Cellular senescence: Molecular mechanisms and pathogenicity
Abstract
Cellular senescence is the arrest of normal cell division. Oncogenic genes and oxidative stress, which cause genomic DNA damage and generation of reactive oxygen species, lead to cellular senescence. The senescence-associated secretory phenotype is a distinct feature of senescence. Senescence is normally involved in the embryonic development. Senescent cells can communicate with immune cells to invoke an immune response. Senescence emerges during the aging process in several tissues and organs. In fact, increasing evidence shows that cellular senescence is implicated in aging-related diseases, such as nonalcoholic fatty liver disease, obesity and diabetes, pulmonary hypertension, and tumorigenesis. Cellular senescence can also be induced by microbial infection. During cellular senescence, several signaling pathways, including those of p53, nuclear factor-κB (NF-κB), mammalian target of rapamycin, and transforming growth factor-beta, play important roles. Accumulation of senescent cells can trigger chronic inflammation, which may contribute to the pathological changes in the elderly. Given the variety of deleterious effects caused by cellular senescence in humans, strategies have been proposed to control senescence. In this review, we will focus on recent studies to provide a brief introduction to cellular senescence, including associated signaling pathways and pathology.
1 INTRODUCTION
Cellular senescence was first defined by Hayflick and Moorhead (1961). Cellular senescence is the arrest of normal cell division in response to a variety of cellular stresses or DNA damage along with proinflammatory response, mitochondrial dysfunction, and telomere shortening (Noren & Evans, 2017; Ogrodnik et al., 2017). Senescence is normally involved in the embryonic development (Biran & Krizhanovsky, 2015). Senescent cells can communicate with their neighboring cells by transferring the proteins to the recipient cells (Biran & Krizhanovsky, 2015). Senescent cells can regulate the immune response that facilitates the elimination of senescent cells (Contrepois et al., 2017).
Since its discovery two decades ago, many studies have been done to determine the roles of cellular senescence in human diseases. These studies have revealed that cellular senescence is implicated in an increasing number of pathological processes in many types of cells and tissues. Cell senescence can cause suppression of tumor cell proliferation and invasion by arresting the cell cycle. It is also associated with a variety of diseases, such as nonalcoholic fatty liver disease (NAFLD), diabetes, pulmonary hypertension, osteoarthritis, tumorigenesis, and pathogenic infection. Therefore, cellular senescence affects different pathological conditions in opposite ways (Table 1).
| Diseases | Effects of senescence | Pathological characterization |
|---|---|---|
| Obesity/diabetes | Deleterious | Cellular senescence is involved in insulin resistance. Inhibiting senescence can increase glucose sensitivity. |
| Nonalcoholic fatty liver disease | Deleterious | Mitochondria in senescent cells cannot metabolize fatty acid normally, resulting in accumulation of hepatic fat and steatosis. |
| Pulmonary hypertension | Deleterious | Senescence of PA-SMCs induced by telomerase shortening and oxidative stress contributes to pulmonary hypertension. Osteopontin plays a crucial role in the development of pulmonary hypertension. |
| Idiopathic pulmonary fibrosis | Deleterious | Senescent cell clearance reduces the expression of proinflammatory factors, coinciding with the resolution of fibrosis. |
| Osteoarthritis | Deleterious | Senescent cells cause osteoarthritis in the elderly. A variety of cells within the bone microenvironment become senescent. |
| Tumorigenesis | Beneficial and deleterious | Inducing cellular senescence may be a strategy to inhibit tumor growth. However, the SASP can promote tumorigenesis and confer therapy resistance |
- Note. PA-SMC: pulmonary artery smooth muscle cell; SASP: senescence-associated secretory phenotype.
The causes and mechanisms of cellular senescence are not completely understood. Oncogenic genes and other factors that cause genomic DNA damage and the production of reactive oxygen species (ROS), including RAS (Tu et al., 2011), alcohol (Chen et al., 2017), bacterial lipopolysaccharide (LPS; C. O. Kim, Huh, Han, & Kim, 2012), and sodium butyrate (Itoh, Nakagawa, & Yoshioka, 2013), can induce cellular senescence. Moreover, many signaling pathways are involved in cellular senescence, such as p53, p38 mitogen-activated protein kinase (MAPK), nuclear factor-κB (NF-κB), mammalian target of rapamycin (mTOR), EphA2/ephrin-A1, and transforming growth factor-beta (TGF-β) signaling pathways. Furthermore, cellular senescence is accompanied by chronic inflammation resulting from the production of inflammatory factors, including such as Interleukin-1α (IL-1α), IL-6, TNFα, and IL-10 (Wiley et al., 2016). In addition, the mechanisms of epigenetic regulation of the cellular senescence are being studied (Nacarelli, Liu, & Zhang, 2017).
Given that cellular senescence is linked to many human diseases, studies should be done on how to regulate cellular senescence. Inhibiting ROS production, suppressing senescence-associated signaling, and physical exercise can inhibit cellular senescence. In addition, senescent cell apoptosis can be induced using drugs that inhibit antiapoptotic proteins or activate the p53 pathway. In this review, we will focus on recent studies to provide a brief overview of cellular senescence, including associated signaling pathways and pathology. Previous reviews have detailed the role of cellular senescence in physiology, the immune response, tumorigenesis, and aging (H. Zhang, 2007; Muñoz-Espín & Serrano, 2014; Ohtani & Hara, 2013; Ohtani, Takahashi, Mann, & Hara, 2012; Ovadya & Krizhanovsky, 2014; Sikora, Bielakzmijewska, & Mosieniak, 2014; Vicente, Mausset-Bonnefont, Jorgensen, Louis-Plence, & Brondello, 2016).
2 METHODS FOR DETECTING CELLULAR SENESCENCE
It is challenging to distinguish the senescent cells from normal cells. Senescent cells are characterized by several features, including the constitutive DNA damage response (DDR), senescence-associated β-galactosidase (SA-β-gal) activity, increased expression of cyclin-dependent kinase (CDK) inhibitors p16 (CDKN2A) and p21 (CDKN1A), senescence-associated secretory phenotype (SASP), and the formation of senescence-associated heterochromatin foci (SAHF). Senescent phenotypes are not stable and seen at varying at different time points after the induction of senescence (Hernandez-Segura et al., 2017). Some features, like SAHF formation, may not be observed under certain conditions or not exist in the cell type despite the existence of other cellular senescence phenotypes (Kosar et al., 2011). Therefore, observation of a combination of several different phenotypes is necessary to confirm the existence of cellular senescence. Noren et al. developed in vitro methods to assess senescence by detecting several senescence-related phenotypes including SA-β-gal, γ-H2AX, and SAHF staining (Noren & Evans, 2017). These methods can be used in many models and tissues. Zhao et al. (2017) described methods to identify and quantify cellular senescence using observation of cell morphology, SA-β-gal staining, and monitoring the expression of tumor suppressors in vitro and in vivo.
Together these methods could be used to identify specific senescent phenotypes in a variety of cell types and study their biological functions in normal and disease states.
