Efficient penetration of Scp01-b and its DNA transfer abilities into cells
Abstract
The in vivo application potential of viral-based gene delivery approaches is hindered by a risk of insertional oncogenesis. Of the many delivery methods, cell-penetrating peptides (CPP)-based delivery has good biocompatibility and biodegradability. However, low efficiency is still the disadvantage of CPPs-based nucleic acid transfection, and delivery efficiency may vary from different CPPs. Here, we describe Scp01-b, as a new CPP, which can enter cultured cell lines and primary cultured cells examined by fluorescence microscopy and quantitative assay, the internalization process is a concentration, temperature, and incubation time-dependent manner. Scp01-b does not insert into the membrane directly and its uptake is mediated through endocytosis pathway. Moreover, Scp01-b could mediate the uptake of plasmid DNA into the Caski and HSC-T6 cells, and we noted that Scp01-b-mediated transfection efficiency was nearly the same with traditional liposome (TurboFectin)-mediated transfection. These findings suggest that Scp01-b can act as a useful tool for non-viral-based delivery in further application such as reprogramming and gene editing.
1 INTRODUCTION
To target and modify malicious genes by inserting corrected genes within the genome for genetic disease treatment, an efficient system for gene delivery is required. Numerous viral-based approaches have been widely used in basic research; however, improvements in their in vivo applications are hindered by the risk of their damage to the host’s genes, immune system and their potential for infection (Deng et al., 2015; Guidotti, Brambilla, & Rossi, 2017; Liu et al., 2016). Therefore, delivery of therapeutics, especially biomacromolecules using the nonviral approach, is being investigated as of today. A variety of delivery approaches such as lipoplexes, polyplexes, and cell-penetrating peptides (CPPs, also named as a protein transduction domain) have received great attention as an efficient cellular delivery vehicle for kinds of cargoes (Liu et al., 2016), like conventional drugs, fusion proteins, nanoparticles as well as nucleic acids or plasmid DNA (pDNA), it is worth noting that CPP-based delivery has good biocompatibility and biodegradability compared with other delivery tools.
As nonviral delivery vector, CPPs are still in its infancy and have the potential to grow exponentially. CPPs possess positively charged residue such as arginine and lysine (Rüter & Schmidt, 2017), so they can easily form complex with nucleic acids and then get inside the cells. CPP-DNA condensed particles not only can disrupt the endosomal membrane, but also can escape proteasomal degradation, and further efficiently traffic DNA to serve as a vector capable of packaging and targeting DNA for efficient delivery to the nucleus. Although the advantages of CPP-mediated nucleic acid transfection include low toxicity, reduced immunogenicity and thus safety in use, transfection efficiency is extremely low so that it is very hard to achieve expected therapeutic effects because of their low molecular weight, unstable linear structure, and weak nucleic acid condensation capability (Kurrikoff, Gestin, & Langel, 2016; Lehto, Ezzat, Wood, & El Andaloussi, 2016; Rádis-Baptista, Campelo, Morlighem, Melo, & Freitas, 2017; Zhang, Wang, & Xu, 2016). To improve their delivery ability, various methods have been developed with the substitution optimized residues, conjugating with nonnatural chemical moieties, as well as coincubation with penetration enhancer (Liu et al., 2016; Ma, Wang, Wang, Luo, & Liu, 2015; Wang, Ma, Yang, Song et al., 2016; Wang, Ma, Yang, Zeng, & Liu, 2016; Wang, Zhang, Zeng, & Liu, 2016; Wang, Zhong, Wu, Huang, & Liu, 2010). However, low efficiency is the disadvantage of CPPs-based nucleic acid transfection, so further investigations are required to improve their cystolic delivery efficiency. Based on previous reports (Liu et al., 2016), we noted that most of positively charged CPPs for nucleic acid delivery are shorter than 30 amino acids, although (Wadhwa et al., 1997) systematically investigated the polylysine peptide containing 3–36 lysine residues introducing foreign gene expression, and whether longer CPPs can improve nucleic acid delivery efficiency is still unknown.
