Effects of inhibitors of the renin-angiotensin system on reducing blood pressure and expression of inflammatory factors in CHD patients: A network meta-analysis
Abstract
The renin-angiotensin system (RAS) is an ever-evolving endocrine system with considerable checks and balances on the production and catabolism of angiotensin peptides most likely due to the manifold effects of angiotensins. We aimed to explore the effects of different inhibitors of RAS on blood pressure and expression of inflammatory factors in patients with coronary heart disease (CHD). We initially searched PubMed, EMBASE and Cochrane Library electronic databases with nine eligible randomized controlled trials enrolled. Direct and indirect evidence was combined to calculate the weighted mean difference value and draw surface under the cumulative ranking curves. The results demonstrated that, compared with placebo and enalapril, ramipril had a better effect on reducing systolic blood pressure after short-term usage of drugs (<12 months), while perindopril had better effects on reducing diastolic blood pressure and C-reactive protein expression. Furthermore, after long-term usage of drugs (≥12 months), there was no significant difference among olmesartan, quinapril and candesartan in the treatment of patients with CHD. Perindopril and ramipril had better effects on inhibiting blood pressure and expression of inflammatory factors among eight inhibitors after short-term usage of drugs (<12 months); while quinapril had better effects on reducing blood pressure and expression of inflammatory factor after long-term usage of drugs, and there was little difference in the effects between olmesartan and candesartan (≥12 months). Perindopril may have better short-term effects on reducing blood pressure and expression of inflammatory factor, while quinapril may have better long-term effects on reducing blood pressure and expression of inflammatory factor.
1 INTRODUCTION
Coronary heart disease (CHD), which is generally induced by atherosclerosis due to excessive cholesterol substances accumulated as atheroma on the artery walls making the coronary arteries become narrowed and hardened (Wong, Zhang, & Wang, 2015). CHD is the leading cause of death, and it is estimated that the prevalence of cardiovascular diseases will increase by approximately 10% over the next 20 years (Madjid & Fatemi, 2013). There are varied options to the treatment of CHD, our study aimed to reveal the differences between multiple medical treatments of CHD; its results indicated that angiotensin-converting enzyme (ACE) inhibitors of renin-angiotensin system (RAS) blockade or angiotensin receptor blockers (ARB) in combination with antiplatelet agents, statins and beta-blockers are considered the optimal medical treatment (Cordero et al., 2009). The renin-angiotensin-aldosterone system plays a key role in the regulation of fluid and electrolyte balance (Cheung, 2002). ACE inhibitors inhibit ACE and have been shown to be effective in many cardiovascular diseases (Cheung, 2002). Long-term therapy with the ACE inhibitor perindopril induced structural repair of coronary arterioles that was mainly characterized by the regression of periarteriolar fibrosis and associated with a marked improvement in coronary reserve (Deckers et al., 2006). The long-term treatment with perindopril significantly decreased serum ACE activities and increased plasma bradykinin concentrations (Morishita et al., 2002). Relative to pretreatment measurements, quinapril increased flow-mediated dilation, with significant improvement persisting one week after discontinuation of therapy (Koh et al., 1999). In conclusion, quinapril may interfere with cytokine release by lowering serum interleukin-6 (IL-6) levels, which may be of particular importance for secondary prevention of stable CHD (Gibas et al., 2007).
For simultaneous comparisons of several treatments, the method of network meta-analysis (NMA) can overcome the limitations of traditional pair-wise meta-analysis by including all available evidence into a general statistical framework (Tu & Wu, 2017). A study of the epidemiology of CHD confirmed that hypertension is one of the risk factors of causing CHD (Gupta, 2008). C-reactive protein (CRP) is a plasma protein synthesized by the liver and plays a role as sensitive and dynamic systemic marker of information, and it was found that the concentration of it has continuous associations with the risk of CHD (Kaptoge et al., 2010). In addition, a previous study has suggested that beneficial long-term effects of interference with the inhibitors of RAS may be related to reduction of oxidative stress (Hornig et al., 2001), indicating that the inhibitors of RAS may be beneficial to reduce the blood pressure and the level of inflammatory factor for the CHD patient. Therefore, based on above evidence, we aim to conduct a NMA to explore effects of the inhibitors of RAS on the reduction of blood pressure and the level of inflammatory factor in CHD patients, and we expect our findings will provide significant guidance for physicians in the treatment of CHD.
