The miR-15a/16 gene cluster in human cancer: A systematic review
Abstract
MicroRNAs (miRNAs) are an important class of endogenous small noncoding single-stranded RNAs that suppress the expression of their target genes through messenger RNA (mRNA) degradation to inhibit transcription and translation. MiRNAs play a crucial regulatory role in many biological processes including proliferation, metabolism, and cellular malignancy. miR-15a/16 is an important tumor suppressor gene cluster with a variety of factors that regulate its transcriptional activity. It has been discovered that a relative reduction of miR-15a/16 expression in various cancers is closely related to the occurrence and progression of tumors. miR-15a/16 takes part in a wide array of biological processes including tumor cell proliferation, apoptosis, invasion, and chemoresistance by binding to the 3′-untranslated region of its target gene's mRNA. In this review, we will examine the complex regulatory network of miR-15a/16 gene expression and its biological functions in human cancers to further elucidate the molecular mechanisms of its antitumor effects.
1 INTRODUCTION
MicroRNAs (miRNAs) are an abundant class of regulatory noncoding single-stranded RNA molecules that are about 20–23 nucleotides in length. MiRNAs lead to the degradation of target mRNAs or the inhibition of their translation by binding with imperfect complementarity to the 3′-untranslated region (3′-UTR) resulting in posttranscriptional regulation of the expression of target genes (Dai et al., 2012). MiRNAs hybridize with RNA-induced silencing complex to suppress the expression of target genes (Muniategui, Pey, Planes, & Rubio, 2013), and this complex plays an important role in various biological processes including tissue development, immune responses, cardiovascular diseases, metabolic diseases, and tumorigenesis (Bartel, 2004; Dugo et al., 2018; J. Wu, Ding, Yang, Guo, & Zheng, 2018; Tao et al., 2017; Templin et al., 2017). miR-15a/16 was the first cluster of miRNA found to function as a tumor suppressor in chronic lymphocytic leukemia (CLL; Cho, 2007).
miR-15a and miR-16 are important members of the miR-15 family, which includes miR-15a, miR-15b, miR-16-1, miR-16-2, miR-195, and miR-497 (Hullinger et al., 2012). miR-15a and miR-16-1 share the same seed sequence which indicates that they may have similar biological functions (Yue & Tigyi, 2010). The miR-15a/16-1 gene cluster is located on human chromosome 13 (13q14) and resides in an intron of the long noncoding RNA (lncRNA) gene DLEU2. miR-15a/16-1 is involved in the regulation of cell differentiation and maturation (Bianchi et al., 2012; Cai et al., 2018; Lindner et al., 2017) as well as the response to infections and the promotion of tissue repair (Jia et al., 2016; Lu et al., 2018; Moon, Yang, Zheng, & Jin, 2014). Significantly, miR-15a/16 is a critical tumor suppressor and plays a pivotal role in the regulation of tumor cell proliferation, apoptosis, differentiation, and angiogenesis.
2 REGULATION OF miR-15A/16 EXPRESSION
Abnormal expression of miR-15a/16 was found across many types of tumors including CLL, multiple myeloma (MM), nonsmall cell lung cancer (NSCLC), and breast cancer (BC; Braga et al., 2017; Luo et al., 2013; L. Zhang, Zhou, Shi, Kuang, & Fang, 2018; T. Yang et al., 2015). Numerous studies have shown that the expression of miR-15a/16 is regulated by multiple factors including chromosomal deletions, epigenetic modifications, and transcription factors (Figure. 