and then wounds were introduced to the confluent monolayer of cells with a sterile 200 µl plastic pipette tip to create a denuded area in the center. Cell movement into the wound area was photographed at 0 and 48 hr under a microscope (Yu, Tsai, Wang, et al., 2013).
2.7 Immunofluorescent image
After fixation with 4% paraformaldehyde for 10 min, cells were incubated with the primary antibody against Snail or α-SMA (Santa Cruz Biotechnology) for 3 hr followed by the corresponding secondary antibodies. Cells were also stained with 4′6-diamidino-2-phenylindole (DAPI) to indicate the nuclei (Hsin et al., 2017).
2.8 Lentiviral-mediated RNAi for silencing Slug
The pLV-RNAi vector was purchased from Biosettia Inc. (San Diego, CA). The method of cloning the double-stranded short hairpin RNA (shRNA) sequence was described in the manufacturer’s protocol. Oligonucleotide sequence of lentiviral vectors expressing shRNA that targets Slug was synthesized and cloned into pLVRNAi to generate a lentiviral expression vector. The target sequences for Slug were listed as follows: Sh-Slug-1: 5′-AAAAGGACACACATACAGTGATTTTGGATCCAAAATCACTGTATGTGTGTCC-3′; Sh-Slug-2: 5′-AAAAGCCCAGTCTAGAAAATCTTTTGGATCCAAAAGATTTTCTAGACTGGGC-3.
2.9 Overexpression of Slug
Slug cDNA was cloned into pLV-EF1a-MCS-IRES-Puro (Cat. No: cDNA-pLV01; Biosettia Inc.). Lentivirus production was performed by cotransfection of plasmid DNA mixture with lentivector plus helper plasmids (VSVG and Gag-Pol) into 293T cells (American Type Culture Collection, Manassas, VA) using Lipofectamine 2000 (LF2000; Invitrogen, Carlsbad, CA).
2.10 Luciferase reporter assays
For transactivation assays, luciferase reporter plasmid was cotransfected with Slug into the constructs. A β-galactosidase-expressing vector was included as an internal control for transfection efficiency. After 24 hr, the cells were lysed and both luciferase and β-galactosidase activities were determined with enzyme assay kits (Promega). Luminescence was quantitated using a TD-20e luminometer (Turner Design) from duplicate plates. The representative results from three independent experiments were used.
2.11 Chromatin immunoprecipitation (ChIP) analysis
A ChIP assay will be conducted using the ChIP Assay Kit according to the manufacturer’s instructions (Upstate-Millipore Corporation, Billerica, MA).
2.12 Statistical analysis
Pearson and Spearman’s correlation were used to analyze the relationship between the expression of myofibroblast-related markers and Slug. Data were presented as mean ± SD. A Student’s t test was used to compare the differences. p < 0.05 was considered statistically significant.
3 RESULTS
3.1 The expression of Slug is elevated in OSF
RNA-sequencing and heat map analysis of differential RNA expressions revealed that various fibrosis-associated factors (such as fibronectin and transforming growth factor-β [TGF-β] signaling) were upregulated in OSF tissues (Figure 1a), including Slug (>2-fold). We found that Slug was positively correlated to other myofibroblast-related markers (Figure 1b) and confirmed that the gene expression of Slug was significantly higher in OSF specimens and fBMFs (Figure 1c,d). Results from western blot also showed that the expression of Slug was elevated in OSF samples (Figure 1e).
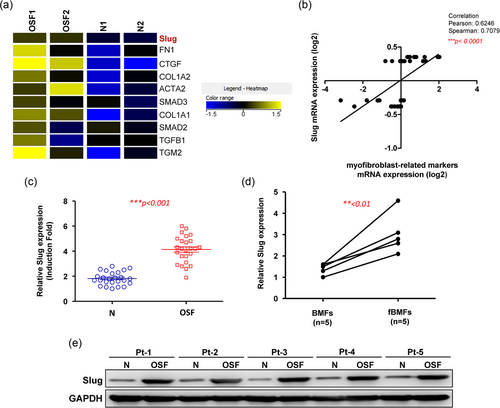
The expression of Slug is upregulated in the OSF tissues. (a) Results from RNA sequencing showed that several fibrosis-associated factors were elevated in OSF tissues. (b) Pearson and Spearman correlation were used to analyze the relationship between the expression of myofibroblast-related markers and Slug. Both methods suggested that there was a strong, positive correlation between Slug and myofibroblast markers. The higher expression of Slug in OSF tissues (c) and fibrotic BMFs (d) was validated by RT-PCR (n = 25). (e) Western blot analysis was used to show that the protein expression of Slug was increased in OSF tissues. Results are means ± SD of triplicate samples from three experiments. OSF: oral submucous fibrosis; RT-PCR: real-time polymerase chain reaction
3.2 Downregulation of Slug attenuates the hallmarks of fibrosis
To examine the significance of Slug in myofibroblasts, knockdown of Slug was used to study its functional role (Figure 2a). Given that myofibroblasts migrate to the injured cites and participate in the wound healing process, cell mobility and contractility are common features to investigate (Hinz et al., 2007). Our results showed that the behavioral phenotypes of fBMFs, including collagen gel contractility (Figure 2b), migration (Figure 2c), invasion (Figure 2d), and wound healing (Figure 2e) were all reduced following downregulation of Slug, indicating that modulation of Slug was sufficient to inhibit the myofibroblast activation. Additionally, the relative protein expression levels of myofibroblast markers, including α-SMA, type I collagen, and vimentin were inhibited in the Slug-knockdown fBMFs (Figure 2f). Together, these data suggested that Slug was critical to the existence of myofibroblasts characteristics.
