Adipokines, adiposity, and bone marrow adipocytes: Dangerous accomplices in multiple myeloma
Abstract
Obesity has become a global epidemic influencing the establishment and progression of a wide range of diseases, such as diabetes, cardiovascular disease, and cancer. In 2016, International Agency for Research on Cancer reported that obesity is now associated with 13 different cancers, one of which is multiple myeloma (MM), a destructive cancer of plasma cells that predominantly reside in the bone marrow. Obesity is the accumulation of excess body fat, which causes metabolic, endocrine, immunologic, and inflammatory-like changes. Obesity is usually associated with an increase in visceral and/or subcutaneous fat; however, an additional fat depot that also responds to diet-induced changes is bone marrow adipose tissue (BMAT). There have been several studies over the past few decades that have identified BMAT as a key driver in MM progression. Adipocytes secrete numerous adipokines, such as leptin, adiponectin, resistin, adipsin, and visfatin, which when secreted at normal controlled levels have protective properties. However, in obesity these levels of secretion change, coupled with an increase in adipocyte number and size causing a profound and lasting effect on the bone microenvironment, contributing to MM cell growth, survival, and migration as well as potentially fueling bone destruction. Obesity is a modifiable risk factor making it an attractive option for targeted therapy. This review discusses the link between obesity, monoclonal gammopathy of undetermined significance (a benign condition that precedes MM), and myeloma, and the contribution of key adipokines to disease establishment and progression.
1 INTRODUCTION
Obesity is now being described as a global epidemic. In 2014, it was estimated that 640 million adults were suffering from obesity worldwide (a six-fold increase since 1975). Obesity increases the risk of many serious diseases and health conditions, such as type 2 diabetes, heart disease, stroke, and cancer. Lifestyle and environmental factors, rather than inherited genetic defects, are now thought to regulate the development of 90%–95% of all cancers (Anand et al., 2008), and around 20% of these have been attributed to obesity (Calle, Rodriguez, Walker-Thurmond, & Thun, 2003). Over a decade ago, the International Agency for Research on Cancer confirmed the association of excess body fatness with the risk of cancers of the colon and rectum, esophagus, kidney, breast, and endometrium (Vainio, Kaaks, & Bianchini, 2002). However, in a report published on August 2016, this list was reviewed and a further eight cancer types were added to include cancers of the gastric cardia, liver, gallbladder, pancreas, ovary, and thyroid, and meningioma, and multiple myeloma (MM) (Lauby-Secretan et al., 2016).
2 OBESITY
Body-mass index (BMI) is widely used as a measurement of adiposity. By definition, people with a BMI between 25 and 29.9 kg/m2 are considered overweight, whereas a BMI of 30 kg/m2 and above is considered obese (National Institutes of Health, 1998). Obesity is due to an imbalance between energy intake (diet) and expenditure (physical activity). When energy intake exceeds energy expenditure in a chronic manner, obesity ultimately occurs. According to estimates from Public Health England, two-thirds of adults and a quarter of children between 2 and 10 years old are overweight or obese, and obese children are more likely to become overweight adults. These statistics highlight the growing problem and the need for prevention strategies. Obesity is still very much on the rise as food becomes more available and the average level of physical activity decreases (Hill, Wyatt, & Peters, 2012). Even though obesity is now well recognized as a disease in its own right, it is often not thought of as a stand-alone condition, as excess body fat not only causes direct effects on the body but is also known to be a key driver of many other severe health issues. Obesity causes metabolic, endocrine, immunologic, and inflammatory-like changes that may amplify cell mutation rate, dysregulate gene function, disrupt DNA repair, and/or induce epigenetic modifications. In turn, these changes may create a favorable environment for abnormal neoplastic transformation (inductive) or a permissive environment in which pre-existing clones that are dormant are permitted to emerge (selective) (Lichtman, 2010). It is unclear whether obesity creates an inductive or selective environment or, as is most likely, a combination of both. Nonetheless, the end result is that people who suffer from the condition have a higher risk of developing a number of different malignancies, one of which is MM.