3 DIVERSE SIGNALS INDUCE CELLULAR SENESCENCE
3.1 DNA damage
Increasing evidence indicates that cellular stress, environmental stress, and chronic inflammation can induce cellular senescence through proinflammatory responses (Salminen, Kauppinen, & Kaarniranta, 2012). Oxidative stress (OS), in particular, contributes to DNA damage and telomere shortening (Passos, Saretzki, & von Zglinicki, 2007). For example, Nox4-dependent ROS production induced by treatment with 20% O2 can trigger senescence of rat nucleus pulposus cells by activating p53-p21-Rb and p16-Rb pathways via extracellular signal-regulated kinase (ERK) signaling (Feng et al., 2017).
It has been found that DNA damage not only activates the type I interferon system and inflammation through DNA sensors, such as cyclic guanosine monophosphate (GMP)–adenosine monophosphate (AMP) synthase (cGAS), absent in melanoma 2 (AIM2), and interferon-inducible protein 16 (IFI16), but also leads to cellular senescence (Hartlova et al., 2015). DNA damage results in the accumulation of DNA fragments in the cytoplasm. The sensors that recognize DNA damage and prime cellular senescence are unknown. Recent studies have shown that cGAS is essential for cellular senescence to occur in response to spontaneous immortalization and DNA damaging agents (Glück et al., 2017; H. Yang, Wang, Ren, Chen, & Chen, 2017). The activation of cGAS stimulator of interferon genes (STING) induces the appearance of SASP (Glück et al., 2017). It is still unknown whether downstream effectors, TBK1 and IRF3, are involved in cGAS-induced senescence and the SASP (Figure 1).
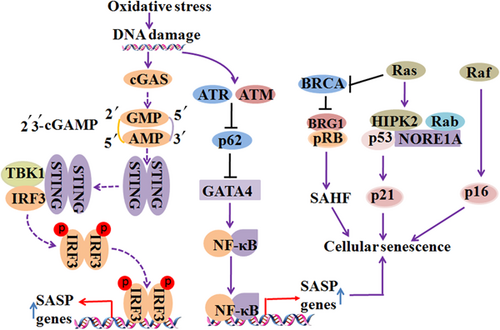
Diverse signals induce cellular senescence. DNA damage occurs during OS, which results in the accumulation of DNA fragments in the cytoplasm. Cyclic GMP–AMP synthase (cGAS) is essential for responding to spontaneous immortalization and DNA damaging agents. The activation of cGAS induces the appearance of senescence-associated secretory phenotype (SASP) through the stimulator of interferon genes (STING). DNA damage regulators, ataxia telangiectasia mutated (ATM) and ATM-Rad3 related (ATR), are required for the activation of GATA4. GATA4 activates transcription factor NF-κB to initiate the SASP. Moreover, Ras has the potential to induce cellular senescence by signaling for the accumulation of p53 and p16. Raf, BRG1, NORE1A, and other molecules play an important role in regulating Ras-mediated cellular senescence. Solid lines represent confirmed interactions and dashed lines represent uncertain interactions. OS: oxidative stress; NF-κB: nuclear factor-κB [Color figure can be viewed at wileyonlinelibrary.com]
Regulators of DNA damage play a role in maintaining cellular senescence. GATA4 was found to activate transcription factor NF-κB to initiate the SASP (C. Kang et al., 2015). DNA damage regulators, ataxia telangiectasia mutated (ATM) and ATM-Rad3 related (ATR), are required for the activation of GATA4, which is stabilized during cellular senescence (Kang et al., 2015; Figure 1). GATA4 is degraded by p62-mediated autophagy under normal conditions, but cellular senescence inhibits the degradation of GATA4 (C. Kang et al., 2015; Figure 1). Another study showed that CDK inhibitor p21 maintains the viability of DNA damage-induced senescent cells and inhibition of p21 can induce the apoptosis of senescent cells by activating ATM and NF-κB kinase (Yosef et al., 2017).
Long noncoding RNA (lncRNA) also plays a role in cellular senescence by regulating the DDR. For example, the lncRNA HOX transcript antisense RNA (HOTAIR) can induce cell senescence and influence the sensitivity of tumors to platinum (Özeş et al., 2016). HOTAIR expression is increased in recurrent platinum-resistant ovarian tumors, compared with primary ovarian tumors (Özeş et al., 2016). HOTAIR can induce the DDR after platinum treatment and activate NF-κB signaling by reducing the expression of NF-κB inhibitor Iκ-Bα (Özeş et al., 2016).
3.2 Oncogenic genes
Two decades ago, the oncogenic gene Ras was found to have the potential to induce cellular senescence by signaling for the accumulation of p53 and p16 (Serrano, Lin, McCurrach, Beach, & Lowe, 1997; Figure 1). Subsequently, Zhu, Woods, Mcmahon, and Bishop (1998) found that the oncogenic gene Raf also can regulate senescence through MAPK signaling (Figure 1). The molecular mechanism of Ras action and Ras-mediated p53 and p16 accumulation is still unknown. It has been known that Ras inactivates the BRCA1 DNA repair complex by separating BRCA1 from chromatin, which leads to the accumulation of DNA damage and arrest of the cell cycle (Z. Tu et al., 2011; Figure 1). In addition, BRG1 acts downstream of BRCA1 and is involved in SAHF formation and Ras-mediated senescence (Z. Tu, Zhuang, Yao, & Zhang, 2013; Figure 1). NORE1A, a member of the Ras-associated domain family, also plays a role in Ras-mediated cellular senescence by interacting with p53 and Rb (Donninger, Barnoud, & Clark, 2016; Figure 1). Besides, Ras induces the formation of the NORE1A and HIPK2 complex and enhances the interaction between HIPK2 and p53 (Figure 1). Furthermore, NORE1A promotes the prosenescence by promoting the acetylation of p53, and inhibiting the proapoptotic phosphorylation of p53 (Donninger et al., 2015).
4 CELLULAR MACHINERY CHANGES DURING CELLULAR SENESCENCE
4.1 Nuclear lamina
It is well established that telomere shortening due to cellular proliferation leads to senescence (Victorelli & Passos, 2017). A previous study showed that centromeres and telomeres are localized by nuclear lamina in senescent cells and telomeres are progressively aggregated during cellular senescence (Raz et al., 2008). In fact, telomere aggregation is associated with the changes in lamina spatial organization (Raz et al., 2008). Another study showed that there is a difference between the shape of nuclear lamina in healthy and senescent cells. As such, lamina morphology can be used as a phenotype for identification of senescent cells (Raz et al., 2008). As a result, some methods have been developed to quantify the intensity and curvature of nuclear lamina for classification of cell populations (Raz et al., 2008; Righolt, Hoff, Vermolen, Young, & Raz, 2011). Both lamin B1 and lamin B1 receptor (LBR) were found to be depleted during the tumor cell transition to senescence after gamma irradiation (Lukášová, Kovarˇík, Bacˇíková, Falk, & Kozubek, 2017). The reduction of lamin B1 and LBR leads to the release of heterochromatin from the lamina, resulting in changes in the chromatin architecture and gene expression (Lukášová et al., 2017). These results were is in line with those of a previous study, which showed that silencing lamin B1 can induce cellular senescence, which is dependent on activation of p53 and pRb (Shimi et al., 2011). However, another study showed that, although both lamin B1 overexpression and depletion can inhibit proliferation, only lamin B1 overexpression can induce cellular senescence, which can be hindered by inactivation of p53 and telomerase expression (Dreesen et al., 2013). The result of this study is consistent with an early study that found that OS increases the expression of lamin B1 by activating the p38 MAPK pathway which induces cellular senescence (Barascu et al., 2012). The molecular mechanisms by which lamin B1 silencing and overexpression induce cellular senescence are still elusive. Christopher J Hutchison et al. suggested that the difference in the lamin B1/lamin A ratio may lead to the change in nuclear lamina structure, which causes cellular senescence (Hutchison, 2012).