2 MATERIALS AND METHODS
2.1 Search strategy
PubMed, EMBASE and Cochrane Library electronic databases were searched from the inception to February, 2017. With the combination of keywords and free words, the search items included: coronary heart disease, randomized controlled trial, inhibitors of the RAS, angiotensin converting enzyme inhibitors (ACEI), ARB, enalapril, perindopril, irbesartan, telmisartan, telmisartan, ramipril, olmesartan, quinapril, candesartan, and so forth. In addition, a manual search was also conducted to other potential and relevant references.
2.2 Inclusion and exclusion criteria
The inclusion criteria were: (a) study subjects must be patients with CHD and accordingly clinical manifestation; (b) studies with two or more groups with at least one group was an inhibitor of the RAS; (c) interventions: ACEI (Enalapril, Peripental, Ramipril, Quinapril) and ARB (Olmesartan, Irbesartan, Telmisartan, Candesartan); (d) outcomes included systolic blood pressure (SBP) (mmHg), diastolic blood pressure (DBP) (mmHg) and CRP (mmHg); the evaluation was divided into short-term efficacy (<12 months) and long-term efficacy (≥12 months), and differences before and after the treatment were used for statistical analysis; (e) research with complete and desirable data. The exclusion criteria were: (a) studies included non-English reference, non-human study, conference report, systematic reviews and summaries, duplicated publications; (b) patients with heart failure (ejection fraction < 45%), severe liver and kidney function deficiency.
2.3 Data extraction and quality assessment
With the standard data collection forms, the risk of bias of included randomized controlled trials (RCTs) was assessed according to the Cochrane Collaboration's tool (Higgins et al., 2011). This tool included six items: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and anything else ideally prespecified. The assessment includes assigning a judgment of “yes,” “no,” or “unclear” for each domain to designate a low, high, or unclear risk of bias, respectively. If one or no domain was deemed “unclear” or “no,” the study was judged as having a low risk of bias. If four or more domains are deemed “unclear” or “no,” the study was regarded as having a high risk of bias. If two or three domains were deemed “unclear” or “no,” the study was considered as having a moderate risk of bias (Chung & Lee, 2013). Review Manager 5 (RevMan 5.2.3, Cochrane Collaboration, Oxford, UK) was utilized to carry out the quality assessment and investigation of publication bias.
2.4 Statistical analysis
First, we conducted traditional pairwise meta-analyzes for studies that compared different treatment arms directly. The pooled estimates of weighted mean difference (WMD) and 95% confidence intervals (CIs) were reported in our results. We used the Chi-square test and I-square test to test heterogeneity among the studies (Chen et al., 2015). Secondly, R 3.2.1 software was used to draw a network diagram, in which each node represented an intervention measure, the size of node represented sample size and the thickness of line between nodes represented the number of included studies. Thirdly, Bayesian network meta-analyzes were used to compare different interventions to each other. Each analysis was done on the basis of non-informative priors for effect sizes and precision. After four chains and a 20,000-simulation burn-in phase, we checked and confirmed the convergence and lack of auto correlation; ultimately, direct probability statements were derived from an additional 50,000-simulation phase (Tu, Needleman, Chambrone, Lu, & Faggion, 2012). To assist in the interpretation of WMDs, based on a Bayesian approach using probability values summarized as surface under the cumulative ranking curve (SUCRA), we calculated the probability of each intervention being the most effective treatment method, the larger the SUCRA value, the better the rank of the intervention (Chaimani, Higgins, Mavridis, Spyridonos, & Salanti, 2013; Salanti, Ades, & Ioannidis, 2011). Cluster analyzes were used to group the treatments according to their similarity regarding both outcomes (Chaimani et al., 2013). R (V.3.2.1) package gemtc (V.0.6) as well as the Markov Chain Monte Carlo engine Open BUGS (V.3.4.0) was used to do all computations.