3 MULTIPLE MYELOMA
MM is a condition caused by the clonal proliferation of abnormal plasma cells in the bone marrow (BM). MM represents around 10% of all hematological malignancies, and despite a vast improvement in treatment strategies over the last few decades, it remains an incurable disease. Patients often suffer with bone pain, pathologic fractures, weakness, anemia, infection (often pneumococcal), hypercalcemia, spinal cord compression, and renal failure. There are a number of risk factors associated with the disease, such as age, race, gender, family history, and, more recently, obesity (Beason, Colditz, Mittelman, & Berger, 2012). The association between obesity and MM began to emerge in the 90's as large cohort studies became a popular way to study disease correlations (Friedman & Herrinton, 1994). Since then, these initial findings have been validated using larger, more in-depth studies (Birmann et al., 2017; Teras et al., 2014); and over the years as more data have been collected, a dose-response effect has been reported suggesting that as BMI increases from normal to overweight to obese, there is an increase in the associated risk (Teras et al., 2014).
MM is consistently preceded by a premalignant condition known as monoclonal gammopathy of undetermined significance (MGUS). Patients suffering from MGUS have abnormal immunoglobulin protein in the blood or urine without myeloma-related organ damage or bone disease; these patients are asymptomatic and so are usually diagnosed as a result of routine blood screening for an unrelated health issue. There is no treatment for MGUS; it can only be monitored. This condition is prevalent in around 3.2% of adults over 50 years of age, and it is estimated that around 1% of these patients will progress to MM each year (Wadhera & Rajkumar, 2010). Obesity has also been correlated as a risk factor for progression with obese patients being 20% more likely to progress from MGUS to MM than patients of normal weight (Chang et al., 2017; Lauby-Secretan et al., 2016). This is an important finding as it provides evidence of a potentially modifiable risk factor associated with progression. One question that remains unanswered is whether obesity is correlated with MGUS incidence. The link between MGUS and obesity and MM progression is a powerful one as this allows intervention on diagnosis. However, if future studies reveal a link between obesity and MGUS development, this would need a population-wide intervention, which would be unrealistic. Unfortunately, evidence suggests that obesity in early adulthood could be significantly associated with MM (Hofmann et al., 2013.; Teras et al., 2014) and may even have an influence in childhood (Birmann, Suppan, & Colditz, 2014). However, the highest risk of MM mortality appears to be among those who were heavier in both young adulthood and later adulthood (Teras et al., 2014). Since MGUS develops before MM, it could be postulated that the changes that occur during prolonged periods of obesity may promote the development of MGUS and ultimately MM in a subset of the population. However, because MGUS is an asymptomatic condition, it is difficult to accurately assess the influences that promote its development.
4 ADIPOSE TISSUE
Adipose tissue is now recognized as the body's largest endocrine organ, capable of secreting more than 50 different adipokines, cytokines, and chemokines (Deng, Lyon, Bergin, Caligiuri, & Hsueh, 2016). Adipose tissue is often classified into two main subtypes: white adipose tissue (WAT) and brown adipose tissue (BAT). WAT is the most abundant and is found both subcutaneously and viscerally; its main function is to store energy in the form of lipids. BAT is localized in more distinct sites and mediates adaptive thermogenesis through an abundance of uncoupled mitochondria (Sulston and Cawthorn, 2016). In addition to these two well-studied tissue types, there is a third type of adipose tissue: BM adipose tissue (BMAT). This has been the least studied fat depot; although in recent years this has started to change as evidence has begun to emerge that suggests BMAT may have an important contribution to cancer progression, particularly in cancers that metastasize to the bone, such as breast and prostate, as well as cancers that arise in the bone, such as myeloma.
