Formulation and evaluation of anticancer and antiangiogenesis efficiency of PLA–PEG nanoparticles loaded with galbanic acid in C26 colon carcinoma, in vitro and in vivo
Abstract
Galbanic acid (GBA) is an active sesquiterpene coumarin derivative, with various medicinal benefits, including anticancer properties. However, the low solubility of GBA is the main limitation of its clinical applications. In this study, we used a nanosystem based on poly (D, l-lactide)–polyethylene glycol (PLA–PEG), for the delivery of GBA to C26 colon carcinoma cells. The physicochemical characteristics of nanoparticles (NPs) prepared by the emulsification–evaporation method were evaluated. MTT assay was used to compare the anticell proliferation of GBA and PLA–PEG–GBA against C26 cell lines. PLA–PEG-NPs with an average size of about 140 nm had an enhanced release of GBA at a pH of 5.5 compared with a pH of 7.4. Cytotoxicity studies showed that the IC 50 of the PLA–PEG–GBA NPs (8 µM) was significantly lower than free GBA (15 µM). In the in vivo study, PLA–PEG–GBA NPs exhibited remarkable efficacy and reduced in vivo toxicity in C26 colon carcinoma tumor-bearing female BALB/c mice. To study the antiangiogenesis effect of the NPs, tumor sections were stained with an anti CD34 antibody. The results show the CD34 (+) vessels were decreased in the GBA and PLA–PEG–GBA treated mice by more than 75% and 90%, respectively. These results suggest that the encapsulation of GBA into the PLA–PEG could potentially be used for the treatment of colorectal cancer.
1 INTRODUCTION
Chemotherapy as the main strategy for cancer treatment is limited by its unsatisfactory efficacy, high cytotoxicity, and drug resistance (Pilkington et al., 2015). In recent years, numerous studies have focused on using natural products in cancer therapy because of their high efficacy and low toxicity (Cragg & Newman, 2001; Emami et al., 2015; Fernández, 2006; Kim et al., 2011). Genus Ferula belonging to the family Apiaceae has been reported to be an excellent source of biologically active compounds such as terpenoid coumarins and sesquiterpene derivatives (Iranshahi et al., 2008). Galbanic acid (GBA) as a sesquiterpene coumarin isolated from the resin of Ferula has drawn special attention for its interesting biological properties including antithrombotic, anticoagulant, antiviral, antileishmanial, anti-inflammatory, and anticancer activities. GBA inhibits the growth of tumor cells via the permeability glycoprotein (P-gp) inhibitory effect and, antiangiogenic activity, as well as inducing apoptosis by reducing androgen receptor (AR) abundance in AR (+) prostate cancer (PCa) cells (Kasaian, Iranshahy, & Iranshahi, 2014).
However, low solubility of the GBA is the main problem for its clinical application. One of the effective strategies to overcome its solubility problem is the formulation of lipophilic natural compounds using appropriate carriers (Liang, Friedman, & Nacharaju, 2017). Poly (D, l-lactide; PLA) is a biocompatible and biodegradable polymer that can be loaded with a variety of bioactive agents such as hydrophobic drugs (Avgoustakis, 2004). However, the application of PLA as a drug delivery system is limited due to hydrophobic surface properties and lack of readily reactive side-chain groups (Xiao, Wang, Yang, & Gauthier, 2012). Various researchers have modified PLA nanoparticles with different agents such as, cholesterol and graphene oxide (Jin, Wang, Ke, Wang, & Dai, 2013; Kumari et al., 2017). PLA surface coating with the hydrophilic polymeric chain and, polyethylene glycol (PEG), leads to its steric stabilization against phagocytosis by macrophages, and increases blood circulation following intravenous administration (Gref et al., 1995). In this study, a nanosystem was utilized based on PLA–PEG nanoparticles (PLA–PEG-NP) containing GBA to evaluate its in vitro cytotoxicity and in vivo antitumor activity in C26 tumor-bearing mice.