3 RESULTS
3.1 Baseline characteristics of included studies
A total of 637 related literatures were initially retrieved in this study, and one of them was manually searched. After reviewing the titles and abstracts, we excluded 57 non-English studies, 68 for non-human studies, 197 for case reports, letters and reviews. After full-text review, of the rest 315 literatures, 80 unrelated to CHD, 74 non-drug research, 94 non-RCTs were ruled out. Upon further assessment, we excluded 20 studies in the same clinical trial, and 32 literatures irrelevant to the outcome measure, six articles without data or incomplete data. Finally, nine eligible RCTs were incorporated in this NMA from 2002 to 2014 (Bohm, Beltran, & Pernow, 2005; Hirohata et al., 2010; Kondo et al., 2003, Krysiak & Okopien, 2012; Link, Lenz, Legner, Böhm, & Nickenig, 2006; Miyoshi et al., 2014; Morishita et al., 2002; Tousoulis et al., 2008; van Haelst et al., 2003) (Figure 1). A total of 1,102 patients with CHD were recruited into the meta-analysis, among which the majority of patients were treated with placebo regimen. Four RCTs were in Asians, and five RCTs were in Caucasians. Furthermore, seven included studies were two-arm trials, and the rest were three-arm trials. Part of patients included in this study was with hypertension or high risk of hypertension; there were more male patients than female patients, and the mean age of patients was around 60 years. The characteristics of the included studies are summarized in Supporting Information Table 1 and the Cochrane risk of bias assessment was shown in Figure 2.
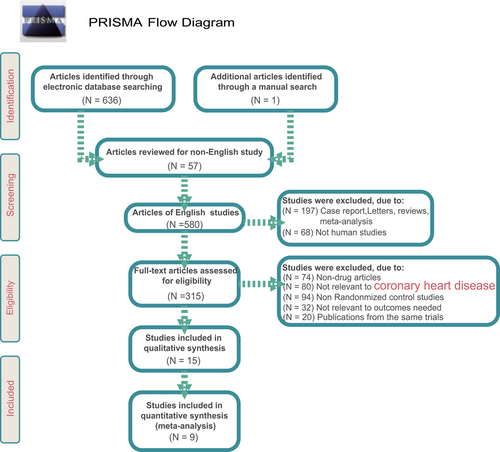
Flowchart showing literature search and study selection [Color figure can be viewed at wileyonlinelibrary.com]

Cochrane system bias evaluation of included literatures [Color figure can be viewed at wileyonlinelibrary.com]
3.2 Pairwise meta-analysis of the effects of different inhibitors of RAS on the treatment of patients with CHD
The study explored the effects of eight inhibitors of RAS on blood pressure (SBP and DPB) and expression of inflammatory factor (CRP) in patients with CHD. Pairwise meta-analysis suggested that, compared with placebo, perindopril had a better effect on reducing SBP, DBP, and CRP after short-term usage of drugs (<12 months) (WMD = −3.00, 95%CI = −4.31 to − 1.69; WMD = −4.80, 95%CI = −5.87 to − 3.73; WMD = −1.51, 95%CI = −1.68 to − 1.34, respectively); but there was no significant difference in the therapeutic effect of each drug in patients with CHD after long-term usage of drugs (≥12 months) (Table 1).
| Pairwise meta-analysis | ||
|---|---|---|
| Studies | Comparisons | WMD (95%CI) |
| SBP (<12 months) | ||
| 3 studies | Perindopril vs Placebo | −3.00 (−4.31 to −1.69) |
| DBP (<12 months) | ||
| 3 studies | Perindopril vs Placebo | −4.80 (−5.87 to −3.73) |
| CRP (<12 months) | ||
| 2 studies | Perindopril vs Placebo | −1.51 (−1.68 to −1.34) |
| SBP (≥12 months) | ||
| 2 studies | Olmesartan vs Placebo | 0.62 (−4.05~5.29) |
| DBP (≥12 months) | ||
| 2 studies | Olmesartan vs Placebo | 0.22 (−2.30~2.75) |
- Note. 95%CI: 95% confidence intervals; CRP: C-reactive protein; DBP: diastolic blood pressure; SBP: Systolic blood pressure.
3.3 Evidence network of the effects of different inhibitors of RAS on the treatment of patients with CHD
According to the index for SBP, DBP and CRP, in short-term usage of drugs (<12 months), of the majority of CHD patients received perindopril, while patients who received irbesartan were relatively less. In long-term usage of drugs (≥12 months), most patients underwent olmesartan and candesartan treatment, while fewest patients took quinapril (Figure 3).