5 BM ADIPOSITY
Adipocytes are a major component of human BM, comprising up to 70% of BM volume, and accounting for more than 10% of total adipose mass in a healthy lean adult (Cawthorn et al., 2016). BM adipocytes are smaller than their visceral counterparts, although their fatty acid uptake is similar due to enhanced triacylglycerol synthesis (Trubowitz & Bathija, 1977). These cells were first thought of as inert space filling cells, however, over the years it has become clear that they are both an endocrine target and have endocrine-like functions: responding to growth hormones, insulin, and thyroid hormone, as well as releasing cytokines, such as IL-6, IL-1β, and TNF-α (Caers et al., 2007). BM adipocytes also secrete adipokines, such as leptin and adiponectin, which regulate calorie uptake and insulin sensitivity, respectively. They are now considered an influential component of the bone microenvironment with the ability to influence neighboring cells via autocrine, paracrine, and endocrine signaling (Rosen et al., 2009). BMAT is made up of two types of adipocytes: constitutive (cBMAT) and regulated (rBMAT), which reside in different locations in the bone. cBMAT is found in the distal areas of the bone and more closely resembles the densely packed arrangement of WAT. In contrast, rBMAT is found in the more metabolically active areas of the bone, these adipocytes are found scattered among neighboring hematopoietic cells. The exact contribution to circulating and local levels of cytokines and adipokines from these two cell types is not clear. However, the lipid content is thought to differ with rBMAT preferentially storing saturated fats and cBMAT unsaturated fats (Scheller et al., 2015), this could suggest that they have distinct functions that may influence the adipokines they secrete. The ratio of adipocytes to other cells in the marrow has become of great interest in the field; as more data are collected, the importance of maintaining a healthy level of adiposity in the bone is becoming clearer. BM adipocytes support and regulate the hematopoietic cells they surround by preventing hematopoietic progenitor expansion while preserving the hematopoietic stem cell pool (Naveiras et al., 2009). They originate from a common mesenchymal progenitor that has the potential to differentiate into an osteoblast or an adipocyte. In childhood, the differentiation decision favors osteoblastogenesis, however, as we age this preference changes in favor of adipogenesis. In old age, the percentage of fatty marrow is much higher, and these changes alone may cause our ageing population to be more vulnerable to disease establishment (Takeshita, Fumoto, Naoe, & Ikeda, 2014). Therefore, a change in adipocyte size and number in response to environmental ques, such as diet, coupled with the effect of ageing, could have a profound impact on the functionality of neighboring cells. It is well established that obesity increases WAT cells in both number and size, overwhelming their storage capacity, and causing a level of dysfunction (Skurk, Alberti-Huber, Herder, & Hauner, 2007). Obesity is associated with low-grade and chronic levels of inflammation in WAT. As adipocytes increase in size, some become apoptotic, which results in the infiltration of immune cells, such as macrophages, mast cells, and neutrophils (Wang, Liu, & Xie, 2017). Interactions between adipocytes and immune cells can enhance lipolysis and secretion of lipids, as well as the adipocyte and immune cell production of multiple proinflammatory factors. This in turn can have a negative effect on peripheral tissues inducing insulin resistance, hyperglycemia, and hyperlipidaemia, which are associated with oxidative stress and cancer development and/or progression. These changes can cause sustained proliferative signaling and activation of migration and metastasis (Hanahan and Coussens, 2012). Although these are changes that have been studied in the context of WAT, a similar response was observed in the BM of mice fed on a high-fat diet (HFD) suggesting that obesity may have a detrimental effect on adipose tissue as a whole (Scheller et al., 2016).
6 BM ADIPOSITY AND MM
