The role of hypoxia-inducible factor-2 alpha in angiogenesis
Abstract
Angiogenesis is a key enabling feature of mammalian embryonic development and tumor progression, which provides oxygen and nutrients that are required for vessel growth and tumor cell growth, respectively. Hypoxia is a driver of this phenomenon and is considered to be one of the most potent initiators of angiogenesis both in vitro and in vivo through stabilization of the transcription factors, hypoxia-inducible factor-1 and -2 (HIF-1 and HIF-2). Although these proteins are highly homologous, emerging evidence suggests that they have unique transcriptional targets and differential impact on angiogenesis. Although HIF-1α is the best known and widely described isoform, recent studies suggest that HIF-2α is a critical regulator of physiological and pathophysiological angiogenesis and, at least, the similiarly important as HIF-1α. Indeed, HIF-2α has been shown to regulate multiple aspects of angiogenesis, including cell proliferation, migration, maturation of blood vessels, and metastasis. In this review, we focus on recent insights into HIF-2α expression, activation, and function under hypoxic and nonhypoxic conditions. We also summarize the current knowledge on the crosstalk between HIF-2 and angiogenesis, describing reported phenotypical changes of HIF-2α genetic models and HIF-2 target genes implicated in angiogenesis. Finally, we provide a survey of recent pharmacologic strategies to specifically target HIF-2 activity.
1 INTRODUCTION
The maintenance of molecular oxygen (O2) homeostasis is essential for the survival of all eukaryotic organisms because O2 is a universal key substrate for mitochondrial ATP production and numerous intracellular biochemical reactions and pathways (Semenza, 2007). Hence, cells or organisms exposed to reduced oxygen concentration (hypoxia) respond by changing the expression of a great number of genes mediating hypoxia adaptation and survival (Semenza, 2012; Simon, 2016). Hypoxia-inducible factor-1 and -2 (HIF-1 and HIF-2) perceive oxygen availability and have a central role in regulating the transcription of most hypoxia-target genes, such as those involved in angiogenesis, metabolism, erythropoiesis, cell survival/apoptosis, migration, and other tissue-specific functions including inflammation and fibrosis (Dengler, Galbraith, & Espinosa, 2014).
Localized tissue hypoxia as a consequence of rapid oxygen consumption and/or poor circulation is the key driving force for new blood vessel formation. HIF-1 and HIF-2 can directly induce the expression of a plethora of genes whose products modulate vascular functions and angiogenesis in different cell types (Fong, 2008), as well as the vascular endothelial growth factor (VEGF), an angiogenic factor essential in the initiation stage of vasculate branching (Pugh & Ratcliffe, 2003). Moreover, intensive studies with HIF-1α- or HIF-2α-deficient (knockout) mice have demonstrated the critical role of hypoxia and HIF signaling in embryonic vascular and cardiovascular development (Krock, Skuli, & Simon, 2011).
Deregulation of O2 homeostasis leads to angiogenesis (Fraisl, Mazzone, Schmidt, & Carmeliet, 2009; Krock et al., 2011), not only in physiological conditions, including embryonic development, wound healing (Gurtner, Werner, Barrandon, & Longaker, 2008), or vessel penetration into avascular regions (Chung, Lee, & Ferrara, 2010), but also in pathological conditions, such as solid tumors (Chung et al., 2010) and various ischemic and inflammatory diseases (Carmeliet, 2005; Costa, Incio, & Soares, 2007). Therefore, significant scientific research has been focused on attempting to understand the mechanisms governing regulation, function, and pathogenic involvement of hypoxia and the ubiquitously expressed HIF-1 in angiogenesis (Hirota & Semenza, 2006; Semenza, 2014). However, much progress has been made toward understanding the role of the less-studied and cell-type-specific HIF-2 in angiogenesis (Keith, Johnson, & Simon, 2011). Indeed, HIF-2α has been shown to regulate multiple aspects of angiogenesis, including cell proliferation, migration, maturation of blood vessels, and metastasis. These findings make HIF-2α a critical regulator of physiological and pathological angiogenic phenotype.
In this review, we describe the current understanding of the role of HIF-2 in response to hypoxia and discuss the crosstalk between hypoxia and angiogenesis and the most recent potential therapeutic approaches targeting HIF-2.
2 HYPOXIA-INDUCIBLE FACTOR-2
HIF-2 is the second member of the HIF family. It is a dimeric protein complex that plays an integral role in the organism's response to hypoxia. HIF-2 is a transcriptional activator, essential for adaptation to low-oxygen conditions as well as for embryogenesis and tumor progression (Lofstedt et al., 2007; Patel & Simon, 2008).
2.1 Structure and function of HIF-2α
HIF-2 consists of two different subunits, ð > ¼ and ð > ¼ (also known as aryl hydrocarbon nuclear receptor translocator [ARNT]); both subunits are members of the basic helix-loop-helix (bHLH) Per-ARNT-Sim (PAS) transcription factor family (Scheuermann, Yang, Zhang, Gardner, & Bruick, 2007; Wang, Jiang, Rue, & Semenza, 1995). HIF-2α is also known as endothelial PAS domain protein 1 (EPAS1) (Tian, McKnight, & Russell, 1997), HIF-related factor (Flamme et al., 1997), HIF-like factor (Ema et al., 1997), and member of PAS family 2 (Hogenesch et al., 1997).