As some DNA viral infections can disrupt nuclear lamina, it would be interesting to study whether the viruses can induce cellular senescence because cell cycle arrest during cellular senescence is beneficial for viral replication (Cano-Monreal, Wylie, Cao, Tavis, & Morrison, 2009; C.-P. Lee et al., 2008; Sharma, Kamil, Coughlin, Reim, & Coen, 2014; W. Wei et al., 2016; X. Zhang et al., 2017).
4.2 Mitochondrial dysfunction
Increasing evidence has shown that mitochondria play an important role in cellular senescence. Mitochondrial DNA damage leads to mitochondrial dysfunction and increased ROS production (Passos et al., 2007). ROS produced by mitochondria may contribute to cellular senescence by inducing telomere dysfunction (Passos et al., 2007). Cigarette smoke (CS) can also induce senescence by damaging mitophagy and increasing the number of impaired mitochondria in the lung fibroblasts and small airway epithelial cells. CS interferes with the translocation of Parkin into mitochondria by inducing the accumulation of cytoplasmic p53 which interacts with Parkin. Mitochondria-specific antioxidants can delay cellular senescence (Ahmad et al., 2015). Cells lacking hFis1, a mitochondrial fission molecule, showed sustained mitochondrial elongation and a low rate of cell proliferation. In these cells, SA-β-gal activity is also elevated (S. Lee et al., 2007). Peroxisome-proliferator-activated receptor-γ coactivator 1α deficiency promotes vascular cellular senescence due to increased OS, reduced telomerase activity, and mitochondrial dysfunction (Xiong et al., 2013). Reduced expression of dynamin-related protein 1, a mitochondrial fission protein, in senescent endothelial cells (ECs) aggravates EC dysfunction due to impaired autophagic flux (Lin, Shen, Yan, & Gao, 2015).
Mitochondrial-dysfunction-associated senescence (MiDAS) triggers the SASP with the production of TNFα, IL-10, and CCL27. Lower NAD+/NADH ratios seen in MiDAS are caused by activation of the AMP-activated protein kinase (AMPK)-p53 pathway activation (Wiley et al., 2016). Caveolin-1 (Cav-1) deficiency also induces premature senescence by causing mitochondrial dysfunction. Cav-1 deficiency leads to decreased mitochondrial respiration and NAD/NADH ratios, reduced activity of oxidative phosphorylation complex, and inactivation of Sirtuin 2 (Sirt2). Cellular senescence caused by Cav-1 deficiency is dependent on p53-p21 pathway (D. M. Yu et al., 2017). Under OS, Cav-1 interacts with Sirt1 in the caveolar membrane and inhibits the activity of Sirt1. In agreement with this, a previous study found that ROS induces cellular senescence by increasing the acetylation of p53 and IL-6 production in wild type, but not Cav-1 knockout mouse embryonic fibroblasts (Volonte et al., 2015).
5 SIGNALING PATHWAYS THAT ARE INVOLVED IN CELLULAR SENESCENCE
5.1 p53 signaling
The roles of p53 signaling in the cellular senescence have been studies for many years. Some proteins are found to promote p53-mediated cell senescence, such as Aurora B kinase (H.-J. Kim, Cho, Quan, & Kim, 2011), secretory phospholipase A(2) (H. J. Kim et al., 2009), and interferonγ (K. S. Kim, Kang, Seu, Baek, & Kim, 2009), whereas other proteins suppress p53-mediated cell senescence, such as Sirt2 (Langley et al., 2002), Hsp27 (O'Callaghan-Sunol, Gabai, & Sherman, 2007), and MAD2 (Lentini, Barra, Schillaci, & Di, 2012). p53 was found to act as a molecular switch regulating IGF-1-induced premature senescence (Tran et al., 2014). Short exposure of IGF-1 promotes cell proliferation, whereas prolonged exposure to IGF-1 induces cellular senescence (Tran et al., 2014). Prolonged exposure to IGF-1 inhibits Sirt1 deacetylase activity and increases p53 acetylation, resulting in p53 stabilization (Tran et al., 2014). Besides, Akt and p21 are required for the induction of cellular senescence downstream of p53 (Y. Y. Kim et al., 2017). Moreover, Foxp3 also accelerates senescence downstream of p53 signaling in fibroblasts and epithelial cancer cells. Foxp3 overexpression can increase the amount of p21 and ROS, which leads to the initiation of cellular senescence (J. E. Kim et al., 2017; Figure 2(a)).
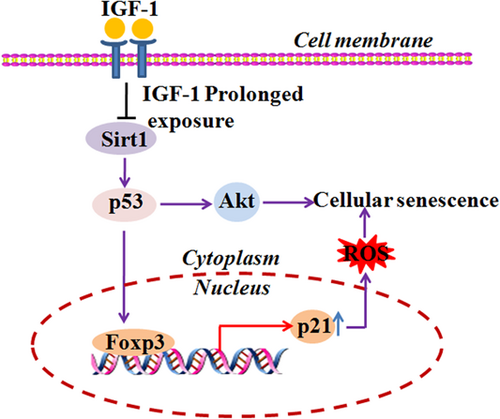
p53 signaling is involved in cellular senescence. Akt and p21 are required downstream of p53 for the induction of cellular senescence. Foxp3 overexpression can increase the amount of p21 and ROS, which lead to the initiation of cellular senescence. Prolonged IGF-1 exposure induces cell senescence by inhibiting Sirt1 deacetylase activity and increasing p53 acetylation. p53 acetylation leads to its stabilization. ROS: reactive oxygen species [Color figure can be viewed at wileyonlinelibrary.com]
5.2 p38 MAPK signaling
p38 signaling mediated by AMPK and transforming growth factor-beta-activated protein kinase (TAK) binding protein 1 drives the senescence of human T cells by inhibiting telomerase activity, T-cell proliferation, and expression of the T cell receptor (TCR) signalosome molecules (Lanna, Henson, Escors, & Akbar, 2014). CD45RA-expressing CD8+ T cells have elevated levels of both ROS and p38 MAPK. Inhibiting p38 MAPK signaling can induce mTOR-independent autophagy and enhance telomerase activity in the senescent CD8+ T cells (Henson et al., 2014; Henson, Macaulay, Riddell, Nunn, & Akbar, 2015). p38 MAPK activation also triggers the SASP, independent of the DDR (Freund, Patil, & Campisi, 2011). Blocking signaling by p38 and PD-1 can enhance cell proliferation (Henson et al., 2015). Similarly, p38 MAPK inhibitor was found to increase the number of human corneal ECs in vitro by suppressing cellular senescence, providing evidence for treatment of corneal endothelial dysfunction (Hongo, Okumura, Nakahara, Kay, & Koizumi, 2017). However, another study showed that AMPK activation can inhibit OS-induced senescence by restoring autophagic flux and NAD+ levels in the senescent fibroblast cells (Han et al., 2016; Figure 3). The difference in the results may be due to the different cell types used or the mechanisms of action of AMPK and p38 MAPK pathways.