1; C. Q. Chen et al., 2013; Hernandez-Alcoceba, del Peso, & Lacal, 2000; X. Zhang et al., 2012). The deletion of chromosome 13q14 is associated with the pathogenesis of CLL as well as a significant reduction in the expression of miR-15a/16 (Cutrona et al., 2017). In pediatric acute myelogenous leukemia (AML), a significant reduction in the expression of DLEU2 was observed because of hypermethylation in the gene's promoter (Morenos et al., 2014). Regulation of miR-15a/16 expression has also been attributed to acetylation modifications. C. Q. Chen et al. (2013) found that treatment with the histone deacetylases (HDACs) inhibitors trichostatin A (TSA) and sodium butyrate in lung cancer (LC) led to enhanced expression of miR-15a/16-1 through an increase in histone acetylation in the DLEU2/miR-15a/16-1 promoter region. Furthermore, siRNA knockdown of HDAC3 increased the levels of hyperacetylation in the DLEU2/miR-15a/16-1 promoter region, resulting in higher expression of miR-15a/16-1 (C. Q. Chen et al., 2013). Myb (c-Myb) is an important transcription factor in the Myb family, which consists of Myb, Mybl1 (a-Myb), and Mybl2 (b-Myb). Transcription of miR-15a/16-1 is negatively regulated by c-Myc and HDACs in mantle cell lymphoma (MCL). Moreover, c-Myc and HDAC3 have been found to colocalize in the two promoters of the miR-15a/16-1 cluster gene DLEU2 (X. Zhang et al., 2012). In leukemia K562 cells, miR-15a downregulates c-Myb protein expression through transcriptional inhibition, suggesting the existence of a negative feedback loop between c-Myb and miR-15a/miR-16-1 (Chung et al., 2008; Zhao, Kalota, Jin, & Gewirtz, 2009). The B-cell specific activator protein directly interacts with the DLEU2 promoter and inhibits miR-15a/16-1 expression through the negative regulation of the DLEU2 promoter (Kasar et al., 2014). In addition, Lu et al. (2018) found that overexpression of miR-15a/16-1 depresses interleukin-22 (IL-22) through the inhibition of the aryl hydrocarbon receptor. By contrast, IL-22 downregulates miR-15a/16-1 expression through the activation of phosphorylated signal transducer and activator of transcription 3-c-Myc signaling (Lu et al., 2018). This indicates that there is a negative feedback loop between miR-15a/16-1 and IL-22. Z. Liu et al. (2016) found that a knockdown of cyclin-dependent kinase 4 (CDK4) resulted in the transcriptional suppression of c-Myc, which inhibits the expression of miR-15a via binding to DLEU2 in nasopharyngeal cancer (NPC; Z. Liu et al., 2016). In summary, knockdown of CDK4 promoted miR-15a expression as a result of c-Myc inhibition, and overexpression of miR-15a inhibited CDK4. A feedback loop was also identified between miR-15a/16 and p53 in CLL. Deletion of p53 resulted in a significant decrease in miR-15a/16-1 expression and a significant increase in the expression of Mcl-1, Bcl-XL, and Bcl-2. Thus, suppression of miR-15a/miR-16-1 may be a critical mechanism of the p53 deletion and the enhancement of Mcl-1, Bcl-2, and Bcl-XL expression (J. Liu et al., 2014). Finally, Janaki Ramaiah et al. (2014) found that miR-15a/16 was a downstream target of the p53 signaling pathway.
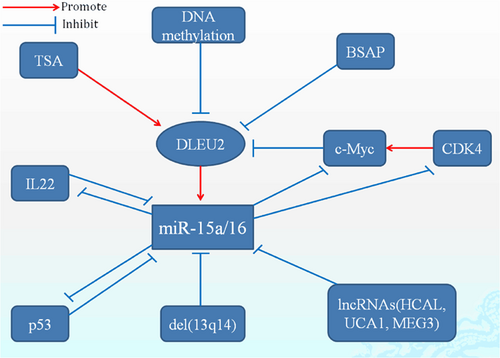
The regulation of miR-15a/16 gene expression. TSA and DLEU2 drive the expression level of miR-15a/16. In contrary, miR-15a/16 was downregulated by many factors involved in 13q14 deletion, DNA methylation, transcription factors such as c-Myc, and lncRNAs. Also, there are two feedback between miR-15a/16 and p53 or IL-22. IL-22: interleukin-22; lncRNA: long noncoding RNA; TSA: trichostatin A [Color figure can be viewed at wileyonlinelibrary.com]
The lncRNAs are a class of RNA with important regulatory functions, including the ability to transcriptionally regulate target genes by functioning as miRNA sponges. In hepatocellular carcinoma (HCC) tissue, upregulation of the HCC-associated lncRNA (HCAL) was associated with poor cellular differentiation, intravascular cancer embolus, decreased patient survival, and direct inhibition of miRNA expression including miR-15a, miR-196a, and miR-196b (Xie et al., 2017). In both chronic myelogenous leukemia (CML) and bladder cancer, a regulatory interaction between miR-16 and the urothelial carcinoma–associated 1 gene (UCA1) was observed. UCA1 functioned as a competitive endogenous RNA of MDR1 through the complete binding of miR-16, and this miRNA-sponge effect on miR-16 presented in a dose-dependent manner (H. J. Li et al., 2015; Xiao et al., 2017). MEG3 was significantly downregulated in osteoarthritis, and disinhibition of miR-16 led to the transcriptional suppression of SMAD7, which in turn resulted in the promotion of cell proliferation and the inhibition of apoptosis (Xu & Xu, 2017).