At the N-terminal end, HIF-2α contains PAS and bHLH motifs that are required for the heterodimerization between HIF-2α and ARNT (Card, Erbel, & Gardner, 2005; Scheuermann et al., 2009) (Figure 1). In addition, the bHLH domain of the HIF-2α/ARNT dimer is essential for DNA binding on hypoxia responsive elements (HREs) with the consensus sequence (G/ACGTG) in the promoters or enhancers of target genes (Wu, Potluri, Lu, Kim, & Rastinejad, 2015). HIF-2α has two transcriptional activation domains (TADs), an N-terminal activation domain (N-TAD) and a C-terminal activation domain (C-TAD), which interact with transcriptional coactivators such as CBP/p300 (CREB-binding protein/E1A binding protein p300) and are responsible for its transcriptional activity. N-TAD confers target gene specificity of HIF-2α, whereas C-TAD promotes the expression of HIF-1α and HIF-2α common target genes (Hu, Sataur, Wang, Chen, & Simon, 2007). Among N-TAD and C-TAD lies an inhibitory domain, the function of which needs to be further elucidated. Also, an oxygen-dependent degradation domain (ODDD), which overlaps with the N-TAD, is responsible for HIF-2α degradation in normoxic conditions (Ema et al., 1997; O’Rourke, Tian, Ratcliffe, & Pugh, 1999) (Figure 1). HIF-2α exhibits significant homology with HIF-1α in the primary amino acid sequence (48%) (Tian et al., 1997), and they share 83% and 70% sequence identities in their DNA binding and dimerization domains, respectively (Hu et al., 2007). Also, HIF-2α contains two nuclear localization signals (NLS), the N-NLS (amino acids 14–50) and the C-NLS (amino acids 705–742) (Luo & Shibuya, 2001). Under hypoxia, HIF-2α is localized in the nucleus in speckles near the active polymerase RNA, which gives better access to the promoters of target genes, in contrast to HIF-1α, which is homogeneously distributed in the nucleus (Taylor et al., 2016).
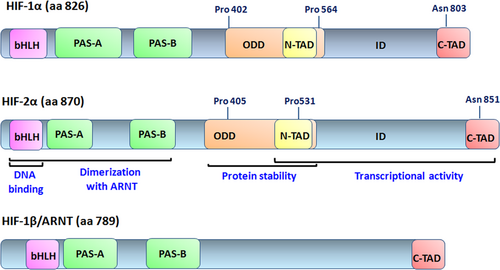
The structural domains of HIF-1α, HIF-2α, and HIF-1β (or ARNT) proteins. The bHLH and PAS domains are involved in DNA binding and heterodimerization. The ODD domain is required for oxygen-dependent hydroxylation and degradation and contains two hydroxylation-sensitive proline residues; and the N-TAD and C-TAD domains are required for transcriptional activation. ARNT, aryl hydrocarbon nuclear receptor translocator; bHLH, basic helix loop helix; HIF, hypoxia-inducible factor; PAS, Per-ARNT-Sim; ODD, oxygen-dependent degradation domain; C-TAD, C-terminal activation domain; N-TAD, N-terminal activation domain [Color figure can be viewed at wileyonlinelibrary.com]
HIF-2α expression is tissue specific and restricted primarily to the endothelium, liver, lungs, kidneys, heart, brain, and intestine (Wiesener et al., 2003). HIF-2 regulates the transcription of hypoxia-target genes, which is involved in several biological processes such as erythropoiesis (erythropoietin [EPO]) (Kapitsinou et al., 2010; Rankin et al., 2007), stemness/self-renewal (octamer-binding transcription factor-4) (Covello et al., 2006), proliferation (transforming growth factor alpha and cyclin D1 [CCND1]) (Raval et al., 2005), apoptosis (Qi et al., 2015), invasion (plasminogen activator inhibitor-1 [PAI-1]) (Hu et al., 2007; Pawlus, Wang, Ware, & Hu, 2012), epithelial to mesenchymal transition (Yang et al., 2016), redox homeostasis (superoxide dismutase-2 [SOD-2]) (Scortegagna et al., 2003), and angiogenesis (discussed in the section 4).