2 MATERIALS AND METHODS
2.1 Materials
Methoxy-PEG–PLA (5/16 kD) and PLA–PEG-COOH (16/5 kD) were obtained from Polyscitech (West Lafayette, IN) and, polyvinyl alcohol (PVA) (31000–50000 Da) was purchased from Sigma-Aldrich (Darmstadt, Germany). All chemicals including acetone, methanol, acetonitrile, and dichloromethane were purchased from Merck (Darmstadt, Germany). The dialysis membrane Spectra/pro (MWCO, 10 kDa) was bought from Spectrum Laboratories Inc. (Rancho Dominguez, CA). The BCA protein quantification kit was obtained from Pars Tous Biotechnology (Mashhad, Iran). Protease inhibitor cocktail tablets were purchased from Roche Diagnostics GmbH (Berlin, Germany). Nonidet P40 substitute was purchased from (Sigma-Aldrich). Amicon® Ultra-15 centrifugal filter devices were obtained from Millipore (Darmstadt, Germany). The primary antibody of CD34 was bought from Abcam, (Berlin, Germany) and, enzyme-labeled polymer EnVisionTM was obtained from Dako A/S (San Francisco, CA). DAB (3,3′-diaminobenzidine) for the visualization of the peroxidase reaction was purchased from Sigma-Aldrich. Galbanic acid was provided by Golexir Pars Co. (Mashhad, Iran).
2.2 Cell culture
The C26 cell line (C532, murine colon carcinoma cell) was purchased from the Pasture Institute (Tehran, Iran). Cells were maintained in RPMI-1640 medium, supplemented with 10% fetal bovine serum and 1% penicillin streptomycin (Sigma) in the standard humidified environment of 37°C and 5% CO2.
2.3 Preparation of PLA–PEG nanoparticles containing GBA
PLA–PEG nanoparticles were prepared by a single emulsion–solvent evaporation method. Briefly, the PLA–PEG-COOH (25 mg) and GBA (1.25 or 2.5 mg) were dissolved separately in the organic phase by mixing together acetone and dichloromethane (1:4 ratio, 1 ml), and stirring for 30 min. The resulting preparation was then added gradually to the water phase (polyvinyl alcohol [PVA] 5%), and sonicated using an ultrasonic probe at 80% amplitude for 20 min (ultrasonic processor UP200Ht, Hielscher Co., Germany).
The resulting NPs suspension was diluted with an aqueous solution (0.1% PVA). To evaporate the organic phase, the mixture was magnetically stirred overnight and then centrifuged (18,000 rpm for 20 min). The obtained NPs were washed three times with deionized distilled water. Finally, NPs were resuspended in 1 ml of deionized water containing sucrose as a cryoprotectant agent (5% w/v), and then lyophilized into a fine cake (freeze dryer, Operon Co, Korea).
2.4 Characterization and morphology evaluation of synthetized NPs
Size, polydispersity index (PDI), and zeta potential of NPs were determined using dynamic light scattering (DLS) (Malvern Zetasizer, ZS, Malvern, UK). In this regard, NPs (1 mg) were suspended in 1 ml of double-distilled water, sonicated to form a homogeneous suspension, and analyzed in triplicate. NPs were also coated with gold under vacuum-dried condition and their surface morphology was observed by a scanning electron microscopy (SEM) (MIRA3/TESCAN).
2.5 Determination of the entrapment efficiency of GAB in NPs
2.6 In vitro release of GBA from PEG–PLA
The dialysis method was used to evaluate the in vitro release kinetics of GBA from PEG–PLA. In this study, NPs were suspended in phosphate-buffered saline (PBS; 0.1 M, pH 7.4, 2 ml) or citrate buffer (0.1 M, pH 5.5, 2 ml) was dialyzed (cut-off: 10 kDa) against each buffer containing 0.1% w/v Tween-80, whereas shaking for over 6 days at 37°C. Ten aliquots were removed at selected time intervals and the concentration of released GBA was calculated by spectrofluorimetry (JASCO FP-6200) at excitation and emission wavelengths of 330/390 nm. The GBA content was measured using a calibration curve of GBA solution in the buffers.