3 THE ROLE OF mIR-15A/16 IN TUMOR CELL PROLIFERATION
Numerous studies have found that miR-15a/16 inhibits cell proliferation through the regulation of a number of proliferation-related targets (Figure 2). Cyclin and CDK have been shown to play pivotal roles in cell proliferation. Cyclins (CCND1, CCND2, CCND3, CCNE1, CCNE2) and CDK were considered to be the “rate-limiters” for entering the G1 phase (Wilczynska, Git, Argasinska, Belloc, & Standart, 2016). In CLL, BC, NPC, and LC tumors, DLEU2 negatively regulates CCND1, CCND2, and CCNE1 through the upregulation of miR-15a/16-1, leading to cell-cycle arrest in the G1-G0 phases (C. Q. Chen et al., 2013; Lerner et al., 2009; Luo et al., 2013; Z. Liu et al., 2016). D. W. Wang, Wang, and Shu (2018) found complementary binding sites between miR-16 and MAPK/ERK kinase 1 (MEK1). In pituitary adenomas, miR-16 was downregulated, whereas the expression of p-ERK1/2, Survivin, and Cyclin-D1 increased along with rates of cellular proliferation and the ratio of cells in the S or G2/M phase. Transfection with miR-16 or si-MEK1 resulted in a marked inhibition of the expression of MEK1, p-ERK1/2, Survivin, and CCND1, as well as the suppression of cell proliferation and the induction of apoptosis and cell-cycle arrest (D. W. Wang et al., 2018). In addition, overexpression of miR-15a suppressed cell proliferation and arrested cell-cycle progression through the inhibition of CDK4 expression (Z. Liu et al., 2016).
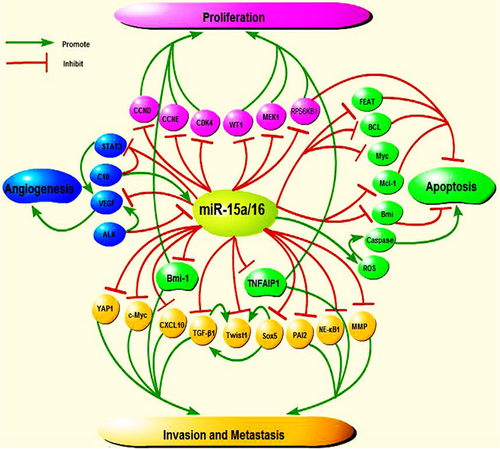
The important roles of miR-15a/16 in proliferation, apoptosis, invasion and metastasis, and angiogenesis. (a) MiR-15a/16 represses tumor proliferation by inhibiting CCND1, CCND2, CCNE1, CDK4, and so on. Moreover, miR-15a/16 regulates tumor proliferation, invasion, and metastasis by inhibiting Bmi-1 and TNFAIP1. (b) MiR-15a/16 suppresses tumor invasion and metastasis through Smad3 / Sox5 / Twist1, NF-κB1/MMP-9 signaling pathway, YAP1, PAI-2, and so on. (c) MiR-15a/16 induces apoptosis of tumor cells by regulating caspase-3, Bax, Bcl-2, and so on. (d) MiR-15a/16 suppresses tumor angiogenesis by inhibiting STAT3 and VEGF. Also, the C-10 is associated with angiogenesis by upregulating the expression of miR-15/16 and inhibiting STAT3 protein. And ALK is contributed to reduction of miR-16 and upregulated VEGF levels. All these demonstrate that miR-15a/16 induces tumor cells apoptosis and inhibit tumor proliferation, angiogenesis, invasion, and metastasis. ALK: anaplastic lymphoma kinase; TNFAIP1: tumor necrosis factor α-induced protein 1; VEGF: vascular endothelial growth factor [Color figure can be viewed at wileyonlinelibrary.com]
In AML, there is a significant inverse correlation between miR-15a/16-1 and WT1 expression levels, indicating that miR-15a/16-1 may inhibit leukemic cell proliferation through the downregulation of the WT1 oncogene (Gao et al., 2011). In osteosarcoma cells, miR-15a inhibits cell proliferation through the downregulation of tumor necrosis factor α-induced protein 1 (TNFAIP1) expression (Tian, Zhang, Yan, Dong, & Guo, 2015). Janaki Ramaiah et al. (2014) also found that the mammalian target of rapamycin (mTOR) kinase controls cell division by regulating protein synthesis, specifically through phosphorylation and inactivation of 4E-BP1 and phosphorylation and activation of S6 kinase 1. These two molecules are important downstream effectors of mTOR signaling and control cellular growth and metabolism (Janaki Ramaiah et al., 2014). Overexpression of miR-15/16 might lead to reductions in the mRNA levels of RPS6KB1 and influence the activity of mTOR. By targeting RPS6KB1, exogenous miR-15/16 can control proliferation and induce apoptosis in a caspase-dependent manner even in the absence of functional p53 (Janaki Ramaiah et al., 2014). In addition, in pancreatic ductal adenocarcinoma (PDAC), gastric cancer (GC), and ovarian cancer, the expression of Bmi-1 is inversely correlated with miR-15a expression, and miR-15a suppresses the proliferation of tumor cells through the downregulation of Bmi-1 (Bhattacharya et al., 2009;C. Wu et al., 2016; Guo et al., 2014).