2.2 Oxygen-dependent regulation of HIF-2α
Under normoxic conditions, HIF-2α is constitutively transcribed and translated. When the oxygen tissue supply is sufficient, de novo synthesized cytoplasmic HIF-2α is hydroxylated at two conserved proline residues proline (Pro-405 and Pro-531) located within the ODD domain by a family of prolyl hydroxylase enzymes (PHDs). Prolyl hydroxylation of HIF-2α results in the binding of the von Hippel-Lindau (VHL) tumor suppressor, which recruits the Elongin C-Elongin B-Cullin 2-E3-ubiquitin-ligase complex, leading to polyubiquitination and proteasomal degradation of HIF-2α with a half-time of approximately 5 min (Shen & Kaelin, 2013). The second major mechanism that modulates HIF-2α activity is the hydroxylation of asparagine residue 847 in the C-TAD domain. HIF-2α is hydroxylated under normoxic conditions by factor-inhibiting HIF (FIH), which inhibits HIF-2α interaction with the transcriptional coactivator CBP/p300 (Kaelin and Ratcliffe, 2008) (Figure 2). Hydroxylases (PHDs and FIH) are strictly Fe(II)- and α-ketoglutarate-dependent dioxygenases, which are activated only in the presence of molecular oxygen. However, during hypoxia, PHDs and FIH are inactive or have reduced activity, which leads to the inhibition of HIF-2α hydroxylation. Under these conditions, HIF-2α is stabilized and is subsequently translocated into the nucleus where it dimerizes with ARNT. The HIF-2α/ARNT dimer binds to hypoxia responsive elements (HREs) and regulates target gene expression (Mole et al., 2009; Schodel et al., 2011) (Figure 2).

O2-dependent HIF-2α regulation. In the presence of oxygen (normoxia), HIF-2α is hydroxylated on proline 405 and 531 residues located within ODDD by prolyl hydroxylase enzymes (PHDs). Hydroxylation results in the binding of the von Hippel-Lindau (VHL) E3 ligase complex, which ubiquitinates HIF-2α, targeting it to proteasomal degradation. The hydroxylation of the asparagine residue prevents CBP/p300 binding to HIF-2α. Under hypoxic conditions, HIF-2α subunit is stabilized because of a lack of hydroxylation and is subsequently translocated into the nucleus where it dimerizes with ARNT. The HIF-2α/ARNT dimer binds to HREs and regulates target gene expression. CBP/p300; CREB-binding protein/E1A binding protein p300; HIF, hypoxia-inducible factor; HRE, hypoxia response element; ODDD, oxygen-dependent degradation domain [Color figure can be viewed at wileyonlinelibrary.com]
2.3 Oxygen-independent regulation of HIF-2α
Apart from oxygen tension, multiple oxygen-independent mechanisms can also regulate HIF-2α at the level of transcription, translation, protein stability, and posttranslational modification.
Relatively, little is known about HIF-2α transcriptional activation. In M2-polarized macrophages, Th2 cytokines interleukin (IL)-4 and IL-13 activate EPAS1 (gene encoding HIF-2α) expression, resulting in the induction of Arginase1 expression and the suppression of nitric oxide (NO) production (Takeda et al., 2010). Moreover, currently published data have reported that HIF-2α transcription is controlled by epigenetic modifications. In breast cancer, methylated CpG binding protein 3 binds to the EPAS1 promoter and enhances its transcription by demethylating CpG located around transcriptional start site, causing the activation of HIF-2α-mediated angiogenesis (Cui et al., 2016). In addition, it has been reported that EPAS1 transcription is regulated by the IGF-induced PI3K-mTORC2 system promoting vascularization in neuroblastoma (Mohlin et al., 2015). The deubiquitylase Cezanne (also known as OTUD7B) is also involved in the regulation of HIF-2α mRNA synthesis via E2F1, which directly binds to the EPAS1 promoter (Moniz et al., 2015). Finally, Nakazawa et al. (2016) has showed that HIF-2α expression is epigenetically downregulated via class I/II HDACs, resulting in increased Anoctamin 1 (ANO1), calcium signaling, and elevated mTORC1 activity in tumors promoting sarcoma proliferation (Nakazawa et al., 2016).
At the level of HIF-2α mRNA translation and protein stability, it has been reported that the PTEN/PI3K/AKT signaling axis regulates HIF-2α expression and stability via a mTORC2-dependent manner (Nayak et al., 2013; Toschi, Lee, Gadir, Ohh, & Foster, 2008). In addition, hypoxia-induced HIF-2α stabilization in macrophages is controlled by the PTEN/PI3K/AKT promoting tumor growth, angiogenesis, and metastasis (Joshi, Singh, Zulcic, & Durden, 2014). A second mechanism of regulation HIF-2α mRNA translation has also been reported demonstrating that the iron response element (IRE) binding protein 1 binds specifically to an IRE in the HIF2α 5′-UTR causing inhibition of translation (Sanchez, Galy, Muckenthaler, & Hentze, 2007; Zimmer et al., 2008; Zimmer et al., 2010).
The stabilization of HIF-2α and subsequently its activity are also described to be affected by chemical agents called “hypoxia mimetics,” such as 2-oxoglutarate-dependent oxygenase inhibitors such as dimethyloxaloylglycine (DMOG) (Elvidge et al., 2006), the iron chelator desferrioxamine (DFO) (Befani et al., 2013), and transition metals such as cobalt (Befani et al., 2013). All the compounds mentioned above are routinely used in vitro and in vivo to stimulate HIF-2α in normoxic conditions. However, the action especially of cobalt is not at all equivalent to true, low-oxygen hypoxia response and, therefore, may cause different and oxygen-independent biological effects. This issue was clarified by our previous work in which we demonstrated that in hepatic-derived cancer cell lines, cobalt, unlike hypoxia, increases HIF-2α protein expression but it cannot activate HIF-2-dependent EPO mRNA and protein production in Huh7 cells. In addition, the presence of cobalt does not generally affect HRE-dependent transcription but rather specifically inhibits the activity of HIF-2. Moreover, this specific effect of cobalt on HIF-2 signaling was accompanied by reduced interaction of the HIF-2α with upstream stimulatory factor 2 (USF2), suggesting that HIF-2α, but not HIF-1α, requires the assistance of a cobalt-sensitive factor to activate transcription of its target genes in liver cancer cells (Befani et al., 2013).