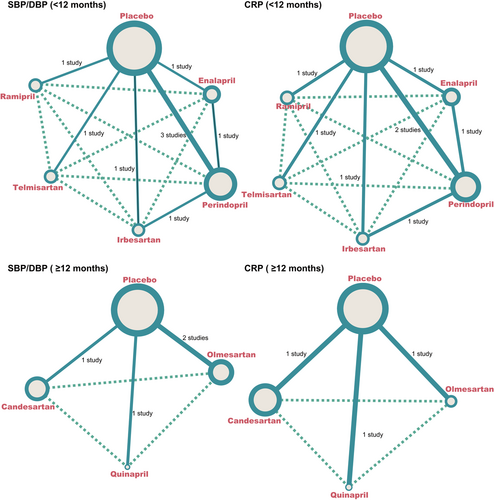
The evidence network diagrams of SBP/DBP (<12 months), CRP (<12 months), SBP/DBP (≥12 months) and CRP (≥12 months). CRP: C-reactive protein; DBP: diastolic blood pressure; SBP: systolic blood pressure [Color figure can be viewed at wileyonlinelibrary.com]
3.4 The main results of NMA
The NMA indicated that compared with placebo and enalapril, ramipril had lower SBP level after short-term usage of drugs (<12 months) (WMD = −12.83, 95%CI = −22.51 to −3.33; WMD = −17.66, 95%CI = −30.86 to −4.51, respectively), and perindopril had lower levels of DBP and CRP (DPB: WMD = −4.45, 95%CI = −7.29 to −0.77; CRP: WMD = −1.51, 95%CI = −2.62 to −0.45, respectively) (Table 2, Supporting Information Figures 1–3). However, after long-term usage of drugs (≥12 months), there were no significant differences among olmesartan, quinapril and candesartan in the treatment of CHD (Table 3, Supporting Information Figures 1–3).
| WMD (95%CI) | |||||
|---|---|---|---|---|---|
| SBP (<12 months) | |||||
| Placebo | 4.69 (−4.01, 14.13) | −3.03 (−9.01, 2.86) | −3.12(−12.28,5.86) | −3.13(−14.53,9.40) | −12.83(−22.51,−3.33) |
| −4.69 (−14.13,4.01) | Enalapril | −7.67 (−17.22, 0.86) | −8.07(−20.18,3.21) | −8.00(−22.79,7.21) | −17.66(−30.86,−4.51) |
| 3.03 (−2.86, 9.01) | 7.67 (−0.86, 17.22) | Perindopril | −0.13 (−9.02, 8.53) | −0.09(−12.52,13.36) | −9.84 (−20.64, 1.24) |
| 3.12 (−5.86, 12.28) | 8.07 (−3.21, 20.18) | 0.13 (−8.53, 9.02) | Irbesartan | −0.19(−14.48,15.65) | −9.82 (−22.98, 3.36) |
| 3.13 (−9.40, 14.53) | 8.00 (−7.21, 22.79) | 0.09 (−13.36, 12.52) | 0.19(−15.65,14.48) | Telmisartan | −9.99 (−25.17, 4.97) |
| 12.83 (3.33, 22.51) | 17.66 (4.51, 30.86) | 9.84 (−1.24, 20.64) | 9.82 (−3.36, 22.98) | 9.99 (−4.97, 25.17) | Ramipril |
| DBP (<12 months) | |||||
| Placebo | −4.13 (−9.13, 1.06) | −4.45 (−7.29, −0.77) | −3.71 (−8.35, 0.89) | −0.88 (−6.99, 5.16) | −3.04 (−8.05, 1.99) |
| 4.13 (−1.06, 9.13) | Enalapril | −0.26 (−5.14, 5.00) | 0.46 (−6.01, 6.62) | 3.27 (−4.75, 11.17) | 1.13 (−6.14, 8.32) |
| 4.45 (0.77, 7.29) | 0.26 (−5.00, 5.14) | Perindopril | 0.76 (−4.15, 5.00) | 3.51 (−3.55, 10.14) | 1.40 (−4.97, 7.19) |
| 3.71 (−0.89, 8.35) | −0.46 (−6.62, 6.01) | −0.76 (−5.00, 4.15) | Irbesartan | 2.76 (−4.64, 10.29) | 0.66 (−5.99, 7.69) |
| 0.88 (−5.16, 6.99) | −3.27 (−11.17, 4.75) | −3.51 (−10.14, 3.55) | −2.76 (−10.29, 4.64) | Telmisartan | −2.20 (−9.91, 5.77) |