4 THE ROLE OF miR-15A/16 IN TUMOR CELL APOPTOSIS
Numerous studies indicate that abnormal regulation of apoptosis is closely related to the pathogenesis of tumors. In eukaryotic cells, apoptosis is induced through mitochondrial and death receptor pathways that activate caspase-3 and caspase-7. The Bcl-2 family has a critical function in cell apoptosis including proapoptosis proteins (e.g., Bax and Bak) and antiapoptosis proteins (e.g., Bcl-2 and Bcl-xL; Pan et al., 2017). Patel et al. (2016) found that overexpression of miR-15a/16 increases the levels of mitochondrial reactive oxygen species in BC cells, which impairs the mitochondrial membrane potential. Caspase-3 and caspase-6/9 are activated by the release of cytochrome C into the cytoplasm, and this activation leads to apoptosis (Patel et al., 2016). Reduced expression of miR-16 leads to a reduction in its inhibitory effect on the Bcl-2 gene and consequently a reduced rate of apoptosis in tumor cells (Xia et al., 2008).
Previous studies have reported that Bcl-2 is an important target of miR-15a/16 in CLL and that the 13q14 deletion leads to both decreased expression of miR-15a/16 and a significant overexpression of Bcl-2 (Pekarsky & Croce, 2015). miR-15a/16 induces apoptosis by negatively regulating the expression of Bcl-2 at the posttranscriptional level (Aqeilan, Calin, & Croce, 2010). Moreover, miR-15a/16-1 plays a role in the regulation of the antiapoptotic protein Mcl-1. Downregulation of miR-15a/16 results in the disinhibition of Mcl-1 expression and a subsequent elevation of Mcl-1 protein levels. P53 inhibits the transcription of Mcl-1, and the absence of p53 may enhance Mcl-1 activity. Therefore, the p53-miR-15a/16-1-Mcl-1 axis may be a critical pathway in the regulation of apoptosis and drug resistance in CLL (J. Liu et al., 2014).
Myc typically represses the expression of miR-15 and the let-7 families in LC, BC, and CM. By contrast, HDAC inhibition (HDACi) results in the transcriptional activation of the tumor-suppressive miR-15 and let-7 families that target and decrease the expression of the crucial prosurvival genes Bcl-2 and Bcl-xL, respectively. Myc is also required for the downregulation of Bcl-2 and Bcl-xL after HDACi. Inhibition of the binding sites of the miR-15 and let-7 families in the 3′-UTR of Bcl-2 and Bcl-xL resulted in protection from HDACi-induced apoptosis (Adams & Eischen, 2016). In MCL, the downregulation of miR-16 is associated with the upregulation of Bmi-1, a proto-oncogene (a transcription factor) that inhibits the expression of proapoptotic proteins including Bim and Noxa. In lymphoma cells, miR-16 promoted apoptosis through targeting Bmi-1 (Huang, Liu, & Chu, 2015). Moreover, overexpression of miR-16 promoted apoptosis in the A549 cell line through the inhibition of (FEAT protein is encoded by METTL13 gene) FEAT expression that is highly expressed in the cells and for which miR-16 can directly bind with its 3′-UTR (Liang et al., 2015).
5 THE ROLE OF miR-15A/16 IN TUMOR INVASION AND METASTASIS
The development of mesenchymal characteristics, the loss of epithelial markers, and cell motility are critical factors that promote tumor cell migration and invasion during the epithelial-mesenchymal transition (EMT). Furthermore, tumor cells have the characteristics of increasing apoptosis and developing resistance to chemotherapeutic agents during the course of EMT. Studies have shown that the transcription factor Twist1 plays an important role in the regulation of the EMT process. Significant changes in cell morphology and the ability to invade and metastasize were found in overexpressed Twist1 tumor cells. Interestingly, increased levels of miR-15a-3p and miR-16-1-3p significantly inhibited the activity of the Twist1-3′-UTR and reduced the activity of EMT-related genes including Twist1, N-cadherin, E-cadherin, α-SMA, and fibronectin, while also repressing the activity of matrix metalloprotein (MMP-9) and MMP-2 and reducing cell migration and invasion (T. Wang et al., 2017). In GC and NSCLC tissues, miR-15a-3p and miR-16-1-3p were significantly downregulated, whereas Twist1 mRNA and protein were significantly increased, suggesting that abnormal downregulation of miR-15a-3p and miR-16-1-3p may be linked with the aberrant regulation of Twist1 and the EMT process during tumor development (J. He, 2017; T. Wang et al., 2017). miR-15a inhibits EMT through the downregulation of Bmi-1 expression, which in turn inhibits the invasion and metastasis of PDAC cells (Guo et al., 2014). In addition, miR-16 inhibits transforming growth factor-β1 (TGF-β1)-induced EMT through the activation of autophagy in NSCLC (H. Wang, Zhang, Wu, Wang, & Wang, 2018). TGF-β-induced EMT is accompanied by enhanced expression of Sox5 in prostate cancer (PCa), BC, and pituitary tumors. Interestingly, knockdown of Sox5 can attenuate TGF-β-induced EMT. Furthermore, Smad3 can regulate Sox5 expression by binding to the promoter of Sox5 (Hu et al., 2018). Sox5 can also bind directly to the Twist1 promoter and induce EMT by transactivation of Twist1 (Pei, Lv, & Li, 2014). Therefore, Smad3, Sox5, and Twist1 enhance EMT and contribute to the progression of PCa, and miR-15a/16 can inhibit the proliferation, invasion, and metastasis of tumor cells by directly targeting Sox5 (Renjie & Haiqian, 2015).