p38 MAPK signaling is involved in cellular senescence. p38 signaling mediated by AMP-activated protein kinase (AMPK) and TAK binding protein 1 (TAB1) drives the senescence of human T cells by inhibiting telomerase activity. Meanwhile, AMPK activation can inhibit oxidative stress-induced senescence by restoring autophagic flux and NAD+ levels in senescent fibroblast cells. AMP: XXX; MAPK: mitogen activated protein kinase; TAK: transforming growth factor-beta-activated protein kinase [Color figure can be viewed at wileyonlinelibrary.com]
5.3 NF-κB signaling
Many studies have correlated NF-κB signaling with cellular senescence. NF-κB signaling is implicated in indoxyl sulfate-induced cellular senescence via the ROS-NF-κB-p53 pathway (Shimizu et al., 2011). Indoxyl sulfate can induce the expression of NF-κB p65 and the phosphorylation of p65 at Ser-276. Inhibiting NF-κB signaling can suppress SA-β-gal activity as well as expression of p53, TGF-β, and α-smooth muscle actin (Shimizu et al., 2011). DNA damage can also activate NF-κB signaling, which stimulates the appearance of the SASP (Salminen et al., 2012). However, noncanonical NF-κB signaling was recently found to regulate the expression of the enhancer of zeste homolog 2 (EZH2) in melanoma and that inhibiting NF-κB signaling can reduce the expression of EZH2 and restore cellular senescence, resulting in the reduction of melanoma growth (De Donatis et al., 2016). EZH2 is directly involved in chromatin DNA methylation (Viré et al., 2006). Also, activation of EZH2 inhibits expression of p21, resulting in suppression of cellular senescence (Fan et al., 2011; Figure 4). More studies are still required to elucidate the potential downstream effectors of EZH2 during cellular senescence.
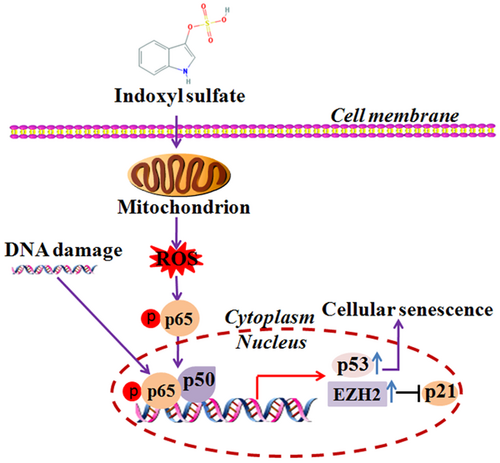
NF-κB signaling is involved in cellular senescence. NF-κB signaling is implicated in indoxyl-sulfate-induced cellular senescence via the ROS-NF-κB-p53 pathway. DNA damage can activate NF-κB signaling, which stimulates the appearance of the SASP. Noncanonical-NF-κB signaling regulates expression of the enhancer of zeste homolog 2 (EZH2) in melanoma. Activation of EZH2 inhibits p21 expression, resulting in suppression of senescence. NF-κB: nuclear factor-κB; ROS: reactive oxygen species; SASP: senescence-associated secretory phenotype [Color figure can be viewed at wileyonlinelibrary.com]
5.4 mTOR signaling
mTOR signaling involves cellular senescence through NF-κB, STAT3, and Akt signaling, as well as autophagy. mTOR inhibitor rapamycin was found to regulate cellular senescence by suppressing the translation of membrane-bound IL-1α which reduces the transcriptional activity of NF-κB. The authors proposed that rapamycin can ameliorate age-related diseases by inhibiting the senescence-related inflammation (Laberge et al., 2015). Besides, rapamycin can inhibit cellular senescence by upregulating the expression of Nrf2 and downregulating STAT3 pathway (Wang et al., 2017). Moreover, rapamycin can decelerate cellular senescence by restoring autophagy, as impairment of autophagy has been found to cause cellular senescence (Tai et al., 2016). In particular, autophagic flux is impaired during the development of OS-induced senescence. Rapamycin can restore the autophagic flux by restoring mitochondrial and lysosomal functions (Tai et al., 2016; Figure 5).
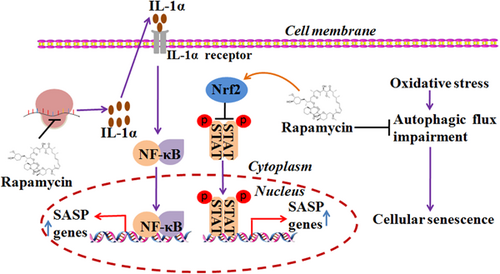
mTOR signaling is involved in cellular senescence. Rapamycin can inhibit senescence by upregulating the expression of Nrf2 and downregulating STAT3 pathway. Rapamycin can regulate cellular senescence by suppressing the translation of membrane-bound IL-1α to reduce the transcriptional activity of NF-κB. Rapamycin can restore the autophagic flux by rejuvenating mitochondrial and lysosomal functions. IL: interleukin; mTOR: mammalian target of rapamycin; NF-κB: nuclear factor-κB [Color figure can be viewed at wileyonlinelibrary.com]
5.5 TGF-β signaling
Human pluripotent stem cells (hPSCs) have great potential for use in repairing ischemic tissue and treating of vascular disease. However, hPSCs display increased senescence under serum-free in vitro conditions, which affects vessel forming. TGF-β signaling is involved in the cellular senescence in hPSCs-derived ECs. Inhibiting TGF-β signaling can promote proliferation of hPSC-derived ECs and ameliorate cellular senescence (Bai, Gao, Hoyle, Cheng, & Wang, 2017). Mechanically, TGF-β may be involved in the formation of NF1/Smad complexes, which can repress the expression of adenine nucleotide translocate-2 (ANT2) by binding to the ANT2 promoter (Kretova et al., 2014). TGF-β-mediated suppression of ANT2 leads to increased ROS and DNA damage during the initiation of cellular senescence (Kretova et al., 2014). Integrin beta 3 (ITGB3) was found to regulate cellular senescence by activating TGF-β signaling in a paracrine and autocrine manner. In addition, polycomb protein CBX7 regulates the expression of ITGB3 during oncogene-induced senescence (Rapisarda et al., 2017). Furthermore, endogenous Notch1 may also mediate TGF-β-related cellular senescence (Kagawa et al., 2015; Figure 6).