The relationship between obesity and BM adiposity is still unclear, although there is mounting evidence to suggest that obesity is positively correlated with an increase in BMAT. Scheller et al., (2016) showed that mice fed on a HFD had a significant expansion of BMAT. Interestingly, they also demonstrated that mice fed on a HFD and subsequently fed on a normal diet to mimic weight loss did not exhibit an expansion in BMAT, suggesting that BMAT volume was modifiable through changes in diet. However, using this model of diet-induced obesity and weight loss they measured changes in bone morphology and mechanical strength, and concluded that HFD causes long-term, persistent changes in bone quality, despite prevention of marrow adipose tissue accumulation (Scheller et al., 2016). The observation that the bone microenvironment is affected by diet is an interesting one. A study by Lwin et al. (2015) showed how a HFD could detrimentally modify the environment to promote myeloma. This study demonstrated that C57Bl6 mice fed on a HFD before inoculation with 5TGM1 MM cells developed a myeloma-like condition, when compared with control mice (Lwin et al., 2015). Moreover, Trotter et al., (2016) showed that MM cells could also influence cell lineage determination by causing osteoblast progenitors to shift toward adipogenesis. Patients suffering from MM were found to have a higher proportion of preadipocytes, coupled with significantly larger mature adipocytes (Trotter et al., 2016). These findings support the notion that an increase in adiposity is pro-tumorigenic, therefore, coupled with an increase in adiposity induced by diet, this would further exacerbate disease progression. BMAT acts as an energy supply to fuel bone physiological functions, including bone remodeling. Adipose-rich areas of the bone are found near the more metabolically active areas of the bone, such as the growth plate and around the trabeculae, therefore an increase in BMAT may lead to elevated levels of bone resorption. These changes in bone turnover could be advantageous to tumor cell growth and survival. The way in which MM cells interact with and use their environment is often described in the context of a vicious cycle. The MM cells release factors that activate osteoclasts to break down the bone matrix, releasing bone-derived growth factors, such as transforming growth factor-β, insulin-like growth factor 1, and raising extracellular calcium concentrations. The growth factors bind to receptors on the cell surface of the tumor cells and activate SMAD and MAPK signaling, extracellular calcium binds and activates calcium pumps leading to tumor cell proliferation. Therefore, this increase in BMAT could potentially fuel cancer progression by increasing osteoclast activity and, thus, bone resorption, leading to the development of associated bone disease. Future studies need to be carried out to address whether there is a correlation between the location of a patient's bone lesions and the level of adiposity in the surrounding area. It could be that the secretions from the neighboring BM adipocytes are contributing to local bone disease, raising the question as to whether areas of high adiposity contain more and/or larger lesions. Adipocytes have also been implicated as drivers of chemoresistance, one proposed mechanism for this is that mature adipocytes activate autophagy and upregulate the expression of autophagic proteins resulting in the suppression of chemotherapy-induced caspase cleavage and apoptosis in myeloma cells (Liu et al., 2015). There have been several studies demonstrating that adipocytes support MM cell growth and survival as well as promoting migration (Bullwinkle et al., 2016; Caers et al., 2007). These effects are mainly due to adipokine secretion levels. There is a fine balance in the levels of adipokines secreted by a healthy individual, promoting homeostasis and protecting against disease development. However, in the event of increased adiposity, imbalance occurs, which has an opposing effect; the protective function is lost, resulting in the promotion of disease establishment and progression.
7 ADIPOKINES: GOOD OR BAD?
7.1 Leptin
Leptin is a peptide hormone that acts centrally via the hypothalamus to regulate food intake and energy expenditure, and on peripheral organs. Its main function is to mediate satiety, stimulate lipolysis, and suppress lipogenesis. The levels of serum leptin correlate with fat mass as leptin secretion increases in conjunction with adipocyte size. Leptin has been shown to induce pro-tumor effects in several cancers (Alshaker et al., 2015; Andò & Catalano, 2011) one of which being MM. A study carried out by Yu et al. (2016) demonstrated that the upregulation of leptin could stimulate proliferation of MM cells and reduce the antitumour effect of chemotherapy via activation of protein kinase B (AKT) and signal transducer and activator of transcription 3 (STAT3) pathways. Leptin was also shown to induce an upregulation of Bcl-2 expression and the inhibition of caspase-3 activation resulting in protection from chemotherapy-induced apoptosis (Yu et al., 2016). Leptin was shown to upregulate autophagy, again leading to suppression of chemotherapy-induced apoptosis (Liu et al., 2015). Interestingly, several studies have found elevated leptin in patients suffering from MM (Pamuk et al., 2006; Reseland et al., 2009). However, this elevated expression was not found to be correlated with risk (Dalamaga et al., 2009; Hofmann et al., 2012) suggesting that leptin levels are potentially associated with MM progression but not development.