Chemokine ligand 10 (CXCL10) was a predicted target of miR-15a-5p, and it was later confirmed that miR-15a-5p negatively impacts survival and the metastasis of CML by targeting CXCL10 (D. Chen et al., 2017). Levels of c-Myb were positively correlated with cell mobility in HCC cells, and this mobility was inhibited by the overexpression of miR-15a, which targets c-Myb. In addition, the use of a miR-15a inhibitor promoted the migration of HCC cells through the disinhibition of c-Myb expression (B. Liu et al., 2017). miR-15a represses tumor cell migration and invasion by posttranscriptional inhibition of Bcl-2 expression (Leng, Song, Zhao, & Wang, 2018). miR-15a can also repress the migration and invasion of osteosarcoma cells by targeting TNFAIP1 (Tian et al., 2015). The expression of PAI-2 was higher in most patients with cholangiocarcinoma than in normal liver tissue, leading to increased tumor cell migration that can be mitigated by miR-15a through the transcriptional inhibition of PAI-2 (Utaijaratrasmi et al., 2018). T. Q. Yang et al. (2014) discovered a novel mechanism by which miR-16 reduces invasiveness in glioma through the repression of the NF-κB1 and MMP-9 signaling pathways. In addition, in cholangiocarcinoma (CCA), neuroglioma, and NPC, miR-16 inhibits the migration and invasion of tumor cells through the regulation of a number of targets including oncoprotein YAP1, Sal-like protein 4, and fibroblast growth factor 2 (Han et al., 2017; Lin et al., 2016; Q. He et al., 2016; Zhou, Liu, Hu, & Jiang, 2016).
6 THE ROLE OF miR-15A/16 IN THE TUMOR MICROENVIRONMENT
It has been now recognized that the tumor microenvironment is a critical component of tumorigenesis, the metastatic progression, and the patient's therapeutic response. The tumor microenvironment is composed of an extracellular matrix and numerous stromal cells including blood, endothelial cells, mesenchymal stem cells (MSCs), lymphatic endothelial cells, immune cells, cancer-associated fibroblasts (CAFs), leukocytes, tumor-associated macrophages (TAMs), and adipocytes (Y. He et al., 2018). There are reduced levels of miR-15/16 in the fibroblasts surrounding prostate tumors. Downregulation of miR-15/16 in CAFs increases FGF-2 and its receptor FGFR1 that act on stromal and cancer cells to increase survival, proliferation, and migration during the development and growth of prostate cancer (Musumeci et al., 2011). In the BC tumor microenvironment, upregulation of exosomal miR-16 by epigallocatechin gallate treatment induces IκB accumulation in TAM and inhibits macrophage polarization (Jang, Lee, Jeon, & Kim, 2013).
Previous studies suggest that the pathogenesis of MM can be mostly attributed to the interaction between myeloma cells and the bone marrow microenvironment (L. Zhang et al., 2016). MM bone marrow mesenchymal stem cells (BM-MSCs) release exosomes that are transferred to the MM cells, thereby leading to modulation of tumor growth in vivo. There are differences in the levels of exosomal miRNA between MM and normal BM-MSCs with lower levels of the tumor suppressor miR-15a in MM BM-MSCs. Moreover, when comparing exosomes from different cells of origin, MM BM-MSC-derived exosomes have higher levels of cytokines, oncogenic proteins, and adhesion molecules. Importantly, although MM BM-MSC-derived exosomes promote the growth of MM tumor cells, normal BM-MSC exosomes inhibit it (Roccaro et al., 2013).