2.7 In vitro toxicity assay
C26 cells were seeded in 96-wells plates with a density of 5 × 103 cells per well and incubated for 24 hr at 37°C. Cells were treated with different concentrations of free GBA in dimethyl sulfoxide (DMSO), PLA–PEG, and PLA–PEG NPs containing 20–120 µM GBA for 48 hr. Afterward, cells were exposed to 20 μl of MTT reagent (5 mg/ml in PBS) and incubated for 3 hr at 37°C. The culture media was then removed and 100 μl DMSO was added to each well to dissolve the formazan crystals. The optical density of the obtained DMSO solution was measured at 570 nm using a microplate reader (microplate reader Infinite® 200 PRO, Tecan Trading AG, Switzerland). The untreated cells were considered as the control with 100% viability.
2.8 Cellular uptake study
To evaluate cellular uptake, PLA–PEG NPs loaded with GBA (20 and 40 µM) were added to each of the wells of the 48-well plates containing C26 (8 × 104 cells/well). After 6 hr and 24 hr of incubation at 37°C, the cells were rinsed twice with cold PBS, (pH 7.4). Then, the cells were lysed with cold lysis buffer (300 µl NaCl 5 M, 1 ml NP-40 lysis buffer 10%, 500 µl Tris 1 M pH 8, protease inhibitor cocktail ½ tablet, and DW qs 10 ml), and probe sonicated in triplicate for 30 s. The amount of total protein in the lysate solution was determined by the BCA test. In the next step, acetonitrile was added to the above solution in the bath sonication condition to digest the polymeric NPs and to release GBA. Supernatant was collected using centrifugation and the content of the free drug was analyzed by a spectrofluorimetry at excitation and emission wavelengths of 330/390 nm.
2.9 In vivo antitumor efficacy
2.10 In vivo toxicity
At the experimental endpoint, the mice were euthanized by an overdose of ether inhalation. Then, the lungs, heart, liver, spleen, and kidneys were fixed in a 10% neutral-buffered formalin solution and embedded in paraffin. The organs embedded in paraffin were cut at 5 µm thickness and stained with hematoxylin and eosin (H&E). The images were prepared at a magnification of ×40.
2.11 Immunostaining for CD34
To observe the vasculature, immunostaining was performed for the detection of CD34 as described previously. Briefly, the primary antibody of CD34 was added to the tissue section and incubated at room temperature for 30 min followed by adding the enzyme-labeled polymer and further incubation for 30 min. In the final step, the tissue sections were incubated with DAB to visualize the peroxidase reaction by producing a brown stain wherever primary antibodies and enzyme molecule conjugate (Dabbs, 2002; Kumar, Abbas, Fausto, & Robbins, 2005).
The sections were observed using a Nikon light microscope (Nikon Corp., Tokyo, Japan). Images of five randomly selected microscopic fields were quantitatively analyzed.
2.12 Statistical analysis
Analysis of variance was used to evaluate the data of the current study. A value of p < 0.05 was considered significant and the results are expressed as mean standard deviation (SD).
3 RESULTS
3.1 Synthesis and characterization of PLA–PEG containing GBA
To efficiently deliver GBA to the cancer cells, GBA was encapsulated into mPEG-PLA using the single emulsion–solvent evaluation method (Figure 1).
Presentation of PLA–PEG NPs containing GBA. GBA: galbanic acid; NPs: nanoparicles; PLA–PEGs: poly (D, l-lactide)–polyethylene glycols [Color figure can be viewed at wileyonlinelibrary.com]
There was no significant difference in size and encapsulation efficiency of the formulations with the polymer/drug ratio 10:1 and 20:1. The loading capacity of synthesized NPs showed significant difference, therefore, NP formulated with the polymer/drug ratio 10:1 was selected for further experiments (Table 1). Particle sizes of GBA-loaded PLA–PEG NPs was about 140 nm with PDI = 0.06 and zeta potential of −12.3 ± 2.8 mV. The size and morphology of PLA–PEG–GBA were also characterized using SEM. Based on the SEM imagery, the size of PLA NP was 100 nm with a roughly spherical structure (Figure 2). The results of the encapsulation efficiency assay showed that about of 40% of the GBA was loaded into PLA–PEG NPs with the LC at about 2.3%.