Posttranslationally, HIF-2α is regulated either by its interaction with other proteins or its modification by different enzymes affecting its expression and/or transcriptional activity. ETS-1 (Aprelikova, Wood, Tackett, Chandramouli, & Barrett, 2006; Elvert et al., 2003; Fiorito et al., 2014) and ELK-1 (Aprelikova et al., 2006; Hu et al., 2007), which belong to the family of v-ets erythroblastosis virus E26 oncogene homolog (ETS) transcription factors, the NF-κB essential modulator (Bracken, Whitelaw, & Peet, 2005), and USF2 (Pawlus et al., 2012), have been reported to associate with HIF-2α and are required to specifically regulate HIF-2 target gene activation being part of HIF-2-specific enhanceosome (Pawlus & Hu, 2013; Pawlus, Wang, Murakami, Dai, & Hu, 2013). Apart from the above-described transcriptional factors, it is known that HIF-2α is associated with other proteins, such as HIF-associated factor (HAF) (Koh, Lemos, Liu, & Powis, 2011), tumor suppressor Int6 (Chen, Uchida, Endler, & Shibasaki, 2007), Poly (ADP-ribose) polymerase-1 (Gonzalez-Flores et al., 2014), and MYC (Gordan, Bertout, Hu, Diehl, & Simon, 2007), affecting its transcription activity. Especially, HAF has been reported to promote HIF-2 transcriptional activity by binding to its C-terminal domain (Koh et al., 2011) but, on the contrary, HAF destabilizes HIF-1α in proteasome-dependent manner by binding to its N-terminal domain (Koh, Darnay, & Powis, 2008).
Furthermore, HIF-2α is subject to a number of O2-independent posttranslational modifications including SUMOylation, acetylation, and phosphorylation. HIF-2α is sumoylated at K394 of the LK394EE site, and Small Ubiquitin-like Modifier proteins (SUMO)-2-conjugated HIF-2α is rapidly degraded during hypoxia via SUMO-targeted ubiquitin ligases (van Hagen, Overmeer, Abolvardi, & Vertegaal, 2010). Also, HIF-2α can be acetylated at several lysine residues by CPB (Chen et al., 2012) and de-acetylated by SIRT1 promoting HIF-2α signaling and thus participating in regulation of the HIF-2α target gene EPO (Dioum et al., 2009).
It has been reported that HIF-2α is subject to a number of phosphorylation events. Casein kinase 2 can phosphorylate HIF-2α at Thr844, which results in increased HIF-2 transcriptional activity possibly by lowering the affinity of HIF-2α for FIH (Gradin, Takasaki, Fujii-Kuriyama, & Sogawa, 2002). HIF-2α is also phosphorylated at Thr324 by PKD1 and, as a result, its interaction with the SP1 transcription factor is inhibited, thus promoting Nijmegen breakage syndrome 1 gene expression (To, Sedelnikova, Samons, Bonner, & Huang, 2006). In addition, we have recently demonstrated that casein kinase 1 (CK1) phosphorylates HIF-2α and positively affects HIF-2 activity (Pangou et al., 2016). CK1δ targets two conserved serine residues (Ser383 and Thr528) in HIF-2α without affecting its protein stability or its interaction with the USF2 coactivator but controlling CRM1-dependent nuclear export of HIF-2α (Figure 3). CK1δ silencing or inhibition decreases hypoxic induction of two HIF-2-specific target genes, EPO and PAI-1, without affecting HIF-2α protein expression. Furthermore, inhibition of HIF-2α phosphorylation by mutagenesis of the CK1 target sites (conversion of both Ser383/Thr528 into Ala) or treatment with the CK1 inhibitor D4476 impairs nuclear accumulation of HIF-2α and, consequently, decreases its transcriptional activity. However, when cells, expressing the phosphorylation-deficient mutant of HIF-2α, are treated with Leptomycin B (a specific inhibitor of CRM1-dependent nuclear export), nuclear localization of the mutant HIF-2α is restored and its activity is fully recovered, suggesting that lack of CK1-depended phosphorylation reduces nuclear concentration of HIF-2α by excessive nuclear export into the cytoplasm. Either suppression of CK1δ activity or mutations of the CK1δ phosphorylation sites on HIF-2α strongly reduce EPO secretion in two hepatocellular carcinoma cell lines, highlighting the significance of HIF-2 activity for hypoxic adaptation and suggesting a novel regulatory mechanism of HIF-2α by CK1 (Pangou et al., 2016) (Figure 3).