7 THE ROLE OF miR-15A/16 IN TUMOR ANGIOGENESIS
Endothelial proliferation, migration, and capillary formation are important events during angiogenesis. Vascular endothelial growth factor (VEGF) plays a pivotal role in vascular development and cell proliferation in invasive tumors. Signal transducer and activator of transcription 3 (STAT3) is necessary for the transcriptional activation of the VEGF gene. A novel etoposide analog, quinazolino-4β-amidopodo-phyllotoxin (C-10), induces a significant increase in miR-15/16 expression and inhibits the STAT3 protein. miR-15a/16 regulates cell proliferation and angiogenesis by targeting STAT3 and VEGF. Therefore, etoposide and its analog C-10 display a strong antiangiogenic activity by mediating miR-15a/16 expression (Srinivas et al., 2015). miR-15a/16 expression in MM, BC, and PCa cell lines is inversely correlated with the expression of VEGF-A, a direct target of miR-15a/16. In addition, overexpression of miR-15a/16 results in decreased proangiogenic activity of tumor cells (Sun et al., 2013; Terzuoli et al., 2016; Y. Li et al., 2016). Angiogenesis is a pivotal process that contributes to the growth of glioblastoma (GBM). miR-16 downregulates the expression of VEGF-A and VEGF-C, which are closely related to the angiogenesis of GBM (F. Chen et al., 2016). MSC-derived exosomes significantly reduced the expression of VEGF in BC cells, which resulted in the inhibition of angiogenesis (Lee et al., 2013). In anaplastic large cell lymphoma, miR-16 directly hybridized to the 3′-UTR of the VEGF mRNA, resulting in downregulation at translation level and decreased levels of angiogenesis. Furthermore, the expression of anaplastic lymphoma kinase contributed to the reduction of miR-16 and the upregulation of VEGF (Dejean et al., 2011).
8 THE ROLE OF miR-15A/16 IN CHEMOTHERAPY
There is growing evidence that miR-15a/16 is involved in drug sensitivity and resistance. Studies have shown that miR-15a/16 participates in the regulation of drug sensitivity and resistance across various tumors. In MM, patients with lower levels of miR-15a expression were resistant to bortezomib-based therapy and displayed no significant improvements in the rates of progression-free survival and overall survival (OS) in response to treatment (F. Li et al., 2015). In GC, miR-15b/16 may regulate the occurrence of multidrug resistance (MDR) in SGC7901/VCR, a multidrug-resistant GC cell line. In vitro drug sensitivity tests showed that overexpression of miR-15b/16 enhanced the sensitivity of SGC7901/VCR to anticancer drugs, whereas the use of their antisense oligonucleotides inhibited MDR in SGC7901/VCR cells (Xia et al., 2008). miR-15a improved the sensitivity of tumor cells to DDP by mediating the c-Myc/CCND1/CDK4/E2F1 pathway in NPC (Z. Liu et al., 2016). BM-MSCs can protect the MM cells from apoptosis induced by bortezomib through the inhibition of miRNA-15a expression (Hao et al., 2011).
In colorectal cancer (CRC), a lower level of miR-15a expression is associated with a poor prognosis. miR-15a inhibits cancer progression and drug resistance through the inhibition of important target genes such as CCNB1, Bcl-2, Bmi-1, YAP1, and DCLK1 (Dai et al., 2012; Fesler, Liu, & Ju, 2018). 5-Fluorouracil (5-FU) is a traditional chemotherapeutic that improves patients survival primarily by suppressing the rapid proliferation of tumor cells. Moreover, enforced expression of miR-15a inhibits proliferation and enhances the therapeutic effect of 5-FU in colon cancer cells. This drug maintains target specificity, is more effective than an unmodified miR-15a in vitro and suppresses colon cancer metastasis in vivo (Fesler et al., 2018). In addition, systemic delivery of cationic liposomes carrying miR-15a/16-1 plasmids resulted in a significant suppression of subcutaneous cancer growth and angiogenesis in cancer tissues (Dai et al., 2012).
Tamoxifen, generally, is the most prescribed therapy for patients with estrogen receptor (ER) α-positive BC. Providing effective therapy is more difficult when tumors are resistant to tamoxifen, especially in patients who also overexpress human epidermal growth factor receptor 2 (Cittelly et al., 2010). E2F7, a repressive member of E2F family, is responsible for the inhibition of the miR-15a/16 cluster through competition with E2F1 for the E2F binding site at the promoter of DLEU2. Overexpression of E2F7 inhibits miR-15a/16 and increases the expression of CCNE1 and Bcl-2, resulting in tamoxifen resistance in BC (Chu et al., 2015). miR-15a/16 also affects BC cell sensitivity to doxorubicin through the downregulation of Bmi-1 (Patel et al., 2016, 2017).
BL-8040 induces the apoptosis of AML cells in vitro and in vivo. This process is mediated by increased levels of miR-15a/16-1, which leads to the Downregulation of several target genes (Mcl-1, Bcl-2, and cyclin-D1). Overexpression of miR-15a/16-1 directly induces AML cell death. BL-8040-induced apoptosis is mediated via the suppression of survival signals by the AKT/ERK pathways. Bcl-2-inhibitor treatment induces apoptosis and can be combined with BL-8040 to improve the rate of cell death. More significantly, this combined treatment prolongs the survival of tumor-bearing mice and reduces the minimal residual disease in vivo (Abraham et al., 2017). In leukemic cells, curcumin increases miR-15a/16-1 expression, reduces WT1 expression, and effectively suppresses cell proliferation, whereas anti-miR-15a/16-1 oligonucleotides partly reverse the downregulation of WT1 induced by curcumin (Gao et al., 2012).