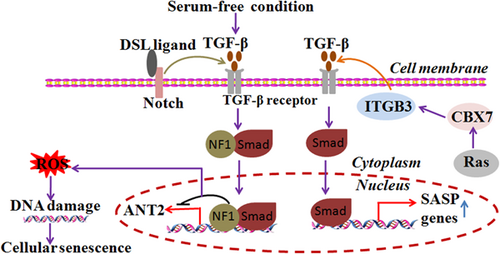
TGF-β signaling is involved in cellular senescence.TGF-β-mediated suppression of ANT2 leads to increased ROS and DNA damage during the initiation of cellular senescence. In addition, polycomb protein CBX7 regulates ITGB3 expression during oncogene-induced senescence. Endogenous ITGB3 regulates cellular senescence by activating TGF-β signaling in a paracrine and autocrine manner. Notch1 may also mediate TGF-β-induced cellular senescence. ROS: reactive oxygen species; TGF-β: transforming growth factor-beta [Color figure can be viewed at wileyonlinelibrary.com]
5.6 Wnt signaling
The role of Wnt signaling in the cellular senescence is still not clear now. It has been reported that melanoma cells with high levels of Wnt5A expression demonstrate senescence-like features, such as increased expression of p21 and classic senescence-related molecules normally present under therapeutic stress induced by irradiation (Webster et al., 2015). However, the cells still have the potential to migrate and invade. Wnt5A knockdown reduces their invasion ability, indicating that Wnt5A drives melanoma invasion and promotes therapy resistance. The author proposed that the appearance of markers of senescence does not mean that the cells are truly senescent, but are exhibiting an adaptive stress response (Webster et al., 2015).
Studies on the molecular mechanisms of cellular senescence are just coming to light. Additional studies are needed to investigate the roles of other signaling pathways in the induction of cellular senescence, such as c-Jun N-terminal kinase (JNK)-signal transducer and activator of transcription 3 (STAT3), Wnt/β-catenin, epidermal growth factor receptor, toll-like receptor, cGAS-STING, and retinoic-acid-inducible gene I, and melanoma differentiation-associated antigen 5.
6 CELLULAR SENESCENCE IS ASSOCIATED WITH AGING-ASSOCIATED DISEASES
6.1 Cellular senescence is associated with chronic inflammation
Senescent cells can normally be recognized and cleared by the immune system (Sagiv & Krizhanovsky, 2013). However, senescent cells accumulate with age, and are involved in certain age-related diseases (Chang et al., 2016). It has been found that the SASP is required for the immune system to recognize senescent cells, which contributes to chronic inflammation and aging-associated diseases (Contrepois et al., 2017). Interestingly, p53 signaling seems to decrease with age, whereas NF-κB signaling is increased. It is proposed that p53 and NF-κB signaling pathways antagonize each other by a negative regulatory loop (Salminen & Kaarniranta, 2011). Also, p53 signaling initiates senescence, whereas NF-κB signaling induces the SASP (Salminen & Kaarniranta, 2011).
Moreover, DNA damage is implicated in senescence-associated inflammatory cytokine production (Rodier et al., 2009). DNA sensors, such as AIM2 and NLR family pyrin domain containing 3 (NLRP3), can recognize cytoplasmic DNA and drive the progression of inflammation. The significance of AIM2 and NLRP3 in senescence stills needs to be explored. Histone variant H2A.J was found to accumulate in senescent cells and can enhance the expression of inflammatory and immune response genes, including SASP-associated genes (Contrepois et al., 2017). It is unknown whether persistent DNA damage can enhance H2A.J accumulation and how H2A.J stimulates SASP-associated gene expression (Jurk et al., 2014).
7 CELLULAR SENESCENCE AND NEURODEGENERATIVE DISEASES
Cellular senescence is related to aging and aging-associated diseases, such as AD, Parkinson's disease (PD), and XFE progeroid syndrome. Amyloid β (Aβ) deposition in senile plaques is a pathological feature of AD. The relationship between Aβ deposition and cellular senescence is not clear. Increased expression of p16 was found in aged mouse, and localized in neurons and astrocytes. The p16 expression is negatively correlated with spatial cognitive impairments. In particular, Aβ (1–42) can induce the production of p16 (Z. Wei et al., 2016). In addition, OS has been linked to AD. In fact, some evidence supports that oxidative damage leads to AD symptoms, such as Aβ deposition, and cognitive decline. Because oxidative damage can cause DNA damage that induces cellular senescence, reducing OS may be a potential way to ameliorate AD symptoms (Bonda et al., 2010). It has been seen that adaption to periodic hypoxia can protect experimental AD rats from neurodegenerative damage by inhibiting oxidative and nitrosative stress (Manukhina et al., 2010). Cilostazol (CSZ), an antiplatelet agent, has been found to inhibit the development of cognitive impairment in AD patients by suppressing OS and the MAPK pathway (Oguchi et al., 2017). Aβ also can increase the phosphorylation of ERK1/2 and p38 MAPK in human neuroblastoma SH-SY5Y cells, whereas CSZ can inhibit this effects (Oguchi et al., 2017). CSZ also reduces superoxide dismutase (SOD) activity and Cu/Zn-SOD content in Aβ-treated SH-SY5Y cells (Oguchi et al., 2017).
It is known that patients with PD have increased levels of OS that may be a risk factor of PD (Nikolova & Mancheva, 2013; Sanyal et al., 2011). Nikolova Galina et al. detected higher levels of malondialdehyde (MDA) products, reduced erythrocyte SOD activity, and increased erythrocyte catalase (CAT) activity in patients with PD (Nikolova & Mancheva, 2013). However, Sanyal Jaya showed that the activity of erythrocyte SOD, CAT, G-Px is lower in patients with a long duration or advanced PD. The difference between the results may be due to the differences in disease course or severity of PD. Recently, senescent cells were found in the astrocytes of brain tissues of patients with PD who are exposed to environmental toxins, like paraquat, which can induce astrocytic senescence (Chinta et al., 2018). Paraquat can cause oxidative damage in dopaminergic neurons and lead to a decrease in the number of neurons, which coincides with the appearance of neurodegeneration (Mccormack et al., 2005).
A study found that XFE progeroid syndrome is caused by dysfunction of DNA repair, which results in the accumulation of endogenous DNA damage (Tilstra et al., 2012). Activation of the NF-κB pathway by damaged can promote aging, whereas inhibiting the NF-κB pathway can ameliorate aging (Tilstra et al., 2012). Another study found that microRNA (miRNA) may also play an important role in the progression of XFE progeroid syndrome. In the old mice or late passage cells, some miRNAs and miRNA processor Dicer are downregulated (Nidadavolu, Niedernhofer, & Khan, 2013).