| Formulation | Polymer/drug (mg)/(mg) | Size(nm) | PDI | Zeta potential (mV) | LC % | EE% |
|---|---|---|---|---|---|---|
| PLA–PEG–GBA | 25/1.25 | 140 ± 20 | 0.06 + 0.04 | −12.3 ± 2.8 | 2.3 ± 0.3 | 40 ± 5 |
| PLA–PEG–GBA | 25/2.5 | 145 ± 20 | 0.06 + 0.03 | −13.0 ± 3 | 4.2 ± 0.3 | 38 ± 6 |
| PLA–PEG | 25/0 | 150 ± 15 | 0.06 ± 0.03 | −14.0 ± 4.5 | – | – |
- Note. EE: encapsulation efficiency; GBA: galbanic acid; PLA–PEG: poly (D, l-lactide)–polyethylene glycol; PDI: polydispersity index.

SEM image of PEG–PLA–GBA NPs. GBA: galbanic acid; NPs: nanoparicles; PLA–PEG: poly (D, l-lactide)–polyethylene glycol; SEM: scanning electron microscopy
3.2 In vitro drug release study
The pattern of GBA release from PLA–PEG was investigated under two conditions (either PBS at pH 7.4 or in acetate buffer at pH 5.5) at 37°C. Our data indicated a pH and time-dependent GBA release pattern from PLA–PEG–GBA (Figure 3). At pH 5.5, about 85% of GBA was released from PLA–PEG–GBA after 40 hr, whereas at pH 7.4 the amount of released GBA decreased to about 60% at the same time.
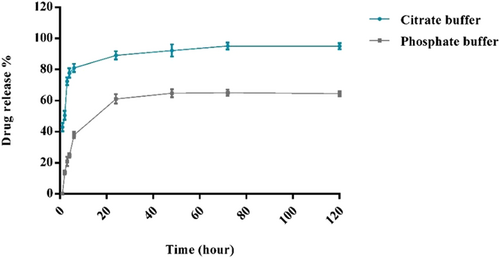
Profile of GBA release from PLA–PEG–GBA in PBS buffer at pH 7.4 and in citrate buffer at pH 5.5. GBA: galbanic acid; PLA–PEG: poly (D, l-lactide)–polyethylene glycol; PBS: phosphate-buffered saline [Color figure can be viewed at wileyonlinelibrary.com]
3.3 In vitro cytotoxicity assay
Escalating-dose studies of GBA (0–40 μM) showed IC50 of GBA in C26 cells after 48 hr of incubation were 15 µM. The cytotoxic activity of PLA–PEG containing GBA against C26 cells was evaluated. GBA-loaded PLA–PEG exhibited greater cytotoxicity than free GBA (IC50 = 8 µM; Figure 4).
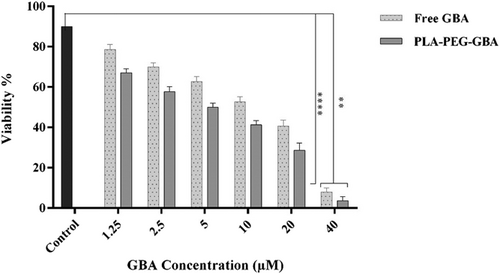
Cell viability of free GBA and PLA–PEG–GBA in C26 cells treated with varying concentrations of GBA by MTT assay (n = 3). GBA: galbanic acid; PLA–PEG: poly (D, l-lactide)–polyethylene glycol
3.4 Cellular uptake study
The results indicate the encapsulation of GBA into PLA NPs could increase cellular uptake of GBA about twice more than GBA at concentrations of 20 and 40 µM (p < 0.0001) as shown in Figure 5.