7.2 Adiponectin
Adiponectin is the most abundant adipokine secreted by adipose tissue accounting for around 0.01% of the total serum proteins. Different fat depots secrete varying concentrations with BMAT having the largest contribution (Cawthorn et al., 2014). It is often referred to as a “good” adipokine as it has anti-inflammatory, antiatherogenic, and insulin-sensitizing properties. Adiponectin acts centrally to enhance energy homeostasis by increasing energy expenditure via activating hypothalamic leptin and insulin signaling pathways. It also improves glucose homeostasis by attenuating insulin resistance. As well as acting centrally, adiponectin also has autocrine and paracrine effects, which influence adipose tissue functions, overexpression of the Adipoq gene increases fat tissue mass via an increase in number of adipocytes rather than size. This increase allows for an expansion of triglyceride storage capacity thereby avoiding the stress that lipid-gorged adipocytes sustain. Increased adiponectin levels also reduce the secretion of proinflammatory factors such as IL-6 and TNF-α, which results in reduction of macrophage infiltration (Kim et al., 2007). Paradoxically adiponectin expression is decreased in obesity. Considering that adipose tissue is the main source of adiponectin in the body, it is counterintuitive that it should reduce as adiposity increases. However, this is a good example of how adipokine secretion changes in response to diet. Low levels of adiponectin have been associated with several obesity-related complications, such as metabolic syndrome and its related disorders: type 2 diabetes (Aleidi, Issa, Bustanji, Khalil, & Bustanji, 2015) and cardiovascular disease (Siasos et al., 2012) as well as several types of cancer (Cust et al., 2007; Goktas et al., 2005; Ishikawa et al., 2005; Tworoger et al., 2007) including myeloma (Dalamaga et al., 2009). The mechanisms behind this downregulation are still unknown, whether the stress sustained by adipocytes due to the overload of lipid causes the cells to become dysfunctional is unclear. However, what is clear is that this alteration in adipokine secretion appears to cause a permissive environment for cancer establishment and progression. A number of studies have reported decreased adiponectin levels in the serum of patients with MGUS and myeloma (Dalamaga et al., 2009; Fowler et al., 2011; Hofmann et al., 2016). As obesity also causes adiponectin downregulation, this could exacerbate and maybe even accelerate progression from MGUS to MM. In vitro studies demonstrated that adiponectin decreases cellular proliferation and increases apoptosis of myeloma cells via the activation of AMP-activated protein kinase (AMPK) and mitogen-activated protein kinase (MAPK) (Fowler et al., 2011). Moreover, adiponectin can also activate cell cycle arrest and apoptosis through the activation of p21 and p53 (Nigro et al., 2014). Adiponectin has garnered much attention, with a number of molecules being engineered to activate its signaling pathways in an attempt to rescue its protective properties; unfortunately, these drugs have had minimal success.
7.3 Resistin
Resistin was first identified as a protein abundantly secreted from the adipocytes of obese mice, providing a link between obesity and insulin resistance, hence the name resistin (for “resistance to insulin”) (Steppan et al., 2001). Since its discovery in 2001, its physiological role has started to emerge as being more complex than originally supposed. Resistin has been shown to participate in inflammation, cancer development, and metastasis (Kim et al., 2011; Kuo et al., 2013). It is also expressed by osteoblasts and osteoclasts as well as BM adipocytes suggesting that it may have a role in bone remodeling (Thommesen et al., 2006). In the context of MM, it has been reported to be both protective and damaging. Low resistin levels were found to be associated with increased risk (Dalamaga et al., 2009) particularly in men (Santo et al., 2017). However, Pang et al., (2017) demonstrated that resistin could play a role in drug resistance, abrogating chemotherapy-induced apoptosis in myeloma cells via the inhibition of chemotherapy-induced caspase cleavage. Taken together these two studies suggest that resistin could be protective against early stages of disease development, whereas detrimental in later stage. Future studies are needed to further elucidate the role of this adipokine, and to understand whether it could be a potential therapeutic target.
7.4 Adipsin
Another adipokine known to play a role in chemotherapy resistance is adipsin. Adipsin was first described in 1987 as a serine protease that was secreted into the bloodstream by adipocytes; due to its abundance in the serum, it was postulated that it could have systemic effects (Cook et al., 1987). Since then it has been shown to be involved in the alternative complement pathway of the complement system, to be a requirement for proper insulin secretion by the pancreatic β-cells, and to stimulate glucose transport for triglyceride accumulation in fat cells as well as inhibiting lipolysis (Lo et al., 2014). Although the levels of adipsin in obesity are less clear, some studies have shown that low levels of adipsin are associated with obesity (Rosen et al., 1989), whereas others have shown elevated levels that positively correlate with fat mass (Vasilenko et al., 2017). It may be that the source of adipsin is important and that patients who suffer from abdominal obesity may have different levels of adipsin compared with those who present with visceral obesity. The levels secreted from the BM remain unclear. The question as to the contribution of adipsin to cancer development and progression has not been extensively studied; one study showed that adipsin secreted by mature human adipocytes activates autophagy, thereby suppressing chemotherapy-induced apoptosis in myeloma cells (Liu et al., 2015). It may be that adipsin levels are variable under different conditions and its contribution to disease states is multifactorial, being influenced by the combination of other secreted factors as well as genetic and environmental ques.