Here, we designed and synthesized a new peptide Scp01-b with longer residue chain than a conventional peptide, and analyzed its structure, penetration efficiency, the probable penetration mechanism, safety, and its mediation of pDNA transfection. Thus, we expect that Scp01-b-mediated pDNA delivery will facilitate CPPs-based transfection.
2 MATERIALS AND METHODS
2.1 Peptide synthesis
Fluorescein isothiocyanate (FITC)-labeled peptides (N-terminal) used throughout this manuscript were synthesized from SBS Genetech (Beijing, China), and purified using reversed phase analytical high-performance liquid chromatography (>99% purity). Peptides were dissolved in phosphate-buffered saline (PBS; 500 mM) and stored in −20°C freezer for further use. Labeled peptide sequence: NCO (KALGISYGRKK), hPP3 (KPKRKRRKKKGHGWSR), TAT(YGRKKRRQRRRK), and hPP10 (KIPLPRFKLKCIFCKKRRKR).
2.2 Bioinformatic assay
Penetrating ability was predicted using a support vector machine-based CellPPD web server (Gautam et al., 2013). The threshold or value to the software was the same as reference described. The three-dimensional (3D) structure of the peptide was predicted by the I-TASSER program (http://zhanglab.ccmb.med.umich.edu/I-TASSER) and Pepstr (peptide tertiary structure; Kaur, Garg, & Raghava, 2007). Schiffer–Edmundson wheel modeling was done by DNASTAR Inc. (Madison, WI) Lasergene 7 program. Energy minimization and molecular dynamic, as well as modeled structures, were validated by the VADAR (volume area dihedral angle reporter) web server (Wishart et al., 2008), and Molegro molecular viewer was used for presenting energy map and surface electrostatics of peptide. Membrane penetration depths and orientations of CPP with respect to the membrane binding affinities were predicted by PPM web server (http://opm.phar.umich.edu/; Lomize, Pogozheva, Joo, Mosberg, & Lomize, 2012).
2.3 Cells and cell culture
Human cervical carcinoma-derived Caski, human ovarian cancer cell line A2780, human osteosarcoma cells MG63 and rat hepatic stellate cell line HSC-T6 were maintained in our laboratory. RPMI 1640 supplemented with 10% fetal bovine serum (FBS) and 1% penicillin–streptomycin was used for cell culture, and cells were maintained in at 37°C in a humidified incubator containing 5% CO2.
2.4 Cellular uptake of Scp01-b
After cells (2 × 105 cells/well) seeded onto 24-well plates for 24 hr, serum-free RPMI 1640 was used to wash cells twice. Indicated concentrations of CPPs were added into the serum-free RPMI 1640 incubation medium. After incubation at 37°C for 1 hr, cells were washed with serum-free RPMI 1640 media over three times. Fluorescence microscope (Nikon, Tokyo, Japan) with a band-pass filter (detects FITC) was used to visualize the cellular uptake of the peptide.
Multimode spectrophotometry (Tecan, Mannedorf, Switzerland) can be used to quantify the intracellular uptake of CPP. The procedure was the same showed above. After the incubation and washing-over, cells were lysed using 0.1M NaOH, centrifuged the lysed cells at 14,000g for 5 min, and the supernatant was used to examine the fluorescence intensity. Total cellular protein concentration of the supernatant was examined using Bradford protein assay Kit. All experiments were performed at least three times.
For hyperosmotic treatment, previous published protocol was followed (Wang, Zhang et al., 2016). Briefly, on the day of treatment, cells were washed by PBS, then incubated with 0.6M glucose or 0.6M sucrose dissolved in RPMI 1640 medium and Scp01-b peptide for 1 hr. After three times wash with PBS, fluorescence quantification were performed following the protocol shown above.