| 3.04 (−1.99, 8.05) | −1.13 (−8.32, 6.14) | −1.40 (−7.19, 4.97) | −0.66 (−7.69, 5.99) | 2.20 (−5.77, 9.91) | Ramipril |
| CRP (<12 months) | |||||
| Placebo | −0.70 (−2.12, 0.67) | −1.51 (−2.62, −0.45) | −0.20 (−1.63, 1.21) | 0.05 (−1.43, 1.72) | −0.23 (−1.71, 1.32) |
| 0.70 (−0.67, 2.12) | Enalapril | −0.80 (−2.24, 0.60) | 0.50 (−1.37, 2.41) | 0.77 (−1.27, 2.93) | 0.48 (−1.62, 2.58) |
| 1.51 (0.45, 2.62) | 0.80 (−0.60, 2.24) | Perindopril | 1.29 (−0.09, 2.82) | 1.59 (−0.30, 3.58) | 1.29 (−0.54, 3.28) |
| 0.20 (−1.21, 1.63) | −0.50 (−2.41, 1.37) | −1.29 (−2.82, 0.09) | Irbesartan | 0.26 (−1.80, 2.43) | −0.02 (−2.13, 2.09) |
| −0.05 (−1.72, 1.43) | −0.77 (−2.93, 1.27) | −1.59 (−3.58, 0.30) | −0.26 (−2.43, 1.80) | Telmisartan | −0.23 (−2.52, 1.86) |
| 0.23 (−1.32, 1.71) | −0.48 (−2.58, 1.62) | −1.29 (−3.28, 0.54) | 0.02 (−2.09, 2.13) | 0.23 (−1.86, 2.52) | Ramipril |
- Note. 95%CI: 95% confidence intervals; CRP: C-reactive protein; DBP: diastolic blood pressure; SBP: systolic blood pressure. Weighted mean difference (95%CI) below the treatments should be read from row to column while above the treatments should be read from column to row.
| WMD(95%CI) | |||
|---|---|---|---|
| SBP (≥12 months) | |||
| Placebo | 0.46 (−4.79, 5.60) | −1.96 (−5.21, 1.37) | −1.10 (−4.95, 2.94) |
| −0.46 (−5.60, 4.79) | Olmesartan | −2.42 (−8.50, 3.60) | −1.52 (−7.85, 5.29) |
| 1.96 (−1.37, 5.21) | 2.42 (−3.60, 8.50) | Quinapril | 0.87 (−4.21, 6.19) |
| 1.10 (−2.94, 4.95) | 1.52 (−5.29, 7.85) | −0.87 (−6.19, 4.21) | Candesartan |
| DBP (≥12 months) | |||
| Placebo | 0.20 (−2.86, 3.39) | 1.99 (−0.50, 4.43) | −0.01 (−2.83, 2.82) |
| −0.20 (−3.39, 2.86) | Olmesartan | 1.75 (−2.21, 5.70) | −0.23 (−4.36, 3.83) |
| −1.99 (−4.43, 0.50) | −1.75 (−5.70, 2.21) | Quinapril | −1.96 (−5.83, 1.73) |
| 0.01 (−2.82, 2.83) | 0.23 (−3.83, 4.36) | 1.96 (−1.73, 5.83) | Candesartan |
| CRP (≥12 months) | |||
| Placebo | −0.71 (−2.40, 0.98) | −1.27 (−2.99, 0.38) | −0.99 (−3.73, 1.73) |
| 0.71 (−0.98, 2.40) | Olmesartan | −0.56 (−2.98, 1.80) | −0.27 (−3.40, 2.86) |
| 1.27 (−0.38, 2.99) | 0.56 (−1.80, 2.98) | Quinapril | 0.27 (−2.83, 3.44) |
| 0.99 (−1.73, 3.73) | 0.27 (−2.86, 3.40) | −0.27 (−3.44, 2.83) | Candesartan |
- Note. 95%CI: 95% confidence intervals; CRP: C-reactive protein; DBP: diastolic blood pressure; SBP: systolic blood pressure.