7.5 Visfatin
Visceral-fat-derived protein, visfatin, also known as nicotinamide phosphoribosyltransferase or pre-B cell colony-enhancing factor (PBEF), is encoded by the PBEF-1 gene and is found both intracellularly and extracellularly. The intracellular form is the rate-limiting enzyme that catalyzes the first step in nicotinamide adenine dinucleotide (NAD) biosynthesis; the extracellular form has a more cytokine-like activity (Dahl et al., 2012). Visfatin was originally thought to be produced mainly by adipose tissue (i.e., adipocytes and infiltrating macrophages) (Fukuhara et al., 2005), however it has now been shown to be secreted by many more tissue types, such as liver, skeletal muscle, BM, and brain. Its widespread secretion is mirrored by its potential involvement in a wide range of disorders, such as inflammatory diseases, metabolic diseases, and cancer (Dahl et al., 2012). Visfatin regulates growth, apoptosis, and angiogenesis of mammalian cells and has been found to be upregulated in breast (Hung et al., 2016), colon (Yang et al., 2016), and prostate cancer (Patel et al., 2010). Moreover, a number of studies have shown that visfatin promotes MM progression; myeloma cells treated with the PBEF1 inhibitor APO866 had a reduction in cellular proliferation coupled with an induction of apoptosis (Venkateshaiah et al., 2013). Visfatin inhibition also enhanced the apoptotic effect of Bortezomib (Cagnetta et al., 2013). Visfatin secretion appears to contribute to MM progression; however, the source of visfatin is unclear, and whether the BM contributes significantly to circulating levels has yet to be established. The dual role of visfatin and its role as an adipokine remains to be fully elucidated, future studies will hopefully shed light on whether it has the potential as a therapeutic target.
8 CONCLUSION
Obesity is becoming one of the most challenging conditions worldwide, with the number of people suffering with the condition rising annually. The growing body of evidence implicating adiposity as a key driver of tumourigenesis is overwhelming. The systemic changes that occur as a result of this condition appear to have profound and lasting effects. MM is a malignancy associated with increased risk due to excess body fat. MM is known to develop from the precursor condition MGUS. It is important to identify factors that increase the risk of progression, thus by modifying patient behavior on diagnosis could decrease their risk of progression. An increase in adiposity causes inflammation and the accumulation of free fatty acids as well as a dysregulation of adipokine secretion. Obesity promotes the increased secretion of adipokines known to play a role in cancer development and progression along with the reduction of adiponectin, an adipokine with protective properties. These changes promote a more permissive environment where myeloma cells are able to thrive (Figure 1). Obesity is one of the few modifiable risk factors, which could have great therapeutic potential. The adipokine secretion profile from each of the different fat depots sheds light on how adiposity promotes cancer. Different types of obesity, such as abdominal and visceral, express different levels of adipokines, which could have a profound effect on the type of cancers that develop. The BM adiposity field is increasing rapidly as it becomes clearer that these adipocytes are contributing significantly to circulating levels of adipokines. BM adipocytes are often overlooked in the study of obesity. However, studies have now shown that even though the bone does not have the potential to increase its marrow space, the marrow itself has the potential to be modified in response to dietary demands. Many studies have already demonstrated that an increase in marrow adiposity can fuel tumor development and metastasis. Future studies are needed not only to develop educational strategies to help patients decrease their risk, but also to develop targeting strategies to manipulate adipokine secretion without compromising adipocyte function.
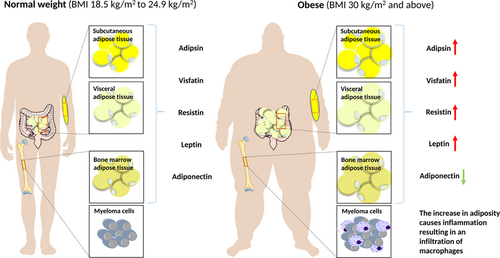
Adipokine secretion is dysregulated in obesity, promoting myeloma progression. Many adipokines are upregulated and are shown to be associated with myeloma cell growth and survival. In contrast, adiponectin, an adipokine known to have protective properties is downregulated creating a permissive environment for tumor cells to thrive. Obesity also causes low-grade chronic inflammation that further contributes to disease progression [Color figure can be viewed at wileyonlinelibrary.com]
ACKNOWLEDGMENTS
This work was supported by Bloodwise, a Marie Curie Career Integration Grant from the European Union Seventh Framework Programme and the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre (BRC). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR, or the Department of Health.