For the penetration mechanism of Scp01-b, cell seeding was the same as described above. Cells were washed cells with PBS for at least three times and then incubated with peptide and different kind of inhibitors serum (10% FBS), NH4Cl (50 μM), NaN3 (40 μM), heparin (50 μg/ml), methyl-β-cyclodextrin (MβCD; 5.0 mM) for 1 hr. The same protocol was used as the one shown above to examine the uptake efficiency.
2.5 Cell viability assay
The 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide (MTT) assay was used to examine the cell viability after treatment with peptide. Cells were seeded into a 96-well plate and cultured for 24 hr, washed with PBS, and incubated with peptide at the indicated time and then cultured for 24 or 48 hr, incubated with 20 μl of MTT (5.5 mg/ml) reagent for another 2 hr. The absorbance of each well was determined at 550 nm using Multiskan Spectrum (Thermo Fisher Scientific, Waltham, MA) reader.
2.6 Lactate dehydrogenase leakage assay
Lactate dehydrogenase (LDH) leakage analysis was used to examine the integrity of the cell membrane. After cells were incubated with CPP at indicated concentrations, the substrate mixture was added in the plate contained with cell-free supernatant and incubated another 10 min. Absorbance of reacted product was measured at 560/590 nm using a Multiskan Spectrum (Thermo Fisher Scientific) plate reader.
2.7 Hemolysis assay
Hemolytic analysis was used to detect the red blood cell (RBC) membrane integrity under physiological conditions. After the whole blood collection, the reference protocol for RBC isolation was followed (Wang, Zhong et al., 2010). The optical density at 414 nm allowed evaluation of hemolysis.
2.8 Formation of pGFP complexes and gel retardation assay
The Scp01-b/pcDNA3.1-GFP complexes were prepared by mixing a pcDNA3.1-GFP (1 μg) at the different nitrogen per nucleic acid (N/P) ratios and incubated for 30 min at room temperature. To confirm the pDNA condensation capability of the Scp01-b, agarose gel retardation assay was performed. All of the samples were prepared as shown above. After 30 min of incubation, each sample was loaded into the well of an agarose gel. Agarose powder (0.4 mg) was dissolved in 40 mL of 1× Tris–acetate–EDTA (TAE) buffer to produce agarose gel.
2.9 Peptide-mediated transfection
B16 and Caski cells (4 × 104 cells/well) were seeded into the 24-well plates and incubated for 24 hr at 37°C. The cells were pretreated with 5% dimethyl sulfoxide (DMSO) for 30 min. The mixture of peptide–pDNA was gently added to the cells, and then incubated with peptide–pDNA complex at different N/P ratio and incubated for 4 hr. The cells were washed with PBS three times, and fresh RPMI 1640 supplemented with 10% FBS was added. The cells were then incubated for 24 or 48 hr. TurboFectin (OriGene China, Beijing, China) was used as a control reagent. After washing with PBS three times, the transfection efficiency was detected by a fluorescence microscope (Nikon) with a band-pass filter (detects FITC).
2.10 Statistical analysis
All values for experimental and control samples are expressed as means ± standard deviation (SD). The statistical analysis (one-way analysis of variance [ANOVA] and post hoc tests or two-way ANOVA for analysis) was performed by GraphPad software Prism 7.0 (GraphPad Software, San Diego, CA). statistical significance was considered when the p < 0.05.