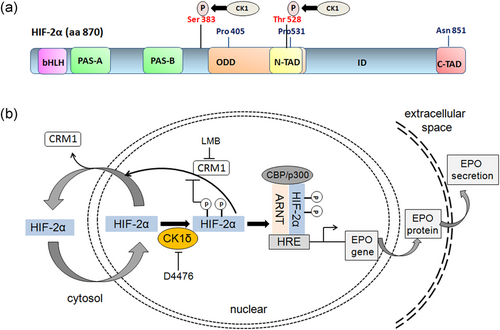
HIF-2α regulation by CK1δ-mediated phosphorylation. (a) CΚ1δ directly phosphorylates HIF-2α on Ser383 and Thr528. (b) Schematic representation of the regulation of HIF-2α-dependent EPO secretion involving CK1δ and CRM1. Under hypoxia, HIF-2α stabilizes and enters the nucleus but can be exported back into the cytoplasm by a CRM1-dependent process. CΚ1δ directly phosphorylates HIF-2α on Ser383 and Thr528, blocks its nuclear export, and promotes formation of a heterodimer with ARNT, binding to HRE sequences in the enhancer regions of its target gene EPO and subsequent stimulation of erythropoietin expression and production. ARNT, aryl hydrocarbon nuclear receptor translocator; EPO, erythropoietin; HIF, hypoxia-inducible factor; HRE, hypoxia response element [Color figure can be viewed at wileyonlinelibrary.com]
In aggregate, these studies support that not only HIF-2α regulation mechanisms are not restricted to cellular adaptation to hypoxic stress, but also HIF-2α is controlled by additional oxygen-independent regulatory networks. These data emphasize the profound contribution of HIF-2α to physiological and pathological processes such as the development of normal tissues or tumors, the determination of cell death or survival, and the adaptation to various stresses.
However, although our understanding for the biology of the HIF-2α pathway is becoming clearer, the oxygen-independent control of HIF-2α and its molecular interactions with other cellular components still need to be investigated in more details.
3 ANGIOGENESIS (STEPS OF SPROUTING ANGIOGENESIS)
Blood vessel formation is a fundamental process for the development of the organism and tissue regeneration. During embryonic development, the primitive vascular network is formed in situ by the differentiation of endothelial progenitor cells called angioblasts, a process known as vasculogenesis (Risau & Flamme, 1995). This vascular plexus is extended and modified through sprouting and nonsprouting angiogenesis (intussusception), the process by which new blood vessels are formed from pre-existing vessels (Carmeliet, 2003).
Sprouting angiogenesis, the first identified form of angiogenesis, is the basic mechanism seen in the growth of new blood vessels. It is characterized by sprouts composed of endothelial cells, which usually grow toward an angiogenic stimulus. On the other hand, intussusceptive angiogenesis involves the formation of blood vessels by a splitting process in which the capillary wall extends into the lumen to break a large blood vessel up into two or more smaller ones (Carmeliet & Jain, 2011; Folkman & Haudenschild, 1980).
The process of sprouting angiogenesis in a normal tissue occurs in several well-characterized stages. Briefly, the blood vessels are composed of an inner lining of closely assembled endothelial cells ensheathed by pericytes, fixed in various stromal cells and extracellular matrix (ECM) (Galley & Webster, 2004). Biological signals, such as hypoxia, inflammation, ischemia, and/or blood vessel damage, activate the quiescent endothelial cells and induce the release of angiogenic growth factors such as VEGF, angiopoietin-2 (Ang-2), fibroblast growth factor (FGF), and chemokines. Then, pericytes first detach from the vessel, in response to Ang-2 signaling, and then endothelial cells loosen their junctions (VE-cadherin) and the vessel dilates (Augustin, Koh, Thurston, & Alitalo, 2009). Vascular permeability increases in response to VEGF, allowing extravasation of plasma proteins, such as fibrinogen and fibronectin, to deposit a provisional ECM, and matrix metalloproteinases degrade and remodel the pre-existing ECM, enabling endothelial cell migration (Lamalice, Le Boeuf, & Huot, 2007). In sprout formation, one endothelial cell, called tip cell, is selected to lead the network of the cells that follow, known as stalk cells. The tip-cell formation is affected by neuropilins, VEGF/VEGFR, and the NOTCH ligands, delta like canonical notch ligand 4 (DLL4) and JAGGED1. Tip cells have filopodia to sense guidance signals, such as semaphorins and ephrins, and adhere to ECM, mediated by integrins α/β, to migrate toward a VEGF-A gradient (De Smet, Segura, De Bock, Hohensinner, & Carmeliet, 2009; Gerhardt et al., 2003). Stalk cells after the tip cell proliferate causing the capillary sprout to elongate and to form a lumen. Also, tip cells establish the junctions among endothelial cells to form continuous lumen through which oxygenated blood can flow. Maturation and stabilization of the new vessel requires deposition of ECM and recruitment of pericytes and smooth muscle cells. In this process, angiogenic factors such as angiopoietin-1 (Ang-1), platelet-derived growth factor (PDGF)-BB and PDGF receptor, ephrin-B2, and FGF participated (Jain, 2003). Once perfusion of the neovessel with oxygen and nutrient is established, the blood vessel becomes quiescent again.