7.1 Cellular senescence and obesity/diabetes
Increasing evidence supports the involvement of cellular senescence in metabolic disorders, such as obesity, insulin resistance, and diabetes. Senescent cells may contribute to obesity-associated inflammation. For example, the protein deleted in breast cancer-1 (DBC1) may regulate cellular senescence in obesity. Deletion of DBC1 can protect preadipocytes from cellular senescence and senescence-related inflammation (Escande et al., 2014). In addition, p38 MAPK signaling is associated with obesity and diabetes as it promotes tissue inflammation and injury. MAP kinase kinase (MKK)3-p38 MAPK signaling was found to be involved in the development of diabetic nephropathy. In diabetic MKK3-deficient mice, p38 MAPK signaling is downregulated, which is indicative of its protective role in the development of nephropathy (Lim et al., 2009). Besides, both high-mobility group protein AT-hook 2 (HMGA2) and p14, an inducer of senescence, are linked with the proliferation of stem and precursor cells of adipose tissue, cellular senescence, and an increased risk of type 2 diabetes (T2D). HMGA2 expression is higher, not only in obese patients, but also in the white adipose tissue of patients with T2D (Markowski et al., 2013). Increased expression of HMGA2 can lead to the increased expression of p14 and p53, followed by an increase in senescent cells (Markowski et al., 2013).
Cellular senescence is also involved in insulin resistance. Cheraghpour et al. studied the role of senescence in the insulin resistance and diabetes. They found that the expression of p53 is increased in fat and other peripheral tissues, whereas the expression of p16 is increased only in fat tissue. In addition, the serum levels of glucose, cholesterol, and triglycerides in the fat tissue of obese rats were found to be higher than in the controls (Cheraghpour, Shahbazi, Homayounfar, H, & Zand, 2015). The authors suggested that cellular senescence in fat tissue may be associated with the development of insulin resistance and age-related diseases (Cheraghpour et al., 2015).
Furthermore, suppressing senescence can increase glucose sensitivity following cold therapy in the elderly. Cold temperatures can be used to control diabesity by inducing progenitor cells to become beige adipocytes. However, the amount of cold-induced beige adipocytes decreases with age because of aging progenitor cells showing senescence-like phenotypes. Inhibiting senescence-associated pathways, like p38/MAPK-p16, can restore cold-induced beige adipocytes and subsequently increase glucose sensitivity (Berry et al., 2016).
7.2 Cellular senescence and liver disease
Senescence of hepatocytes is correlated with the stage of fibrosis (Aravinthan et al., 2013). Senescent cells are found in the livers of patients with NAFLD and cirrhotic, as well as the livers of mice fed a high-fat diet. An increase in the number of senescent cells can promote the development of NAFLD. The mitochondria in senescent cells cannot metabolize fatty acid normally, which results in the accumulation of hepatic fat and steatosis, and contributes to NAFLD. In addition, hepatocytes in patients with NAFLD express more p21 than hepatocytes of control patients (Ogrodnik et al., 2017).
NF-κB signaling plays a crucial role in a variety of liver diseases. For example, hepatic activation of IKK/NF-κB can induce liver fibrosis caused by macrophage mediated-chronic inflammation. Elimination of macrophages can reduce NF-κB- induced liver fibrosis (Sunami et al., 2012). NF-κB signaling is also implicated in trauma-associated damage to liver tissue. Inhibition of NF-κB signaling decreases the secretion of inflammatory cytokines, such as TNFα and IL-6, after liver trauma (W. Yang et al., 2012). A recent study implicates ERK, STAT3, and estrogen receptor α in hepatic steatosis (Choi et al., 2018). It is unknown whether these signaling pathways are involved in cellular senescence and the SASP which may play a role in the progression of liver disease.
7.3 Cellular senescence and lung disease
Senescent cells in the lung are associated with a variety of aging-associated lung diseases. Accumulation of senescent cells and the SASP in the lung of aged patients may lead to mild persistent inflammation, which results in tissue damage and increases the vulnerability to invasive pathogens. This increased vulnerability may be one reason why older people are susceptible to pneumonia (Yanagi et al., 2017). Besides, senescence of pulmonary artery smooth muscle cells (PA-SMCs) induced by telomerase shortening and OS contributes to the pulmonary hypertension (Noureddine et al., 2011). PA-SMCs can stimulate the proliferation and motility of normal PA-SMC cells and the production of paracrine factors (Noureddine et al., 2011). Osteopontin, an extracellular matrix protein secreted by PA-SMCs, was found to play a crucial role in the development of pulmonary hypertension. Osteopontin is highly expressed in the lungs of aged mice and patient with chronic pulmonary disease. Treatment of PA-SMCs from aged mice with an anti-osteopontin antibody can suppress the cell growth (Saker et al., 2016).
Senescence also contributes to the development of idiopathic pulmonary fibrosis (IPF). Alveolar type 2 (ATII) cells in the lungs of patients with IPF express high levels of p21, p16, and plasminogen activator inhibitor 1 (PAI-1). PAI-1 plays an important role in the activation of p53-p21-Rb pathway, ATII cell senescence, and bleomycin-induced fibrosis (Jiang et al., 2017). mTOR and ZEB1 may also be involved in the progression of pulmonary fibrosis. High levels of mTOR and ZEB1 expression are correlated with severe pulmonary fibrosis. STAT3 signaling also contributes to the progression of lung fibrosis. Inhibition of STAT3 reduces the expression of IL-6 and TGF-β-regulated gene expression, and decreases fibroblast-to-myofibroblast differentiation (Pedroza et al., 2015). Because pulmonary fibrosis is caused by chronic lung inflammation or environmental pollutants, it is possible that multiple cellular signaling pathways induce SASP that contributes to lung fibrosis (Park et al., 2014). Treatment with dasatinib and quercetin eliminates senescent cells to restore the pulmonary function and the physical health of aged mice. Senescent cell clearance also reduces the expression of proinflammatory factors, including Mcp1, Mmp12, and TGF-β, which coincides with the resolution of fibrosis (Schafer et al., 2017).
7.4 Cellular senescence and osteoarthritis
Aging and trauma are two etiological factors in the development of osteoarthritis (OA; Jeon et al., 2017). During aging, a subset of cells within the bone microenvironment become senescent (Farr et al., 2016). OS markedly accelerates telomere shortening and promotes senescence of chondrocytes (Brandl et al., 2011). Senescent cells have been shown to cause OA in the elderly, whereas the nonsenescent cells have little effect (Xu et al., 2017). Senescent cells are found in the articular cartilage and synovium after anterior cruciate ligament transaction (Jeon et al., 2017). Local clearance of senescent cells can ameliorate the development of posttraumatic osteoarthritis (Jeon et al., 2017). Xu et al. found that senescent cells transplanted into the knee region can cause symptoms of OA (Xu et al., 2017).
Sirt6/NF-κB signaling plays an important role in the regulation of senescence of chondrocytes (Duarte, 2015). Sirt6 overexpression can inhibit chondrocyte senescence and the NF-κB mediated inflammatory response, which limits the development of OA (Wu et al., 2015). In contrast, Sirt6 inhibition can lead to the increased expression of metalloproteinase 1 (MMP-1), MMP-13, and p16, exacerbated DNA damage and mitochondrial dysfunction, and subsequent premature senescence (Duarte, 2015). IL-6 was found to induce chondrocyte catabolism through STAT3 signaling. Importantly, blocking IL-6 or STAT3 can reduce destabilization of medial meniscus induced OA (Latourte et al., 2017). p38 MAPK is also involved in the senescence of chondrocytes. p38 inactivation can delay the onset of senescence, promote proliferation, and extend the life span of articular chondrocytes in vitro (S. Kang, Jung, Kim, & Shin, 2005).