3 RESULTS
3.1 Scp01-b peptide prediction
Prediction of Scp01-b via bioinformatic tools helps us to understand the penetration ability of Scp01-b systematically. Amino acid sequence of Scp01-b is shown in Supporting Information Figure S1A. Scp01-b has 22 positively charged residues, 9 hydrophobic residues, and 10 hydrophilic residues. Schiffer–Edmundson helical wheel representation of the Scp01-b is shown in Figure 1a, which suggests a possible amphipathic α-helical conformation. Relative surface accessibility (Figure 1b), secondary structure prediction (Figure 1c) were examined using NetSurfP web server, and 3D prediction by I-TASSER server are developed (Figure 1d). Moreover, average hydrophobicity of Scp01-b, TAT, hPP3, and hPP10 was examined by Bachem Peptide Calculator (Supporting Information Figure S1B), and Ramachandran plot analysis of Scp01-b is shown in Supporting Information Figure S1C. Furthermore, the 3D structure, energy map, surface electrostatics, and surface hydrophobicity of Scp01-b are displayed in Figure 1e, had a hand-like-shaped structure, the 3D distribution of all the positive-charged residues will be helpful for the negative-charged molecule binding, such as the DNA, and this may be functionalized for intracellular delivery.

Structure of Scp01-b peptide prediction. (a) Scp01-b peptide was represented as Schiffer–Edmundson helical wheel. (b) Relative surface accessibility prediction of Scp01-b using NetSurfP web server. (c) NetSurfP web server prediction on Scp01-b secondary structure. (d) I-TASSER server predicted structural model of Scp01-b. (e) Representation tridimensional structure, energy map, surface electrostatics, and surface hydrophobicity of Scp01-b. 3D: three dimensional [Color figure can be viewed at wileyonlinelibrary.com]
3.2 Penetration assay of Scp01-b
The documented success of the CPP penetration in in vitro assay can serve as a justification for the concentration range selection (Wang, Zhong et al., 2010; Wang, Zhang et al., 2016; Wang, Ma, Yang, Song et al., 2016; Wang, Ma, Yang, Zheng et al., 2016). FITC-labeled Scp01-b at indicated concentrations from 2.5 to 10 µM was incubated with Caski cells for 1 hr, the fluorescence microscopy was conducted to examine its penetration ability. The fluorescence was apparent at the concentration of 5 µM, although the concentration at 2.5 µM was very weak (Figure 2a). To analyze the precise penetration ability of Scp01-b, quantitative analysis of Scp01-b uptake in Caski cells was conducted using multimode spectrophotometry. As showed in Figure 2b, internalization of Scp01-b was significantly increased from 2.5 to 5 µM compared with NCO group, although the uptake of Scp01-b at 7.5 or 10 µM also increased. The present data does not appear that the cellular uptake is linearly correlated, which may result from the molecule size as well as the 3D conformation of the Scp01-b, even the unknown receptor from the cell membrane, this unknown process may need investigation further. We also analyzed the effect of different incubation time on Scp01-b penetration, Figure 2c clearly indicated that longer incubation promoted the internalization of Scp01-b, although differently from other peptides reported previously of our lab; this may due to the different characteristics of different peptide.
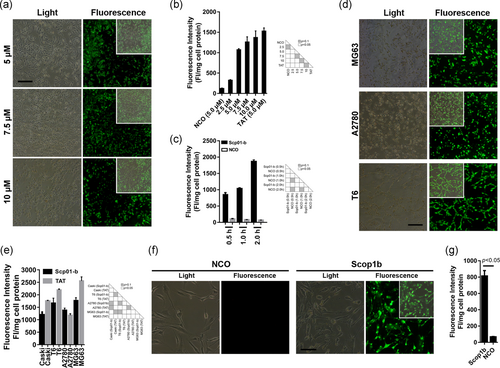
Cellular uptake of Scp01-b in different cells. (a) Fluorescence microscopy of FITC-labeled Scp01-b with different concentrations for 1 hr incubation. (b) Quantization analysis of FITC-labeled Scp01-b corresponding to the fluorescence microscopy. Data are presented as means ± SEM (n = 3), one-way analysis of variance (ANOVA) with Tukey–Kramer’s post hoc test. (c) Fluorescence quantization assays assessing effects of the incubation time on the cellular uptake of Scp01-b. Data are presented as means ± SEM (n = 3), one-way ANOVA with Tukey–Kramer’s post hoc test. (d) Uptake of Scp01-b in cancer cell lines HSC-T6, A2780, and MG63 examined by fluorescence microscope. (e) Fluorescence quantization analysis of Scp01-b in different cell lines. Data are presented as means ± SEM (n = 3), one-way ANOVA with Tukey–Kramer’s post hoc test. (f) Cellular uptake of Scp01-b primary cultured mouse synovial cells. NCO peptide was used for a control. (g) Fluorescence quantization assay of the primary cultured mouse synovial cells’ uptake of Scp01-b. Data are presented as means ± SEM (n = 3), ANOVA with Student’s t test [Color figure can be viewed at wileyonlinelibrary.com]
Moreover, Scp01-b penetration in different cell lines was evaluated. As showed in Figure 2d,e it is clearly suggested that Scp01-b could entry into HSC-T6, A2780, and MG63 cell lines. Next, to examine whether Scp01-b has a penetration ability in primary cultured cells, primary cultured mouse synovial cells were incubated with Scp01-b under the same condition of cancer cells penetration assay. The fluorescence microscopy (Figure 2f), quantization assay (Figure 2g) were conducted, suggesting that Scp01-b can penetrate both cell lines and primary cultured cells.