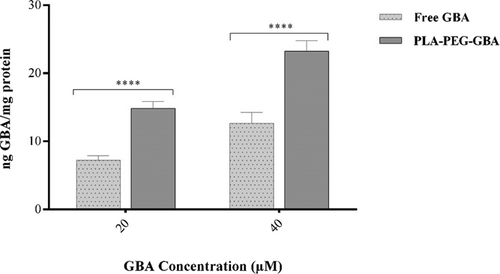
Cellular uptake of free GBA and PLA–PEG–GBA NPs in C26 cells after 24 hr. GBA: galbanic acid; PLA–PEG: poly (D, l-lactide)–polyethylene glycol; NPs: nanoparticles
3.5 Assessment of the antitumor efficacy PLA–PEG containing GBA
For the assessment of the anticancer efficacy of PLA–PEG containing GBA, the in vivo study was carried out in C26 colon carcinoma tumor-bearing female BALB/c mice. As shown in Figure 6, PLA–PEG–GBA is significantly more efficacious in tumor size reduction compared with free GBA at an equal dose. There were no significant changes in body weight in any of the treated groups, which could be due to their low side effects (Figure 7). Hence, PLA–PEG–GBA exhibited lower systemic toxicity based on survival rate during 40 days (Figure 8).
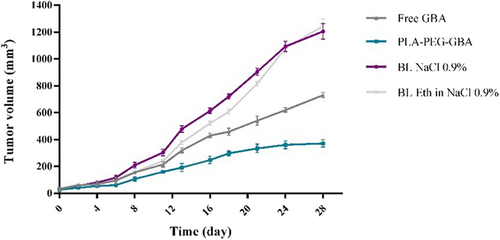
In vivo anticancer efficacy of PLA–PEG containing GBA against mice bearing C26 cells after intravenous administration of PLA–PEG containing GBA (equal to 10 mg/kg GBA), GBA (10 mg/kg), NaCl 0.9%, and ethanol 6.5% in NaCl 0.9% via the tail vein during 28 days. GBA: galbanic acid; PLA–PEG: poly (D, l-lactide)–polyethylene glycol [Color figure can be viewed at wileyonlinelibrary.com]
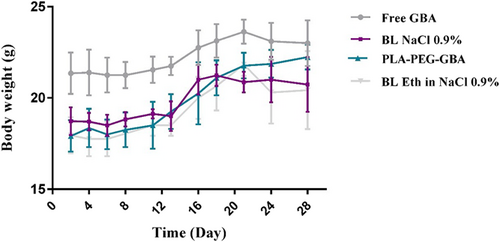
Body weight (g) of C26 tumor-bearing mice (n = 5) treated with PLA–PEG containing GBA (equal to 10 mg/kg GBA), GBA (10 mg/kg), NaCl 0.9%, and ethanol 6.5% in NaCl 0.9%. GBA: galbanic acid; PLA–PEG: poly (D, l-lactide)–polyethylene glycol [Color figure can be viewed at wileyonlinelibrary.com]
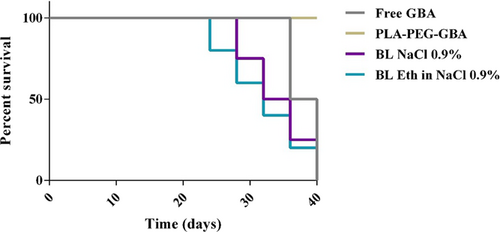
Survival curves of the subcutaneous mouse model of C26 treated with free GBA, PLA–PEG–GBA, NaCl 0.9%, and ethanol in NaCl 0.9%. GBA: galbanic acid; PLA–PEG: poly (D, l-lactide)–polyethylene glycol [Color figure can be viewed at wileyonlinelibrary.com]
3.5.1 In vivo toxicity
To determine the pathological influence and the toxicity of free GBA and PLA–PEG–GBA, H&E staining of tissue sections of the major organs was done. As shown in Figure 9, no histopathological changes were observed in the heart, lung, kidney, spleen, and liver.