7.5 Cellular senescence and tumorigenesis
Senescent tumor cells do not proliferate, and cellular senescence may be a strategy to inhibit tumor growth. For example, TGF-β plays an important role in suppressing the progression of head and neck squamous cell carcinoma (HNSCC) progression by inducing cellular senescence and inhibiting inflammation. Double knockout of TGF-β/pten in mice promotes cell proliferation, decreases apoptosis, and increases the expression of CCND1 in the basal layer of head and neck epithelia, whereas pten only knockout in mice has only a few lesions that progress to HNSCC (Bian et al., 2012). p38 MAPK also plays an important role in cellular senescence in some tumors. p38 MAPK/ROS pathway is involved in physalin A-induced arrest of the cell cycle at the G2/M phase. Physalin A, a Chinese herbal extract, is used to treat tumors and other diseases. Inhibition of p38 can reduce the production of ROS and the proportion of cells in the G2/M phase (N. Kang et al., 2016). It is uncertain whether physalin A can induce cellular senescence by activating the p38 MAPK/ROS pathway. A previous study showed that IL-4 not only induces cell cycle arrest in the G1 phase, but also cellular senescence mediated by STAT6 or p38 MAPK in renal cell carcinoma (H. D. Kim et al., 2013).
Despite the beneficial role of senescence in inhibiting tumor cell proliferation, SASP can promote tumorigenesis and confer therapy resistance. Cytokines or other proteins secreted from senescent cells can promote the proliferation and migration of tumor cells. For example, IL-6 secreted from senescent mesenchymal stem cells has been shown to enhance the proliferation and migration of breast cancer cells by activating of STAT3 in vitro and in vivo (Di et al., 2014). In addition, IL-6 can stimulate the epithelial-mesenchymal transition, promote the migration and proliferation, and inhibit apoptosis of human intrahepatic biliary epithelial cells (HIBECs), delaying of cellular senescence (Li et al., 2018). Moreover, chemokines, like CCL2, which is induced during cellular senescence, play an important role in shaping the tumor-promoting microenvironment (Eggert, Ji, Zender, Wang, & Greten, 2015). For instance, senescent hepatocytes can promote hepatocellular carcinoma cell growth by signaling for the accumulation of immunosuppressive CD11b+Gr1+ myeloid cells which can inhibit T-cell proliferation (Eggert et al., 2015). Importantly, CCL2 plays a crucial role in the accumulation of myeloid cells and accelerates tumor growth (Eggert et al., 2015). Unexpectedly, the expression of p53-dependent CCL2 in senescent tumor cells is needed to recruit natural killer (NK) cells to eliminate senescent tumor cells with induced expression of NKG2D (Iannello, Thompson, Ardolino, Lowe, & Raulet, 2013). Furthermore, senescent tumor cells have a high potential for invasion. For example, senescent tumor cells in papillary thyroid carcinoma (PTC) have high invasion potential through the SASP and are involved in lymph node metastasis. Mechanically, upregulated CXCL12/CXCR4 signaling plays a crucial role in the invasion of PTC cells by graded expression near the invasion border (Y. H. Kim et al., 2017).
It has been found that exosome-like small extracellular vesicles (sEVs) produced by senescent cells promote the tumor cell growth. sEVs-associated EphA2 can bind to the ephrin-A1 in tumor cells and enhance cell proliferation. Further study showed that ROS was found to be involved in sEV sorting of EphA2 through upregulating EphA2 phosphorylation (Takasugi et al., 2017). It is unknown whether senescence-associated sEVs have other contents, such as miRNA, DNA, and proteins, and whether the sEVs play a role in normal and pathological situations (Kadota et al., 2017; Takasugi et al., 2017). It should be noted that, in the human genome, there are ten EphA receptors and six EphB receptors, so it would be interesting to explore whether other ephrin/Eph forward or reverse signaling pathways are involved in cellular senescence (W. Wei, Wang, & Ji, 2017).
7.6 Cellular senescence and pathogen infection
It has been known that bacterial and viral infection can induce cellular senescence of host cells. By now, the roles of bacterial and viral infection in inducing of cellular senescence are still largely unknown.
Human cytomegalovirus (HCMV) infection causes cellular senescence, which might promote chronic inflammation (Wolf, Weinberger, & Grubeck-Loebenstein, 2012). HCMV induces gene expression related to the innate and adaptive immune responses in fibroblasts, some of which are also induced during cellular senescence, such as IL-6 (Wolf et al., 2012). Latent infection of HCMV is linked with age-associated immune dysfunction of T cells, which may contribute to immunosenescence, aging of the immune system, in the elderly (W. Tu & Rao, 2016). Besides, hepatitis B virus (HBV) infection can cause cellular senescence and may require HBcAg for the initiation of cellular senescence (Tachtatzis et al., 2015). In some chronically infected HBV patients, p21 expression is increased and correlates with liver fibrosis and telomere length is reduced. It should be noted that, although HBV can cause cellular senescence, biologically young hepatocytes are the preferred cells for viral replication (Tachtatzis et al., 2015). In addition, hepatitis C virus (HCV) monoinfection and human immunodeficiency virus (HIV)/HCV coinfection can accelerate T-cell immune senescence during early infection in injection drug users, which could contribute to premature morbidity and mortality (Grady, Nanlohy, &...Baarle, 2016). Human papilloma virus (HPV) E2 can induce senescence through the pRb- and p21-dependent pathways (Wells, 2000). The HPV E6 F47R mutant can also induce senescence, but by the p53-dependent pathway (Ristriani, Fournane, Orfanoudakis, Travé, & Masson, 2009). HPV E6/E7 protein can inactivate Notch1 and inhibit Notch1-mediated senescence, which promotes colony formation and xenograft tumor growth (Kagawa et al., 2015). Dengue virus (DENV) infection was found to induce cellular senescence in human umbilical vein ECs, but senescent cells do not support viral infection (Abubakar, Shu, Johari, & Wong, 2014). Another study showed that senescent cells can increase the monolayer permeability of ECs, suggesting that DENV infection may increase vascular permeability, which leads to the plasma leakage observed in dengue hemorrhagic fever and dengue shock syndrome (Krouwer, Hekking, Langelaarmakkinje, Reganklapisz, & Post, 2012).
Bacterial LPS is a potent inducer of inflammation. Interestingly, LPS is also implicated in the induction of cellular senescence. For example, LPS was found to induce cellular senescence in lung alveolar epithelial cells, in a hydrogen-peroxide-dependent manner (C. O. Kim et al., 2012). Another study showed that repeated LPS stimulation can lead to microglia senescence, which may result in failure to respond to detrimental stimuli and subsequent neurodegeneration (H.-M. Yu et al., 2012). In addition, bacterial toxins are also involved in cellular senescence. For example, the genotoxin colibactin, encoded by genomic island of Escherichia coli, can induce cellular senescence and promote the growth of tumor cells and colon cancer (Cougnoux et al., 2014; Secher, Sambalouaka, Oswald, & Nougayrède, 2013).