9 miR-15A/16 AS A POTENTIAL DIAGNOSTIC MARKER
The recent exploration of plasma and serum miRNAs as prospective novel biomarkers for cancers is still a “booming” field. An increasing number of studies indicate that miR-15a/16 may serve as a valuable tool for diagnosis and prognosis as well as a means to predict response to treatment and therapeutic outcomes (J. Zhang, Xiao, & Liu, 2015; Mitchell et al., 2008). Patients with PCa have decreased expression levels of serum miR-15a/16-1 when compared with controls, chronic prostatitis, and benign prostate hyperplasia patients. Downregulation of miR-15a/16-1 is related to a higher tumor stage, a higher Gleason score, greater lymph nodes involvement, and PCa metastasis. Furthermore, PSA combined with miR-15a/16-1 enhanced the specificity and sensitivity for the diagnosis of PCa. Therefore, serum expression levels of miR-15a/16-1 and PSA levels can be combined to be used as useful and specific noninvasive biomarkers for the diagnosis and prognosis of PCa (Zidan, Abdul-Maksoud, Elsayed, & Desoky, 2018). Expression of miR-15a was significantly lower in esophageal squamous cancer (ESCC) tissues and patient's sera. Low expression of miR-15a was significantly associated with shorter OS and disease-free survival of patients with ESCC. Furthermore, serum miR-15a expression levels can be used as a potential diagnostic and prognostic biomarker in the clinic (J. Li, Li, Gao, & Ge, 2017). Serum miR-15a, miR-93, miR-106, and miR-664 are useful biomarkers for the prognosis of patients with GC who received adjuvant chemotherapy, and the risk score derived from these four sera miRNAs was closely related to the OS in these patients (Song et al., 2017). In BC, a machine learning method (Artificial Neural Network) that receives the levels of three circulating miRNAs (miR-15a, miR-101, and miR-144) can automatically classify BI-RADS 4 breast lesions as malignant or benign with approximately 95% specificity,92.5% accuracy, and 88% sensibility (Pezuk et al., 2017). In head and neck squamous cell cancer, the presence of lymph node invasion is related to low levels of miR-15a, miR-199b, and miR-34c, and these miRNAs can serve as prognostic biomarkers and therapeutic targets (Sousa et al., 2016). Some oncomiRNAs were downregulated (miR-155 and miR-106), and some tumor-inhibitor miRNAs were upregulated (miR-16-1, miR-15a, miR-101, miR-568). Very few miRNA can be useful candidates for CML diagnosis on their own due to independently and conflicting results, but when taken together, they can be utilized as an additional method for CML diagnosis. In addition, plasma and serum miRNAs are promising candidates for prognosis, prediction, and therapy (Fallah et al., 2015). A multivariate signature consisting of nine miRNAs (including miR-15a) identifies significant differences between BC patients and healthy controls. By using a particular algorithm based on the nine-miRNA signature, it may be possible to predict an individual's future risk of developing BC. Because of the stability of miRNA in blood components, this signature may be beneficial to the development of a blood-based multimarker test to improve early identification of BC (Kodahl et al., 2014). In addition, the abundant and stable expression of miR-16 in the serum of patients with PCa, BC, GC, and CRC enables it to be used as reference gene for quantitative polymerase chain reaction analysis of other miRNAs profiled in the serum (Chang, Mestdagh, Vandesompele, Kerin, & Miller, 2010; L. Wang et al., 2015; Song et al., 2012; Zhao et al., 2018).
10 CONCLUSIONS
In this review, we discussed the dysregulation of miR-15a/16 observed in human cancers and proposed that miR-15a/16 would be a valuable biomarker for the early diagnosis, evaluation, prognosis, and development of individualized treatments for human cancers. It has been confirmed that miR-15a/16 can inhibit angiogenesis, cell proliferation, cell invasion, and metastasis through its ability to induce cell apoptosis, increase the sensitivity of tumor cells to chemotherapeutic drugs, and inhibit tumors in their own microenvironment. However, additional studies are required to better elucidate the upstream regulatory mechanisms of miR-15a/16 and to identify more of its functional targets. A better understanding of the complex regulatory network involved in the expression and functionality of miR-15a/16 will help to reveal the mechanisms that underlie the occurrence and development of human cancers (Table 1).