3.3 Efficient Scp01-b internalization and its peptide–membrane interaction
To compare the penetration ability of Scp01-b with other CPPs reported previously, Caski cells were incubated with hPP10, TAT, MT23 (Zhou et al., 2017), and RP010. The results indicated that Scp01-b has higher penetration efficiency than RP010 and MT23 (Supporting Information Figure S2), but weaker than hPP10 and TAT (Figure 3a). Using penetration enhancer such as DMSO and hyperosmotic molecules (glucose or sucrose) in previous work suggests that Scp01-b penetration is higher when treated with hyperosmotic treatment (Figure 3b, Supporting Information Figure S3). Next, from the fluorescence quantization assay, our results reveal that Scp01-b has a lower penetration ability in the low temperature (Figure 3c), similar to other peptides reported previously, which suggests that Scp01-b may possibly be mediated by an energy-dependent manner. Cellular uptake of Scp01-b was inhibited as the incubation medium contained serum (Supporting Information Figure S4). The fact that Scp01-b internalization was blocked by serum (10% FBS) and ammonium chloride (50 mM), the endosome release inhibitor of cellular uptake, suggests that endocytosis may be involved in the penetration of Scp01-b (Figure 3d). Oxidative phosphorylation inhibitor sodium azide (40 μM) inhibited Scp01-b cellular uptake, suggesting that internalization of Scp01-b is ATP dependent (Supporting Information Figure S4). To determine the precise mechanism for internalization, we examined the penetration efficiency of Scp01-b being treated by different inhibitors. Scp01-b internalization decreased significantly after being incubated in heparin (50 μg/ml), an inhibitor of heparan sulfate on the cell surface (Wang, Ma, Yang, Zeng et al., 2016). This suggests that Scp01-b internalization requires interaction with glycosaminoglycans (Supporting Information Figure S4). Scp01-b internalization also significantly decreased after being treated with MβCD (5 mM), an inhibitor of lipid raft-mediated endocytosis, suggesting that lipid raft-mediated endocytosis may mediate intracellular delivery of Scp01-b.