Infection by other microbes, such as Escherichia coli (Cuevas-Ramos et al., 2010), Helicobacter pylori (Toller et al., 2011), respiratory syncytial virus (Gibbs, Ornoff, Igo, & Imani, 2009), and transmissible gastroenteritis virus (Ding et al., 2013) can also cause DNA damage and cell cycle arrest. The ability of these pathogens to induce cellular senescence and the corresponding molecular mechanisms are worth studying in the future.
8 ELIMINATION OF SENESCENT CELLS
8.1 Inducing senescent cell apoptosis
Some peptides, chemicals, and small molecules have been developed to induce senescent cell apoptosis by activating apoptotic signaling or inhibiting the activity of antiapoptotic proteins. For example, FOXO4 is required to maintain cell viability by interacting with p53. A peptide that perturbs the FOXO4-p53 interaction can induce cell apoptosis of senescent cells, and restore tissue homeostasis (Baar et al., 2017). Agmatine, a chemical substance, inhibits neuronal senescence by activating p53 and inhibiting p21. Agmatine can also reduce SA-β-gal activity and the expression of IL-6, TNFα, and CCL2 in neuronal cells under high glucose condition (Song et al., 2016). In addition, inhibition of BCL-W and BCL-XL by siRNAs or the drug ABT-737 induces apoptosis of senescent cells in the epidermis and increases the proliferation of hair-follicle stem cells (Yosef et al., 2016). Similarly, ABT263, an inhibitor of BCL-2 and BCL-xL, can selectively eliminate stem cells in culture by inducing cell apoptosis, which is beneficial for rejuvenation of aged tissue stem cells (Chang et al., 2016).
8.2 Inhibiting cellular senescence by suppressing oxidative stress
Natural chemicals with antioxidant potential can suppress OS-induced senescence. For example, curcumin, a natural phenol with antioxidant and anti-inflammatory activities, can attenuate hydrogen peroxide (H2O2)-induced premature EC senescence by activating Sirt1 (Sun, Hu, Hu, Xu, & Jiang, 2015). Some molecules can delay senescence by directly suppressing ROS production. For example, nicotinamide, an amide derivative of vitamin B3, can attenuate senescence by reducing the level of ROS (Kwak, Ham, Kim, & Hwang, 2015). A recent study showed that 27-hydroxycholesterol (27HC) was found to promote the accumulation of ROS and then activate the IL-6/STAT3 pathway, whereas ROS scavenger N-acetylcysteine can inhibit ROS production and activation of the IL-6/STAT3 pathway. Notably, blocking ROS production or inhibiting the IL-6/STAT3 pathway can attenuate 27HC-induced cellular senescence (Liu et al., 2017).
8.3 Inhibiting cellular senescence-associated pathways
Given that several signaling pathways play important roles during senescence, it is possible to inhibit senescence by suppressing these pathways. A study found that therapy can induce tumor cell senescence and Bcl2-associated athanogene 3 (Bag3) is increased in this process (Pasillas et al., 2015). Moreover, Bag3 interacts with the major vault protein (MVP), which upregulates the ERK1/2 signaling, resulting in resistance to chemotherapy (Pasillas et al., 2015). Importantly, Bag3 or MVP knockdown weakens the activation of ERK1/2 and promotes apoptosis of therapy-induced senescent cells (Pasillas et al., 2015). A similar study found that inhibition of the MEK/ERK pathway can promote the elimination of Ras-transformed senescent cells as the cells cannot form the autophagosomes necessary for the clearance of damaged mitochondria, resulting in apoptosis (Kochetkova et al., 2017).
8.4 Immune regulation of cellular senescence
A variety of immune cells, including macrophages, NK cells, and T cells, can be recruited and activated by SASP, including chemokines, cytokines, adhesion molecules. For example, F4/80+ macrophages are involved in the elimination of senescent cells from the uterus of postpartum mice and regulation of the senescence-associated inflammatory microenvironment increases the success rate of pregnancy (Egashira et al., 2017). Moreover, NK cells also eliminate senescent tumor cells, in an NKG2D expression-dependent manner. p53 restoration can promote the secretion of various chemokines that can recruit NK cells (Iannello et al., 2013). Furthermore, type I interferons (IFNs) can amplify DNA-damage responses and promote senescence. It is known that IFNs induce senescence and suppress the development of melanoma (Katlinskaya et al., 2016). Interestingly, IFNs produced by senescent cells can increase the expression of MIC-A and ULBP2 ligands in the NK cells and promote the recognition and clearance of senescent cells by NK cells (Katlinskaya, Carbone, Yu, & Fuchs, 2015).
8.5 Using Oncolytic viruses to destroy senescent cells
Measles vaccine virus (MeV) has been used for lysis of therapy-induced senescent tumor cells. One study found that MeV can efficiently replicate and produce infectious progeny virus particles (Weiland et al., 2014). Another study found that MeV infection can induce cellular senescence in normal and tumor cells (Chuprin et al., 2013). Oncolytic adenovirus, in combination with doxorubicin and ifosfamide, can efficiently eliminate soft-tissue sarcomas cells (Siurala et al., 2015). These viruses could be used to lyse therapy-induced senescent tumor cells.
8.6 Exercise
Nutrient excess contributes significantly to increased expression of senescence-associated proteins, including p16, p53, p21 and increased activity of SA-β-gal (Schafer et al., 2016). Exercise can reduce the expression of the SASP-associated genes and prevent the accumulation of senescent cells caused by the fast-food diet (Schafer et al., 2016). Mechanically, exercise could reduce metabolic and replicative stresses in adipose tissue and limit the transition into a senescent state. Exercise could also trigger protective responses against cellular senescence in adipose tissue cells. Moreover, exercise may promote the clearance of senescent cells by the immune system (Schafer et al., 2016).
9 CONCLUSION AND PERSPECTIVES
Cellular senescence plays an important role in normal and physiological conditions. Since its discovery, many important studies on the effects and molecular mechanisms of cellular senescence have been done. However, more challenge questions have been raised. First, the relationship between cellular senescence and immune response remains elusive. For instance, it is unknown whether cellular senescence plays a role in the selection of B-cell clones during embryonic development. Second, the role of senescence in the pathogenic infection and the corresponding pathological influences are largely unknown. It seems promising that regulation of cellular senescence could control pathogen-associated development of inflammation. For example, inducing cellular senescence of macrophages may make it possible to inhibit inflammation caused by influenza or bacterial infection. Third, it is also possible that other novel signaling pathways may be involved in cellular senescence. Taken together, the study of cellular senescence is just the beginning, and there are more interesting questions to be addressed in the future.
ACKNOWLEDGMENTS
This study was supported by grants from the National Natural Science Foundation of China (31501701 and 31371386), and the Plant Foundation for Young Scientists of Henan University (CX0000A40557).
CONFLICTS OF INTEREST
The authors declare that they have no conflict of interests.