| Target genes | miRNAs | Tumor types | Functions | References |
|---|---|---|---|---|
| CCND1, CCND2 | miR-15a/16 | CLL, BC NPC, LC, AML |
Proliferation apoptosis, and chemotherapy | Luo et al. (2013); Abraham et al. (2017); C. Q. Chen et al. (2013); Lerner et al. (2009); Z. Liu et al. (2016) |
| CCNE1 | miR-15a/16 | NPC, LC, CLL, BC | Proliferation Apoptosis and chemotherapy | Luo et al. (2013); C. Q. Chen et al. (2013); Chu et al. (2015); Lerner et al. (2009); Z. Liu et al. (2016) |
CDK4 |
miR-15a |
NPC | Proliferation |
Z. Liu et al. (2016) |
| Mcl-1 | miR-15a/16 | CLL, AML | Apoptosis | Abraham et al. (2017); J. Liu et al. (2014) |
| RPS6KB1 | miR-15a/16 | BC |
Proliferation and apoptosis |
Janaki Ramaiah et al. (2014) |
| MEK1, p-ERK1/2, Survivin | miR-16 | Pituitary adenoma | Proliferation and apoptosis | D. W. Wang et al. (2018) |
| WT1 | miR-15a/16 | AML | Proliferation |
Gao et al. (2011, 2012) |
| TNFAIP1 | miR-15a | osteosarcoma | Proliferation, migration, and invasion | Tian et al. (2015) |
| Bmi-1 | miR-15a/16 | PDAC,GC, ovarian cancer,GBM,MCL,BC |
Proliferation, apoptosis, migration, invasion, angiogenesis, and chemotherapy | Bhattacharya et al. (2009); C. Wu et al. (2016); F. Chen et al. (2016); Guo et al. (2014); Huang et al. (2015); Patel et al. (2016, 2017) |
| ROS | miR-15a/16 | BC | Apoptosis | Patel et al. (2016) |
| Bcl-2 | miR-15b/16 | GC | Apoptosis | Xia et al. (2008) |
| Bcl-2 | miR-15a/16 |
CLL,LC,BC,AML |
Apoptosis and chemotherapy |
Abraham et al. (2017); Adam and Eischen, (2016); Aqeilan et al. (2010); Chu et al. (2015); Pekarsky and Croce, (2015) |
| FEAT | miR-16 | A549 cell | Apoptosis | Liang et al. (2015) |
| Smad3/Sox5 /Twist1 | miR-15a/16 | NSCLC,GC,PCa,BC |
Migration and invasion | Hu et al. (2018); J. He, (2017); Pei et al. (2014); Renjie and Haiqian (2015); T. Wang et al. (2017) |
| TGF-β1 | miR-16 | NSCLC,PCa | Migration and invasion | Hu et al. (2018); H. Wang et al. (2018) |
| CXCL10 | miR-15a | CML | Migration | D. Chen et al. (2017) |
| c-Myb | miR-15a |
HCC |
Migration |
B. Liu et al. (2017) |
| Bcl-2 | miR-15a | Osteosarcoma | Migration and invasion | Leng et al. (2018) |
| PAI-2 | miR-15a | CCA | Migration | Utaijaratrasmi et al. (2018) |
| NF-κB1/MMP-9 | miR-16 | Glioma | Invasion | T. Q. Yang et al. (2014) |
| YAP1, SALL4, FGF-2 | miR-16 | CCA,NPC, neuroglioma | Migration and invasion | Han et al. (2017); Lin et al. (2016); Q. He et al. (2016); Zhou et al. (2016) |
| Fgf-2, Fgfr1 | miR-15/16 | PCa | Proliferation and migration | Musumeci et al. (2011) |
| STAT3, AEGF | miR-15/16 | BC | Proliferation and angiogenesis | Srinivas et al. (2015) |
VEGF-A, VEGF-C |
miR-15a/16 | MM,PCa,GBM,BC,ALCL,ALK |
Angiogenesis | Dejean et al. (2011); F. Chen et al. (2016); Lee et al. (2013); Sun et al. (2013); Terzuoli et al. (2016); Y. Li et al. (2016) |
| – | miR-15b/16 | SGC7901/VCR | Chemotherapy | Xia et al. (2008) |
| c-Myc/CCND1/CDK4/E2F1 | miR-15a | NPC | Chemotherapy | Z. Liu et al. (2016) |
| – | miR-15a | MM | Chemotherapy | Hao et al. (2011) |
| – | miR-15a | CRC | Migration | Fesler et al. (2018) |
| AKT/ERK | miR-15a/16 | AML | Apoptosis | Abraham et al. (2017) |
- Note. ALK: anaplastic lymphoma kinase; AML: acute myelogenous leukemia; BC: breast cancer; CCA: XXX; CDK4: cyclin-dependent kinase 4; CLL: chronic lymphocytic leukemia; CML: chronic myelogenous leukemia; CRC: colorectal cancer; CXCL10: chemokine ligand 10; GBM: glioblastoma; GC: gastric cancer; HCC: hepatocellular carcinoma; LC: lung cancer; MCL: mantle cell lymphoma; MM: multiple myeloma; NPC: nasopharyngeal cancer; NSCLC: nonsmall cell lung cancer; PDAC: pancreatic ductal adenocarcinoma; ROS: reactive oxygen species; STAT3: signal transducer and activator of transcription 3; TGF-β1: transforming growth factor-β1; VEGF: vascular endothelial growth factor.
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (81870105, 81301774 and 81470362) and Natural Science Foundation of Hunan Province (2016JJ2108).
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.