4 ROLE OF HIF-2α IN ANGIOGENESIS
Hypoxia coordinates not only the earliest steps of vascular development, but also the angiogenesis in adult life in physiological and pathological states. HIF-1α and HIF-2α are the master regulators of O2 homeostasis, but, despite their similarities in structure, function, and regulation, they have different and nonredundant functions during development. In angiogenesis, HIF-1α is the most studied factor, but, on the other hand, although HIF-2α has been less frequently investigated, an increasing number of reports suggest that it is also important in angiogenesis, discussed as follows.
4.1 HIF-2α knockout models
Mouse model studies have demonstrated the participation of HIF-2α in vasculogenesis. Independent groups have created Hif-2α−/− knockout models and obtained strikingly divergent results, supporting the fact that Hif-2α-deficient phenotypes are variable, depending on the genetic background (Table 1).
| Study | HIF-2α phenotype |
|---|---|
| Tian et al. (1998) | Bradycardia, reduced noradrenaline, no vascular defects |
| Peng et al. (2000) | Died between E9.5 and E13, severe vascular defects, and hemorrhaging |
| Compernolle et al. (2002) | Cardiac failure, respiratory distress syndrome, reduced VEGF levels |
| Scortegagna et al. (2003) | Viable Hif-2α-null adult mice, cardiac hypertrophy, hepatic steatosis, retinopathy, significantly reduced EPO levels in the kidney |
| Duan et al. (2005) | Severe vascular defects rescued by endothelium-specific expression of HIF-2α |
| Yamashita et al. (2008) | Impaired development of tumor vessels, reduced expression of ephrin A1 |
| Skuli et al. (2009) | Increased vessel permeability, reduced tumor growth and angiogenesis, impaired expression of angiogenic genes |
- Note. HIF, hypoxia-inducible factor.
Tian, Hammer, Matsumoto, Russell, & McKnight (1998) found that Hif-2α-deficient embryos died at midgestation with bradycardia. This was attributed to reduced amounts of catecholamine (noradrenaline) from the Ηif-2α-expressing organ of Zuckerkandl, possibly due to reduced tyrosine hydroxylase expression, suggesting that HIF-2α regulates embryonic catecholamine synthesis in response to physiologic hypoxia. Almost half of these embryos could be rescued to birth by the supplementation of the drinking water with D,L-threo-3,4-dihydroxyphenylserine, a metabolic intermediate that can be directly converted to noradrenaline (Tian et al., 1998). Despite the initial identification of the Hif-2a gene in endothelial cells, no vascular defects were observed in these embryos. Another group reported that Hif-2α mutant embryos died between E9.5 and E13 exhibiting severe vascular defects and hemorrhaging both in the yolk sac and embryo proper, suggesting that HIF-2α is required for normal remodeling/maturation postvasculogenesis (Peng, Zhang, Drysdale, & Fong, 2000). A third model demonstrated that half of the Hif-2α−/− embryos died of cardiac failure, whereas the Hif-2α−/− neonates died because of respiratory distress syndrome (Compernolle et al., 2002). This is apparently caused by defects in fetal lung maturation because of reduced VEGF levels and insufficient surfactant production by alveolar type 2 cells. This result suggests a role for HIF-2α in the regulation of VEGF expression in the development of the embryonic lung.
In contrast to these previous models, Scortegagna et al. (2003) generated viable Hif-2α-null adult mice although at less than the expected frequency, by crossing mice from two genetic backgrounds. These mice exhibited multiorgan dysfunction, including cardiac hypertrophy, hepatic steatosis, and retinopathy associated with increased oxidative stress. These findings suggest a regulating role of HIF-2α in organ development or homeostasis and the expression of antioxidant enzymes such as superoxide dismutase, catalase, and glutathione peroxidase. In addition, these Hif-2α−/− mice exhibit defects in hematopoietic development due to significantly reduced EPO levels in the kidney, demonstrating that HIF-2α is essential for the regulation of renal EPO expression and thus for global hematopoiesis (Scortegagna et al., 2003, 2005, Rankin et al., 2007, Gruber et al., 2007).
Interestingly, embryonic stem-cell-derived Hif-2α−/− embryos exhibited severe vascular defects, which could be rescued by endothelium-specific expression of HIF-2α (Duan, Zhang-Benoit, & Fong, 2005). Furthermore, conditional deletion of HIF-2α in hepatocytes suppressed vascular tumor development in VHL-mutant livers and revealed that VEGF and other angiogenic factors expression in hepatocytes are predominantly regulated by HIF-2. In addition, knockdown of HIF-2α in endothelial cells resulted in the impaired development of tumor vessels via reduced expression of ephrin A1 (Yamashita et al., 2008). In agreement with these results, Skuli et al., (2009) generated of an EC-specific HIF-2α deletion murine strain, which developed increased vessel permeability, aberrant endothelial cell ultrastructure, pulmonary hypertension, reduced tumor growth, and angiogenesis as well as impaired expression of angiogenic genes encoding fms-related tyrosine kinase-1 (Flt-1), kinase insert domain receptor (KDR/Flk-1), Ang-2, and DLL4.