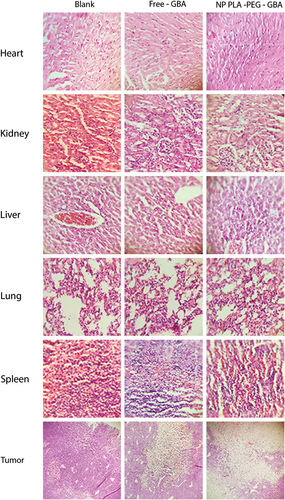
In vivo toxicity after the administration of one intravenous injection of PLA–PEG containing GBA (equal to 10 mg/kg GBA), GBA (10 mg/kg), NaCl 0.9%, and ethanol 6.5% in NaCl 0.9% every two weeks, during the 28-day period with H&E staining. GBA: galbanic acid; H&E: hematoxylin and eosin; PLA–PEG: poly (D, l-lactide)–polyethylene glycol [Color figure can be viewed at wileyonlinelibrary.com]
3.5.2 Antiangiogenesis effect of GBA and PLA–PEG–GBA
To study the antiangiogenesis effect of GBA and PLA–PEG–GBA NPs, tumor sections were stained with an antibody against the endothelial cell surface protein CD34. The results showed that the number of CD34 (+) vessels was decreased by more than 75% and 90%, respectively in the GBA and PLA–PEG–GBA treated mice (Figure 10).
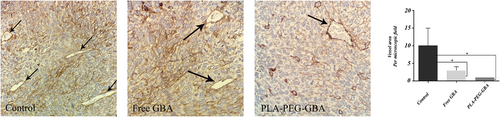
Right, antiangiogenesis effect of the control, GBA, and PLA–PEG–GBA on C26 tumor-bearing mice was investigated by CD34 staining, and normalized in mice treated with either NaCl 0.9% or ethanol 6.5% in NaCl 0.9% as the control. Left, the number denotes the average number of CD-34 positive vessels per microscopic field. Five randomly selected microscopic fields were quantitatively analyzed by light microscopy (x40 magnification). GBA: galbanic acid; PLA–PEG: poly (D, l-lactide)–polyethylene glycol [Color figure can be viewed at wileyonlinelibrary.com]
4 DISCUSSION
GBA as a biologically active sesquiterpene coumarin has antitumor activity in association with antiangiogenic and antiproliferative actions (Kim et al., 2011).
Zhang et al. indicated AR(+) PCa cell growth was suppressed more than AR(−) PCa cells when treated with GBA. This effect could be due to the downregulation of AR protein by GBA followed by the preferential inhibition of the proliferation of AR(+) PCa cells through an induction of G1 arrest (Zhang et al., 2012).
Kim et al. suggested that GBA could significantly inhibit the vascular endothelial growth factor (VEGF)-induced proliferation, migration, and tube formation in human umbilical vein endothelial cells (HUVECs). GBA also decreased Lewis lung cancer (LLC) cells proliferation with an apparent G2/M arrest. An inhibition of tumor-induced angiogenesis was also observed by GBA in LLC allograft bearing mice (Kim et al., 2011).
In another study, it was demonstrated that GBA prevent hypoxia inducible factor-1α (HIF-1α) transcriptional activation through suppressing the EGFR/HIF-1α signaling pathway that inhibits genes donated during hypoxia in lung and ovarian adenocarcinoma (Eskandani, Barar, Ezzati nazhad dolatabadi, Hamishehkar, & Nazemiyeh, 2015).
As low water solubility of the GBA is the main problem for its clinical applications, designing a nanoparticle containing GBA could enable the administration of the drug in an aqueous medium thereby improving its therapeutic efficiency (Aditya, Espinosa, & Norton, 2017; Hafezi-Ghahestani, Alebooye- Langroodi, Mokhtarzadeh, Ramezani, & Hashemi, 2016; Shome, Talukdar, Choudhury, Bhattacharya, & Upadhyaya, 2016).
In a previous study, Eskandani et al. encapsulated GBA in solid lipid nanoparticle formulations (GBA-SLNs) to enhance its solubility in aqueous media, and reported a long-term apoptotic effect compared with free GBA (Eskandani, Abdolalizadeh, Hamishehkar, Nazemiyeh, & Barar, 2015). In this study, we used the nanosystem comprising a PLA core and a hydrophilic PEG shell in which GBA was incorporated physically. It was hypothesized that this nanosystem could increase the solubility of the GBA in the aqueous medium leading to improved pharmaceutical properties. Pegylation of PLA prevents its aggregation and decreases its uptake by the reticuloendothelial system (RES), consequently improving the blood circulation time and the enhanced permeability and retention (EPR effect; Hrkach et al., 2012). Here, we fabricated NPs by a single emulsion–solvent evaporation method. In this technique, organic fraction (oil phase) consists of GBA and PLA and the aqueous phase containing PVA as a stabilizer agent provides the water phase (W) in an O/W emulsion. PLA–PEG-NPs had an average size of 140 nm that was further confirmed by SEM microscopy. Usually the nanoparticle size obtained by DLS is larger than that determined by SEM. This difference may be due to the hydration of particles in the aqueous medium, whereas SEM measures the size of dried samples (Manchanda, Fernandez-Fernandez, Nagesetti, & McGoron, 2010; Wright., 2014). PDI value of 0.06 confirmed relatively monodispersed particle sizes.