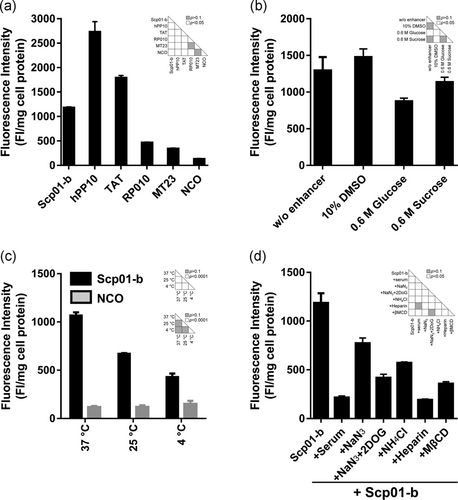
Effects of Scp01-b uptake under different conditions. (a) Comparison of Scp01-b to other CPP reported from our group previously. Data are presented as means ± SEM (n = 3), one-way analysis of variance (ANOVA) with Tukey–Kramer’s post hoc test. (b) Effects of Scp01-b incubated with different penetration enhancers. Data are presented as means ± SEM (n = 3), one-way ANOVA with Tukey–Kramer’s post hoc test. (c) Scp01-b internalization in Caski cells at different temperature. Data are presented as means ± SEM n = 3), two-way ANOVA with Tukey’s multiple comparisons test. (d) Suppressive effect of different inhibitors’ exposure on Scp01-b uptake. Data are presented as means ± SEM (n = 3), one-way ANOVA with Tukey–Kramer’s post hoc test. DMSO: dimethyl sulfoxide; MβCD: methyl-β-cyclodextrin
Moreover, the interaction between Scp01-b peptide and membrane was predicted by PPM web server, as shown in Figure 4a. The widely researched TAT, as well as newly found peptide hPP3 and hPP10, can fully or partially insert into the membrane, which suggested that direct penetration of these peptides may occur under some conditions, although cellular uptake of these peptides may also mediate endocytosis. However, Scp01-b cannot insert into membrane; this result was consistent in the data shown above. Additionally, hydrophobic thickness, ΔGtransfer, tilt angle and embedded residues were shown in Figure 4b.
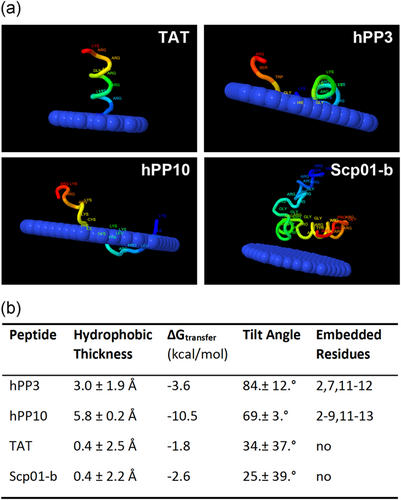
Scp01-b peptide–membrane interaction predicted by PPM web server. (a) Different CPP-membrane prediction, hPP10 and hPP3, is the CPP we reported before. TAT was used as control. (b) Parameters of these four CPP-membrane prediction, embedded residue represents the loci of peptide from N to C terminal. CPP: cell-penetrating peptides [Color figure can be viewed at wileyonlinelibrary.com]
3.4 Assessment of cytotoxicity of Scp01-b
Cytotoxicity assay was conducted to investigate the effect of Scp01-b on cell viability and growth. T6 and Caski cells were treated with indicated concentration of Scp01-b for 1 hr. After 1, 24, 48, and 72 hr later, MTT assay was conducted. The data presented in Figure 5a (T6 cells) and Figure 5b (Caski cells) suggest that a nearly zero repressive effect on cell growth, which indicated that Scp01-b treatment was noncytotoxicity. Next, hemolysis (Figure 5c), LDH release (Figure 5d) assay were conducted to investigate its membrane disturbance. The data showed here indicates no membrane disturbance in RBC or Caski cell line.