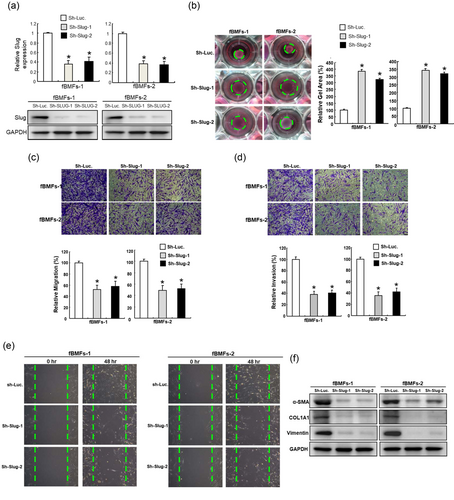
Downregulation of Slug in fibrotic BMFs diminishes the myofibroblast activities and the expression of fibrosis-associated markers. (a) The inhibited expression of Slug was confirmed by Western blot analysis following knockdown of Slug. Downregulation of Slug in fibrotic BMFs repressed the myofibroblast activities, including collagen gen contractility (b), migration (c), invasion (d), and wound healing (e) capacities. Protein expression of α-SMA, type I collagen, vimentin were also decreased. Results are means ± SD of triplicate samples from three experiments. *p < 0.05 compared with Sh-Luc group. BMFs: buccal mucosal fibroblasts; α-SMA: α-smooth muscle actin
3.3 Inhibition of Slug prevents the increased myofibroblast activities induced by arecoline
The main etiology of OSF has been attributed to areca nut chewing and its major component arecoline-induced myofibroblast transdifferentiation has been reported previously (Y. C. Chang et al., 2014). To understand the contribution of Slug in the pathogenesis of OSF, we evaluated the expression of Slug after arecoline treatment. As shown in Figure 3a,b, the gene and protein expression of Slug increased in a dose-dependent manner following arecoline stimulation. In consistent with these results, the immunofluorescent imaging also showed that the expression of Slug and α-SMA-positive stress fibers were dose-dependently enhanced in response to arecoline (Figure 3c). Subsequently, we knockdown Slug (Figure 3d) to investigate whether it could impede the arecoline-induced transdifferentiation of myofibroblasts. Our results demonstrated that inhibition of Slug successfully blocked the myofibroblast hallmarks including increased collagen gel contraction (Figure 3e), migration (Figure 3f), and invasion (Figure 3g). These findings suggested that Slug may be the key factor that involves in the arecoline-increased myofibroblasts activities.
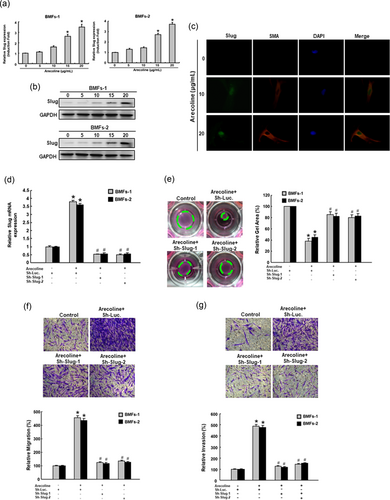
Slug is essential for the arecoline-induced myofibroblast activities in normal BMFs. The mRNA (a) and protein (b) expression of Slug was dose-dependently elevated in response to arecoline stimulation. *p < 0.05 compared with no arecoline treatment. (c) The immunofluorescent images of α-SMA + myofibroblasts expressed Slug following treatment with various concentration of arecoline. (d) The arecoline-induced Slug was abrogated by knockdown of Slug. The arecoline-activated myofibroblast hallmarks, including collagen gen contractility (e), migration (f), and invasion (g) were all impeded by repression of Slug. Results are means ± SD of triplicate samples from three experiments. *p < 0.05 compared with Sh Luc. group. #p < 0.05 compared with arecoline + Sh-Luc. group. BMFs: buccal mucosal fibroblasts; α-SMA: α-smooth muscle actin
3.4 Overexpression of Slug increases the features of myofibroblasts
To verify that Slug was able to induce the myofibroblast activation, ectopic expression of Slug in BMFs was carried out and we showed that increased Slug upregulated the myofibroblast marker, α-SMA, and type I collagen (Figure 4a). Moreover, the contractility of BMFs embedded into a collagen gel was increased (Figure 4b) as well as cell motility, including migration (Figure 4c) and invasion (Figure 4d).