It was demonstrated that nanoparticles <200 nm in size had more uptake and retention in the tumor due to the promotion of the EPR effect in the tumor tissue (Maeda, Wu, Sawa, Matsumura, & Hori, 2000).
The slow release pattern continued within 48 hr only at pH 7.4 indicating that PEGylated PLA remained intact under neutral conditions (i.e., blood) and thus, it could be suggested that the system has a good stability in blood circulation. By switching from pH 7.4 to pH 5.5, the release of GBA from the PEG–PLA was significantly enhanced up to 90% after 48 hr. As pH 5.5 is comparable to the intracellular lysosomes and cancerous tissues microenvironment, so it would be expected to achieve not only more release of GBA within the tumor tissues but also less drug degradation outside the tumor environment (Yazdian-Robati, Ramezani, Jalalian, Abnous, & Taghdisi, 2016).
The obtained results demonstrated that cellular uptake of PLA–PEG–GBA was higher than GBA after 24 hr. However, free GBA showed more uptake by C26 cells after 6 hr (data was not shown) which may be due to the slow release of GBA from NPs.
According to the MTT survival assay results, the treatment of C26 cells with blank PLA–PEG showed negligible cytotoxicity, indicating their biocompatibility, which was consistent with those reported in a previous study (Ignatius and Claes, 1996), The results showed that free GBA was able to inhibit the growth rate of C26 cells with an IC50 value of approximately 15 µM after 48 hr of incubation.
Treatment of C26 cells with PLA–PEG–GBA for 48 hr reduced cell viability (IC50 = 8 µM) compared with free GBA (p < 0.0001). The observed toxicity of PEG–PLA–GBA in C26 cells could be attributed to the nonspecific uptake of particles or alternatively, may be due to the release of GBA into the medium and subsequent higher cellular uptake of NPs compared with free GBA. The antitumor efficacy of PEG–PLA–GBA was also analyzed by allograft mouse models inoculated with C26 cells. Evaluation of the antitumor efficacy showed that tumor size in tumor-bearing mice after treatment with PEG–PLA–GBA significantly decreased in comparison with the other groups.
Recently, angiogenesis has been considered as an attractive target in cancer therapy due to more accessibility to endothelial cells than to tumor cells, independence of tumor cell drug resistance mechanisms, and broad applicability for many tumor types (Tosetti, Ferrari, De Flora, & Albini, 2002). In this study, it was shown that encapsulation of GBA into PLA–PEG NPs could exhibit more antiangiogenesis effects compared with free GBA, which may be due to more internalization of GBA into the endothelial cells when it is encapsulated in PEG–PLA nanoparticles.
5 CONCLUSION
In this study, PEG–PLA–GBA was used for the treatment of tumors, which was comprised of a PLA core and a PEG shell in which GBA was incorporated. The GBA loading and release exhibited a pH-sensitive profile. PEG–PLA–GBA could effectively decrease the cell viability of C26 cells compared with free GBA. Moreover, PEG–PLA–GBA was able to efficiently inhibit tumor growth in vivo and angiogenesis, as well. These results suggest that the encapsulation of GBA into PLA–PEG could potentially be used for the treatment of colorectal cancers.
ACKNOWLEDGMENT
Financial support of this study was provided by the Mashhad University of Medical Sciences (Grant 930626).
CONFLICTS OF INTEREST
The authors declare no conflicts of interest with respect to the research, authorship, or publication of this study.