Interestingly, HIF-2α plays different roles in many respects compared with HIF-1α in vessel morphogenesis, based on their knockout animal models. Targeted inactivation of Hif-1α (encoding HIF-1α) in mouse, results in embryonic lethality during midgestation (Iyer et al., 1998; Ryan, Lo, & Johnson, 1998). Hif-1a−/− mice developed severe defects in yolk sac vasculature, which are associated with increased hypoxia. In addition, loss of Hif-1a resulted in decreased endothelial stem cell proliferation, defects in capillary network formation, and significant cardiovascular abnormalities (Carmeliet et al., 1998; Compernolle et al., 2003; Iyer et al., 1998; Kotch, Iyer, Laughner, & Semenza, 1999; Ryan et al., 1998). Taken together, the results of HIF-specific knockouts imply distinct and nonoverlapping physiological functions of HIF-alpha isoforms in angiogenesis. HIF-1 promotes cell proliferation and migration in early angiogenesis, whereas HIF-2 participates in the remodeling and maturation of the microvasculature controlling vascular morphogenesis, integrity, and assembly.
Overall, these results highlight the essential role of HIF-2α in vascular development, which is associated with the HIF-2α-mediated expression of genes involved in angiogenesis.
4.2 HIF-2α target genes in angiogenesis
HIF-2 upregulates several genes involved in almost every step of angiogenesis, such as in angiogenic switch and destabilization, sprouting, elongation, and maturation (Figure 4). Within the promoters or enhancers of many of these angiogenic genes, HREs or similar sequence elements are recognized by HIF-2. The main angiogenic factor VEGF and its receptors VEGFR-1 (Flt-1) and VEGFR-2 (Flk-1) are induced directly by HIF-2 under hypoxic conditions through their identified HREs (Elvert et al., 2003; Takeda et al., 2004). VEGF is the most critical component in angiogenesis as it regulates almost every step, including the angiogenic switch, the destabilization of the vessel, the tip-cell formation, and the vascular permeability in the initiation of angiogenesis, as well as the migration and proliferation of endothelial cells in elongation and tube formation and cell-to-cell contact in the neovessel formation. Although VEGF is also regulated by HIF-1, it seems that HIF-2 has a stronger transactivation activity than HIF-1 on its promoter (Ema et al., 1997; Rankin et al., 2008; Xia et al., 2001). In addition, HIF-2 activates the transcription of endothelial nitric-oxide synthase gene (eNOS) in HMEC-1 and HUVEC under hypoxia by binding preferentially to the HRE within the eNOS promoter (Coulet, Nadaud, Agrapart, & Soubrier, 2003). Nitric oxide (NO) has an important role not only in the stimulation of angiogenesis, but also in the vasodilation and vessel normalization (Fukumura, Kashiwagi, & Jain, 2006; Sessa, 2004). Other HIF-2 target genes that participate in angiogenesis are EPO (Jaquet, Krause, Tawakol-Khodai, Geidel, & Kuck, 2002; Kertesz, Wu, Chen, Sucov, & Wu, 2004), PAI-1 (Geis et al., 2015; Sato et al., 2004), and adenosine A2A receptor, an angiogenic factor promoting cell proliferation, migration, and angiogenic activity in human lung microvascular endothelial cells (Ahmad et al., 2009). Furthermore, HIF-2 collaborates with Ets-1 transcription factor in activating the transcription of their common genes, which are expressed in endothelial cells during angiogenesis. For example, the junctional molecule VE-cadherin, and VEGF receptor flk-1 are directly controlled by HIF-2 in synergy with Ets-1 (Elvert et al., 2003; Le Bras et al., 2007).
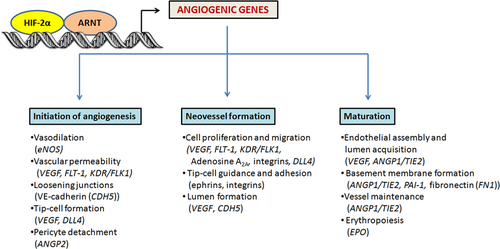
HIF-2 role in angiogenesis. HIF-2 upregulates sever encoding al genes involved in almost every step of angiogenesis including initiation, vessel formation, and maturation. HIF, hypoxia-inducible factor [Color figure can be viewed at wileyonlinelibrary.com]
On the other hand, there are many angiogenic genes with potential nonidentified HREs, which are upregulated by HIF-2α overexpression or downregulated by HIF-2α knockdown. Such examples are the genes encoding integrin β3 in HMEC-1 (Befani & Liakos, 2017), DLL4, and fibronectin (Skuli et al., 2009). Also, the ANG and TIE signaling system, which consists of Ang-1, Ang-2, and Tie-2 receptor and mediates vessel maturation, pericytes detachment, and endothelial cell sprouting, is activated by HIF-2 (Park et al., 2016; Skuli et al., 2009; Tian et al., 1997). These studies demonstrate that HIF-2α regulates almost every aspect of angiogenesis, which has made it an attractive therapeutic target in many human diseases.