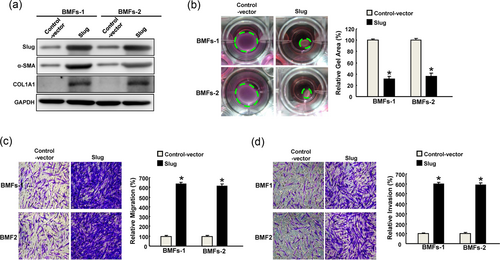
Overexpression of Slug in normal BMFs increases the myofibroblasts activities. (a) The ectopic expression of Slug was confirmed by Western blot analysis following overexpression of Slug. The phenotypic behaviors of myofibroblast, including collagen gen contractility (b), migration (c), and invasion (d) were all increased after overexpression of Slug. Results are means ± SD of triplicate samples from three experiments. *p < 0.05 compared with control vector. BMFs: buccal mucosa fibroblasts
3.5 Slug activates the expression of type I collagen by direct binding to its E-box
We analyzed the data from The Cancer Genome Atlas (TCGA) and found that Slug was positively correlated with several myofibroblast- and ECM-associated markers in oral cancer tissues, including, α-SMA, type I collagen, fibronectin, and vimentin (Figure 5a–d), suggesting that Slug may promote not only fibrosis but also tumorigenesis.
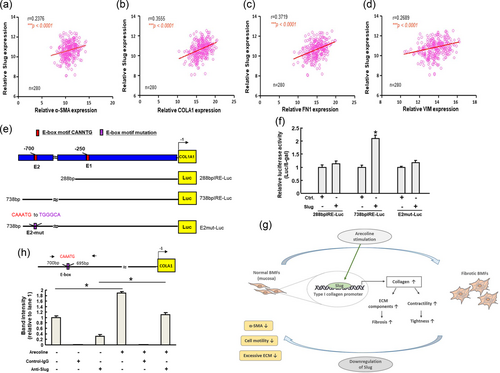
Slug directly binds to the COL1A1 promoter via its E-box. We analyzed the data from TCGA to elucidate the importance of Slug in association with other fibrosis markers in oral cancer. Slug was positively correlated with various fibrosis-associated markers, including α-SMA (a), type I collagen (b), fibronectin (c), and vimentin (d) by Pearson’s correlation. Results are means ± SD of triplicate samples from three experiments. (e) Schematic representation of E-box domain in type I collagen promoter region and reporter constructs. The full-length, deletions, and mutated promoter reporter constructs will be designed and subjected to luciferase reporter assay. (f) Reporter activity of tested reporter plasmids after cotransfection with control vector (pcDNA3), wild-type Slug. (g) An illustration of the region containing the E-box domain in the α-SMA promoter detected by a ChIP assay. Lanes 1 and 4 represented input chromatins from nontreated and arecoline-treated cells, respectively. Lanes 2 and 5 were the control IgG-precipitated chromatins from nontreated and arecoline-treated cells. Lanes 3 and 6 indicated the anti-Slug antibody precipitated chromatins from nontreated and arecoline-treated cells. The binding of Slug to the E-box domain of COL1A1 promoter was increased after arecoline stimulation. The intensities of each band were relative to land 1. Results are means ± SD of triplicate samples from three experiments. *p < 0.05 compared with control group or as indicated. Graphical abstract of the current study. In normal BMFs, arecoline stimulation will elevate the expression of Slug, which directly binds to the promotor of type I collagen, leading to increased collagen deposition and myofibroblasts transdifferentiation. In fibrotic BMFs, inhibition of Slug will reduce the expression of the myofibroblast marker, α-SMA, and the myofibroblast activities as well as downregulate the ECM accumulation, which may relieve the fibrotic condition. BMFs: buccal mucosa fibroblasts; TCGA: The Cancer Genome Atlas; α-SMA: α-smooth muscle actin
Apart from modulating the myofibroblast activities, we sought to further elucidate whether Slug contributes to the excessive ECM accumulation in OSF. We noticed that there were E-box regions in the promoter region of type I collagen (COL1A1; Figure 5e), indicating that Slug may induce COL1A1 expression by directly binding to its promoter. Hence, we performed luciferase-based reporter assay to investigate whether the COL1A1 promoter can be activated by Slug and identify the binding site for Slug within the COL1A1 promoter. This was done using reporter constructs containing wild-type, mutated, and deleted lengths of the COL1A1 promoter (Figure 5e). An increase in COL1A1 promoter activity was observed after transient transfection with Slug (Figure 5f, the “738 bp” group), while deleting a region containing the E2 box (Figure 5f, the “288 bp” group) and site-directed mutagenesis of the E2 E-box hampered COL1A1 activation. By ChIP, we found that arecoline treatment of BMFs increased the binding of Slug to the COL1A1 promoter (Figure 5g). Taken together, our data demonstrated that arecoline stimulation enhanced the direct binding of Slug to the E-box region in the COL1A1 promoter, which may increase the deposition of collagen and further exacerbate the activation of myofibroblast (Figure 5h).