- Weighted mean difference (95%CI) below the treatments should be read from row to column while above the treatments should be read from column to row.
3.5 SUCRA of the effects of different inhibitors of RAS on the treatment of patients with CHD
The SUCRA values of the effects of 8 inhibitors of RAS on CHD were summarized in Figure 4. The results indicated that, after short-usage of drugs (<12 months), in terms of CRP and DBP, the SUCRA values of perindopril was relatively higher (CRP: 97.00%; DBP: 81.00%); as for SBP, the SUCRA values of ramipril was corresponding higher (97.17%), while enalapril was the lowest (21.17%). After long-term usage of drugs (≥12 months), as for SBP, DBP and CRP, the SUCRA values of quinapril were relatively higher (SBP: 84.00%; DBP: 92.00%; CRP: 81.75%.
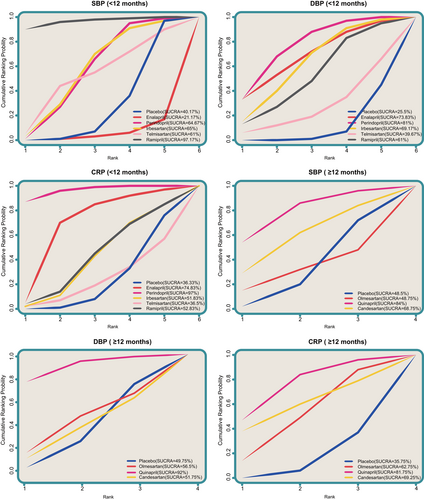
Surface under the cumulative ranking curves of SBP (<12 months), DBP (<12 months), CRP (<12 months), SBP (≥12 months), DBP (≥12 months) and CRP (≥12 months). CRP: C-reactive protein; DBP: diastolic blood pressure; SBP: systolic blood pressure [Color figure can be viewed at wileyonlinelibrary.com]
3.6 Cluster analysis of the effects of different inhibitors of RAS on the treatment of patients with CHD
The efficacy of perindopril and ramipril on reducing blood pressure and expression of inflammatory factor was relative better than other drugs after the short-term usage of drugs (<12 months), while telmisartan was relatively worst. Quinapril may have a better effect after long-term usage of drugs, while effects of olmesartan and candesartan had little difference after long-term usage of drugs (≥12 months). But because of less studies involving ramipril and quinapril and the relevant studies with a small sample size, the effects of them are needed to be further verified (Figure 5).
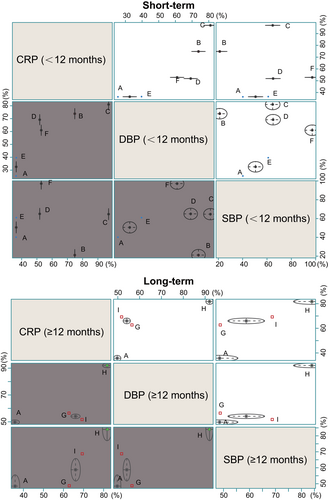
Cluster analysis of SBP, DBP and CRP both after short and long term. (A) placebo, (B) enalapril, (C) perindopril, (D) irbesartan, (E) telmisartan, (F) ramipril, (G) olmesartan, (H) quinapril, (I) candesartan. CRP: C-reactive protein; DBP: diastolic blood pressure; SBP: systolic blood pressure [Color figure can be viewed at wileyonlinelibrary.com]
4 DISCUSSION
After collecting historical cases and analyzing eight different inhibitors of the RAS for CHD by pairwise and NMA, we found evidence that perindopril and quinapril had better short-term effects on reducing blood pressure and the level of inflammatory factor in CHD, while quinapril was correspondingly better in comparison with others after long-term usage of drugs (≥12 months). A previous study assessed whether the ACE inhibitor perindopril reduced cardiovascular risk in a low-risk population with stable CHD and no apparent heart failure or not (Fox & Investigators, 2003). Quinapril was well tolerated in patients after angioplasty with normal left ventricular function (Pitt et al., 2001). Among patients with stable CHD without apparent heart failure, perindopril can significantly improve outcome (Fox & Investigators, 2003). There was no significant difference of the changes in hyperemic blood flow and RH between irbesartan and perindopril treated patients (Tousoulis et al., 2008). The implications of the neutral angiographic effects of ACE inhibition are uncertain, but they deserve further investigation in light of the positive clinical benefits suggested here and seen elsewhere (Teo et al., 2000).