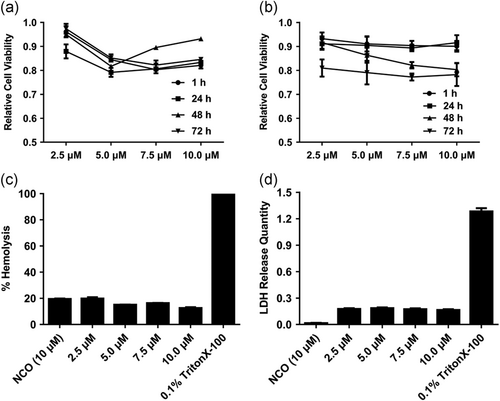
Cytotoxicity and safety evaluation on Scp01-b. (a) Viability of HSC-T6 cells treated with Scp01-b at different test concentrations examined by MTT assay. Data are presented as means ± SEM (n = 3), p > 0.05, two-way analysis of variance (ANOVA) with Bonferroni’s multiple comparison test. (b) Viability of Caski cells treated with Scp01-b at indicated concentrations. Data are presented as means ± SEM (n = 3), p > 0.05, two-way ANOVA with Bonferroni’s multiple comparison test. (c) Hemolysis assay was used to evaluate the cell membrane integrity after different concentration of Scp01-b treatment. Positive control using 0.1% Triton X-100. Data are presented as means ± SEM (n = 3), p > 0.05, one-way ANOVA with Tukey’s multiple comparisons test. (d) LDH release quantity was evaluated in Caski cells treated with Scp01-b at different concentrations, 0.1% Triton X-100 was used as positive control. Data are presented as means ± SEM (n = 3), p > 0.05, one-way ANOVA with Tukey’s multiple comparisons test. MTT: 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide; LDH: lactate dehydrogenase
3.5 Scp01-b-mediated transfection
Before detection of Scp01-b-mediated pcDNA3.1-GFP transfection, the electrostatic peptide-DNA complex was examined by electrophoretic mobility of an 1% (wt/vol) agarose gel stained with ethidium bromide. The tests were conducted by mixing 0.1 µg of pcDNA3.1-GFP plasmid with the peptide at the various N/P ratio. As showed in Figure 6a, no migration of the complex occurred at a ratio of 10:1. This result suggests that Scp01-b can be bound with pDNA and form complex. Then, we also examined the HSC-T6 (Figure 6b), Caski (Figure 6c) cell viability treated by Scp01-b and pDNA complex on 24 and 48 hr and found out that there was no significant cell growth inhibition treated by these complexes at lower N/P ratio. To examine the transfection efficiency, TurboFectin was used as a control reagent (Figure 6d). Efficiency of Scp01-b-mediated pcDNA3.1-GFP transfection in Caski cells (Figure 6e,f) T6 cells (Figure 6g,h) was determined. After 24 (Figure 6e,g) and 48 hr (Figure 6f,h) transfections, the green fluorescent protein (GFP) expression was detected by a fluorescence microscope. In Figure 6e,f, GFP expression could be detected from the ratio of N/P = 5:1 to 40:1, while, at the ratio of N/P = 80:1, nearly no GFP expression could be detected. In Figure 6g,h, GFP expression can be detected from the ratio of N/P = 5:1 to 80:1, although few cells expressed the GFP in the N/P = 80:1 group. Lastly, we found that transfection efficiency of Scp01-b was nearly the same with TurboFectin. Collectively, these data suggested that Scp01-b could form a complex with pDNA, and even can mediate pDNA transfection as well.