4.3 Therapeutic targeting of HIF-2α in angiogenesis
HIF-2 has been considered as a potential therapeutic target in many diseases, and depending on the type of disease and the expected therapeutic effect of HIF-2, targeting has different strategies: on one hand, the activation of HIF-2-dependent angiogenesis in ischemic diseases and on the other hand the inhibition of HIF-2-dependent angiogenesis in malignancy.
The upregulation of HIFs has been used as a potential therapeutic tool for cardiovascular and ischemic diseases. The literature about HIF-2α as a therapeutic potential in ischemic cardiovascular diseases is limited. It has been reported that HIF-2α has a protective role against ischemia of the kidney (Kapitsinou et al., 2014; Kojima et al., 2007) likely through alleviation of oxidative stress (Kojima et al., 2007). In addition, Niemi et al. (2014) have performed gene transfer of adenoviral HIF-1α and HIF-2α into the mouse heart and rabbit ischemic hindlimbs, leading to the induction of angiogenesis in vivo in both myocardium and ischemic skeletal muscles. Especially, HIF-2α gene transfer resulted in the formation of strong capillaries in muscle connective tissue.
Hypoxia is a common feature in tumor development and metastasis, whereas angiogenesis plays an important role in tumor growth and progression. HIF-2α overexpression has been reported to be detected in a variety of tumor types, including non-small-cell lung cancer (Giatromanolaki et al., 2001), endometrial carcinoma (Sivridis, Giatromanolaki, Gatter, Harris, & Koukourakis, 2002), squamous cell head-and-neck cancer (Koukourakis et al., 2002), astrocytomas (Khatua et al., 2003), colorectal cancer (Yoshimura et al., 2004), gallbladder carcinoma Giatromanolaki et al., (2001), and breast carcinomas (Giatromanolaki, Sivridis, Simopoulos et al., 2006). In many of these vascularized tumors, HIF-2α expression positively correlates with expression of angiogenic factors such as VEGF and ECGF, suggesting a critical role for HIF-2α in tumor angiogenesis (Giatromanolaki et al., 2001; Yoshimura et al., 2004). As HIF-2α regulates the induction of many angiogenic factors in tumors and promotes angiogenesis, the ability to inhibit HIF-2 activity appears to be beneficial for cancer therapy.
A large number of molecules have been reported to inhibit HIF-2α activity either directly or indirectly, including inhibitors of HIF-2α mRNA expression and protein synthesis (Ma et al., 2014; Mohlin et al., 2015; Pastorino et al., 2010; Zimmer et al., 2008), inhibitors of HIF-2α transcriptional activity (Cook et al., 2009), and inhibitors of HIF-2α dimerization with ARNT (Chen et al., 2016; Wallace et al., 2016). Especially, on the basis of the structures of HIF-2α PAS and ARNT PAS it was found that HIF-2α PAS-B domain contains an internal cavity that serves as a preformed ligand binding site (Erbel, Card, Karakuzu, Bruick, & Gardner, 2003; Scheuermann et al., 2009). In addition, a number of small-molecule ligands have been found to antagonize HIF-2 heterodimerization and DNA-binding activity in vitro and in cultured cells, reducing HIF-2 target gene expression (Rogers et al., 2013; Scheuermann et al., 2013). On the basis of these ligands, two compounds, PT2385 and PT2399, were identified to be specific antagonists of HIF-2α by blocking its dimerization with ARNT and inhibiting the expression of only HIF-2-dependent genes, including VEGF-A, PAI-1, and CCND1 in clear cell renal cell carcinoma (ccRCC) cell lines and tumor xenografts, but having no effect on HIF-1 function (Chen et al., 2016; Wallace et al., 2016). PT2385 is under clinical investigation for advanced ccRCC in a phase I trial (NCT02293980).
5 CONCLUSION
The HIF pathway has been established as the master regulator of O2 homeostasis controlling the transcription of most hypoxia-target genes, such as those involved in angiogenesis, metabolism, erythropoiesis, cell survival/apoptosis, migration, and other tissue-specific functions. In relation to angiogenesis, HIF-2α is required for proper embryonic development and is critical for the formation of new blood vessels in both physiological and pathophysiological settings by stimulating the expression of angiogenic factors, such as VEGF, angiopoietins, VEGF receptors, and integrins. In addition, HIF-2α expression and activity are increased in tumor angiogenesis leading to enhanced vascularization. Therefore, modulating HIF-2α levels and activity provides novel therapeutic approaches for the treatment of both ischemic disease and cancer. Further studies remain necessary to fully understand the molecular mechanisms of HIF-2α regulation and the role of HIF-2α in angiogenesis.
CONFLICTS OF INTEREST
The authors declare that there is no conflict of interest.
FUNDING INFORMATION
This work is supported by the State Scholarships Foundation (IKY): “IKY Fellowships of Excellence for Postgraduate Studies in Greece-Siemens Programme” to Christina Befani.