High prevalence of serum IgG antibodies reacting to specific mimotopes of BK polyomavirus, a human oncogenic polyomavirus, in patients affected by uveal melanoma
Abstract
The uveal melanoma (UM) is the most common human intraocular tumor. The BK polyomavirus (BKPyV) is a small DNA tumor virus whose footprints have been detected in different human cancers. BKPyV has oncogenic potential. Indeed, BKPyV, when inoculated into experimental animals, induces tumors of different histotypes, whereas in vitro, it transforms mammalian cells, including human cells from distinct tissues. In this investigation, the association between UM and BKPyV was studied employing indirect enzyme-linked immunosorbent assays (ELISAs) using synthetic peptides that mimic BKPyV viral capsid 1 (VP1) antigens. Indirect ELISAs were used to detect serum IgG antibodies against this polyomavirus with oncogenic potential in samples from patients with UM and controls, represented by healthy subjects (HS). It was found that serum samples from patients with UM had a higher prevalence of BKPyV antibodies, 85% (51/60), compared with that detected in HS1, 62% (54/87), and HS2, 57% (68/120). The different prevalence of BKPyV antibodies detected in UM versus the two control groups, HS1 and HS2, is statistically significant (p < 0.005). Our immunologic data suggest a significantly higher prevalence of antibodies against BKPyV VP1 epitopes in serum samples from patients with UM compared with HS. These results indicate an association between UM and BKPyV, suggesting that this small DNA tumor virus may be a cofactor in the UM onset or progression.
1 INTRODUCTION
Melanoma is a cancer that develops in the majority of the cases on the host’s skin (Testa, Castelli, & Pelosi, 2017). However, in 5% of patients, it develops in the eye, mainly in the uveal component, generating uveal melanoma (UM; Singh, Turell, & Topham, 2011).
The onset of this tumor is due to the transformation of melanocytes, which are cells of neural origin specialized in the production of the pigment melanin. Melanocytes, during embryogenesis, migrate from the neural crest to other tissues of the host. In adults, they are present in the skin, striae vascular ear, meninges, and in the uveal component of the eye (Amaro et al., 2017). The function of the melanin, as a pigment, is to protect from sun light. Indeed, melanin is capable of protecting the skin keratinocytes from ultraviolet rays.
In the etiopathogenesis of UM, as for other human tumors, many factors are involved. In the multistep process of oncogenesis, its onset or progression is mainly linked to specific chromosome rearrangements and gene mutations (Amaro et al., 2017). Different chemical, physical, and biological carcinogenic agents, including oncogenic viruses, have been advocated (Bononi et al., 2014; Jovanovic et al., 2013).
Melanoma can be a severe cancer, which, in the presence of metastatic cells, lasts without effective treatments. These key aspects of UM indicate the need for an early diagnosis and a timely treatment (Leyvraz & Keilholz, 2012).
In earlier studies, BK polyomavirus (BKPyV) was found to be associated with human cancers of different types, such as brain and bone tumors, insulinomas, Kaposi’s sarcomas, cancer of the urinary tracts, and genital malignancies (Tognon, Corallini, Martini, Negrini, & Barbanti-Brodano, 2003).
The oncogenic potential of BKPyV is related to the transforming activities of two viral oncoproteins, the large T antigen (Tag) and the small t antigen (tag), whose genes map in the early region of the viral genome (Barbanti-Brodano et al., 2006). The BKPyV Tag serves multiple functions that alter the normal physiological metabolism of cells, ultimately leading to immortalization and neoplastic transformation. Tag is clastogenic and mutagenic. It has the ability to bind tumor suppressor p53 and pRB family proteins, thus abolishing their functions (Tognon et al., 2003). BKPyV Tag can affect cellular growth–control mechanisms, but other additional events are required for the complete transformation of mammalian cells, such as the presence of the activated form of the human oncogene c-H-Ras (Barbanti-Brodano et al., 2006; Pagnani et al., 1988; Shivakumar & Das, 1996). In this instance, the two cofactors, viral and human oncogenes, act synergistically in the full transformation of human cells.