Further analysis of the results of the NMA revealed that, compared with placebo and enalapril, ramipril had a better effect on reducing SBP after short-term usage of drugs (<12 months), while perindopril had better effects on reducing DPB and CRP expression. Short-term treatment with irbesartan and perindopril in normotensive patients with CHD improves forearm vasodilatory response to reactive hyperemia in a similar degree, and decreased serum levels of high sensitivity C-reactive protein (hsCRP) and fibrinogen (Tousoulis et al., 2008). Results of a former study indicated that perindopril was superior to enalapril in producing monocyte-suppressing and systemic anti-inflammatory effects in normotensive patients with CHD (Krysiak & Okopien, 2012). Furthermore, after long-term usage of drugs (≥12 months), quinapril had a better effect on reducing SBP, while olmesartan and quinapril had better effects on reducing CRP expression. In line with our study, a study also revealed that there was a lower expression of CRP with quinapril when characterizing CRP production during treating acute myocardial infarction, which is also a kind of CHD (Tsikouris, Suarez, Simoni, Ziska, & Meyerrose, 2004). This supports the contention that the beneficial effects of ACE inhibition are partly the result of a reduced vascular inflammatory response (van Haelst et al., 2003).
As shown in Figure 4, after short-term usage of drugs (<12 months), the SUCRA values of CRP and DBP of perindopril were relative higher, while enalapril was the lowest. In patients with CHD and well-controlled risk factors, antihypertensive therapy lowered blood pressure and progression of coronary atherosclerosis but did not affect plasma biomarkers of inflammation and remodeling (Zamani et al., 2012); after long-term usage of drugs (≥12 months), the SUCRA curves of CRP and SBP of quinapril were correspondingly higher. Long-term treatment with quinapril could enhance the functions of left ventricular dilatation, mass and hypertrophy regressed and left ventricular in the treatment of aortic regurgitation or mitral regurgitation, notably in the study by Schon and Schomig (1995). The study showed that perindopril, ramipril, and quinapril had better effects on reducing blood pressure and expression of inflammatory factor in patients with CHD.
This study has the following advantages: first, innovation was emphasized. There was no study focused on effects of eight inhibitors of the RAS on reducing blood pressure and expression of inflammatory factor in CHD patients both in short- and long-term so far. Secondly, the statistical method used was scientific and rigorous, the search strategy was comprehensive and the range of retrieval was wide, inclusion and exclusion criteria were strict, and the quality of literature was reliable and the risk of bias is low. Finally, direct comparison and indirect comparison were conducted, and cluster analysis was used, therefore our results are reliable. Nevertheless, several limitations deserve our attentions. Due to the number of enrolled studies about the eight inhibitors of the RAS showed a large difference in short- and long-term, and there are few research focused on the DBP (<12 month) which caused the obvious differences between the results of DBP (<12 month) and other outcomes. In addition, our study just analyzed three outcomes (SBP, DBP, and CRP) because of the insufficient data and information of other outcomes in the included studies, which may affect our results. Also, the sample size of included literatures is small, which may affect the results. But we made strict inclusion/exclusion criteria and conducted scientific, rigorous statistical analysis to try to reduce the heterogeneity to a relative lowest level, which means the results of this study are reliable. However, more and more studies could pay more attention to larger samples and more related outcomes in future. In summary, the results of this NMA suggested that perindopril, ramipril and quinapril were the best choice in the treatment of CHD, which provided evidence toward further development of more effective treatment of CHD and inspiration for further study.
ACKNOWLEDGMENTS
We are grateful for the contributions and useful suggestions of all the reviewers on our article.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.