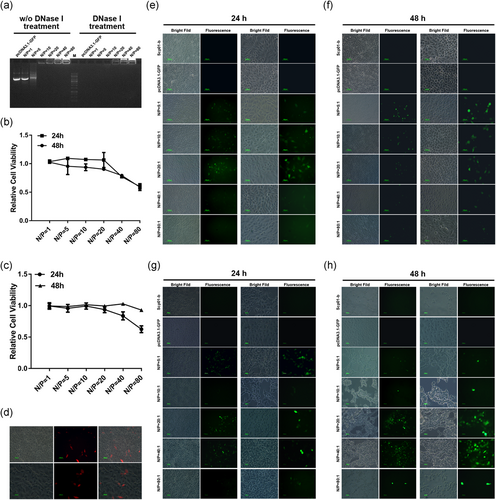
Scp01-b-mediated pcDNA3.1-GFP plasmid transfection. (a) Gel retardation assay on different N/P ratio of Scp01-b and pDNA. (b) Cell viability of HSC-T6 cells treated with Scp01-b and pDNA examined by MTT assay. Data are presented as means ± SEM (n = 3), p > 0.05, two-way analysis of variance (ANOVA) with Bonferroni’s multiple comparison test. (C) Cell viability of Caski cells treated with Scp01-b and pDNA examined by MTT assay. Data are presented as means ± SEM (n = 3), p > 0.05, two-way ANOVA with Bonferroni’s multiple comparison test. (d) pDNA transfection using TurboFectin in Caski cells. (e) Fluorescence microscopy of GFP expression in Caski cell transfected by Scp01-b DNA complex after 24 hr, pcDNA3.1-GFP plasmid incubation only used as negative. The right column represents higher magnification to the left column. (f) Fluorescence microscopy of GFP expression in Caski cell transfected by Scp01-b DNA complex after 48 hr. (g) Fluorescence microscopy of GFP expression in HSC-T6 cell transfected by Scp01-b DNA complex after 24 hr. (h) Fluorescence microscopy of GFP expression in HSC-T6 cell transfected by Scp01-b DNA complex after 48 hr. GFP: green fluorescent protein; MTT: 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide; pDNA: plasmid DNA [Color figure can be viewed at wileyonlinelibrary.com]
4 DISCUSSION
Most of the tools used for gene delivery are a viral-based method, and this integration method may damage the host genes, host immune system stimulation and infection potential, which limit their therapeutic clinical applications (Deng et al., 2015). Although many other organic synthesized macromolecules were developed, their biocompatibility and safety should be considered. Peptide-based delivery has attracted much attention since the first HIV-1 TAT peptide was discovered (Liu et al., 2016; Raucher & Ryu, 2015). Because of the natural characters, CPP-based applications, such as CPP-mediated stem cell reprogramming, have attracted a lot of attention since the clinical trials of CPP application.
In this study, we found that Scp01-b has good penetration ability, using a concentration, and incubation time-dependent manner to enter cells, and its penetration efficiency can be partially enhanced by penetration enhancers. Although the exact mechanism of the Scp01-b was not clarified, our data suggest that it may be mediated by endocytosis. Based on PPM web server prediction, Scp01-b cannot insert into the membrane directly, and this result helps us know more about the penetration mechanism of this peptide. Moreover, Scp01-b internalization did not affect the cell growth and the cell membrane, which means that the internalization is safe for the peptide-based delivery. Additionally, Scp01-b DNA delivery ability was examined. Scp01-b can mediate the pDNA transfection via noncovalent conjugation; this will be convenient for us to deliver any kind of nucleic acid to target cells for further therapeutic applications. We found that Scp01-b can be used for DNA delivery just using noncovalent conjugation, and we noted that Scp01-b-mediated transfection efficiency was nearly the same with traditional liposome (TurboFectin)-mediated transfection. Although the transfection reagent as well as electrotransfection can deliver exogenous DNA, transfection reagents or high pressure that may damage the membrane of the cell, and for most cells, including cells that actively sample their environment, like macrophages, transfection efficiency is still very low. Therefore, we think that Scp01-b-based transfection is an option for other applications, such as DNA delivery of in vitro tissue or organ culture as well as in vivo assay in an animal model. Moreover, modification of Scp01-b and CPP chain is an alternative strategy for efficient DNA delivery.
In summary, we have found a new peptide Scp01-b with more residues compared to conventional CPPs, and this cell-permeable peptide can efficiently mediate nucleic acid intracellular delivery, acting as a powerful DNA carrier for non-viral-based reprogramming in regenerative medicine and the field of gene editing research (Guo, Wang, & Hu, 2013; Liu et al., 2016; Wang, Jiang et al., 2014; Zeng et al., 2015).
ACKNOWLEDGMENTS
We are thankful to the National Nature Science Foundation of China (Grant No. 81501330) and Science Foundation of CTGU (Grant No. KJ2014B066).
CONFLICTS OF INTEREST
The authors declare that there are no conflicts of interest.