4 DISCUSSION
Several studies have shown that areca nut ingredients induced the production of inflammatory factors and cell cycle arrest in oral keratinocytes or oral KB epithelial cells, which may be associated with OSF and oral cancer (M. C. Chang et al., 2001, 2004, 2005; Jeng et al., 2000). Despite it is widely accepted that consumption of areca nut is implicated in the pathogenesis of OSF, the key molecule that contributes to oral fibrogenesis still needs to be identified. TGF-β has been indicated as a major cytokine that involves in the progression of OSF (Pant, Kumar, Khan, Rao, & Kondaiah, 2015) and regulates the expression of α-SMA and type 1 collagen in myofibroblasts (Desmouliere, Geinoz, Gabbiani, & Gabbiani, 1993; Reed, Vernon, Abrass, & Sage, 1994). Besides, it has been demonstrated that TGF-β pathway induces Snail and Slug expression via Smads and HMGA2 (Thuault et al., 2006, 2008). Our previous work has shown that Snail was capable of binding to the promotor of α-SMA (Yang et al., 2018), and the increased expression of α-SMA enhances fibroblast contractile activity (Hinz et al., 2001). The current study revealed that Slug was able to directly bind to type I collagen, the most abundant fibrous protein within ECM. Under pathological condition of ECM remodeling, the accumulating collagen deposition can promote the differentiation of myofibroblasts (Hong et al., 2017). Moreover, since areca nut elevates the expression of both TGF-β (Khan, Kumar, Pant, Narra, & Kondaiah, 2012) and Slug (Figure 3a), it is likely that areca nut increases Slug by upregulation of TGF-β and subsequently affects the myofibroblast activities and collagen expression (Figure 4), leading to excessive accumulation of fibrous tissue.
Additionally, we analyzed the TCGA data of oral cancer and found that Slug was positively correlated with the myofibroblast markers (α-SMA and vimentin) and EMT-associated fibronectin and type I collagen. α-SMA-positive myofibroblasts have been indicated as a prognostic factor for oral cancer (Ding et al., 2014), and vimentin and fibronectin were both associated with the invasiveness of oral cancer (Liu et al., 2016; Ramos Gde et al., 2016). Accordingly, it is possible that Slug also involves in the progression of oral malignancy. Although this hypothesis requires further investigation, it has been previously demonstrated that TGF-β induced EMT and oral cancer migration through Slug (Saito et al., 2013). Besides, numerous studies have shown that Slug was important in tumorigenesis and cancer aggressiveness (Cobaleda, Perez-Caro, Vicente-Duenas, & Sanchez-Garcia, 2007; Perez-Mancera et al., 2005; Wu et al., 2013). It has been demonstrated that TGF-β1 increases Slug via ERK1/2 signaling, leading to invasion of oral cancer cells (Joseph et al., 2009). In addition, the upregulated expression of Slug was found to be associated with drug resistance in oral cancer (Nakamura et al., 2018). TGF-β1 inhibitor treatment significantly reverted arecoline-induced Slug expression in BMFs (data not shown). Altogether, these findings denote the importance to inhibit the abnormal elevation of Slug in myofibroblasts before malignant transformation.
In conclusion, our data suggested that Slug was not simply an EMT driver, it was also crucial for activation of myofibroblasts after arecoline stimulation through affecting phenotypical behaviors and myofibroblast markers. Furthermore, we demonstrated that arecoline treatment enhanced the direct binding of Slug to type I collagen via E-box, which may lead to the accumulation of ECM and further exacerbate the activation of myofibroblast (Figure 5g). Slug was also associated with various fibrosis markers in oral cancer tissues via analyzing TCGA data. Consequently, approaches to eliminate the aberrant Slug expression may be a promising avenue to improve OSF symptoms and prevent the progression to oral cancer.
ACKNOWLEDGMENT
This study was supported by grants from Taipei Medical University-Wan Fan Hospital (106TMU-WFH-09) and Ministry of Science and Technology (MOST 107-2314-B-040-023) in Taiwan.
CONFLICTS OF INTERESTS
The authors declare that there are no conflicts of interest.