The other BKPyV viral oncoprotein, tag, interacts with the phosphatase PP2A, which activates the Wnt pathway (Cardoso et al., 2015). Moreover, tag activates the phosphatidylinositol 3-kinase, an enzyme involved in pathways crucial for cell proliferation and transformation (Verhalen, Justice, Imperiale, & Jiang, 2015).
These activities adversely affect the cellular genome, which accumulates many gene mutations or chromosome aberrations. Then, in the absence of p53 functions, due to Tag binding, the cellular DNA remains unrepaired, with the consequence that the genome “derails” (Verhalen et al., 2015).
So far, the detection of human serum antibodies against BKPyV has been performed by serological methods with the recombinant viral capsid protein 1 (VP1) or with virus-like particles. However, the high homology among the VP1 proteins of the three main polyomaviruses BKPyV, SV40, and JCPyV hampered the final results, which were always influenced by some cross-reactivity (Barbanti-Brodano et al., 2004; Kean, Rao, Wang, & Garcea, 2009; Kjaerheim et al., 2007; Lundstig et al., 2005; Ribeiro et al., 2010; Viscidi et al., 2003).
More recently, the development of a specific and sensitive serologic test for BKPyV has been reported (Pietrobon, Bononi, Mazzoni, et al., 2017; Pietrobon, Bononi, Lotito, et al., 2017), which consists of indirect enzyme-linked immunosorbent assays (ELISAs) using synthetic peptides as mimotopes or antigens of BKPyV VP1. This immunologic assay with VP1 mimotopes was used to detect specific serum antibodies against BKPyV antigens in normal individuals (Pietrobon, Bononi, Mazzoni, et al., 2017) and in patients with choroidal nevi (Pietrobon, Bononi, Lotito, et al., 2017).
In this investigation, the putative association between UM and BKPyV was analyzed by investigating the presence and the titer of serum IgG antibodies against this small DNA tumor virus. Sera were analyzed by indirect ELISAs using two synthetic peptides corresponding to mimotopes of BKPyV viral capsid protein 1 (VP1). Then, to verify the data obtained in ELISA, serum samples were also tested by the hemagglutination inhibition (HAI) assay, which is a BKPyV highly specific assay (Pietrobon, Bononi, Lotito, et al., 2017; Pietrobon, Bononi, Mazzoni, et al., 2017).
2 MATERIALS AND METHODS
2.1 Serum samples
Serum samples (n = 180) were collected from patients affected by uveal melanoma (UM; n = 60) attending the Eye Clinic of the University Hospital of Ferrara, Italy. Serum samples (n = 120) from healthy subjects (HS) were collected at the Clinical Laboratory Analysis of the same University Hospital, after routine analyses from discarded samples, before incineration.
The controls included HS only. No patients affected by any pathologies were included among the controls. These blood analyses were not related to the follow-up of pathologies. All sera from individuals, analyzed at the Clinical Laboratory Analysis, were certified by the Hospital as belonging to HS. Their parameters were within the normal range. HS were not part of the cohort of hospital or hospital outpatients, but just normal individuals who underwent a routine blood analysis for prevention.
To confer more robust power to our statistical analyses, the control group (n = 120) was divided into two subgroups with subjects to equal or double those of UM subjects: HS1 (n = 87) and HS2 (n = 120). Serum samples, belonging to the two cohorts, were from individuals of both sexes, with the same median ± SD (years) age (HS1: 69 ± 13; S2: 68 ± 11).
Written informed consent was obtained from patients or subjects at the time of the hospital admission. The County Ethics Committee approved this study.
All serum samples were analyzed for the presence of IgG antibodies against BKPyV by indirect ELISAs with synthetic peptides, called mimotopes VP1 L and VP1 M. To verify the immunological data obtained with this novel indirect ELISA, the same sera were investigated by a second method, the HAI assay, which is highly specific for the detection of BKPyV antibodies.
2.2 Indirect ELISA
2.2.1 Synthetic peptides
As previously reported (Pietrobon, Bononi, Lotito, et al., 2017; Pietrobon, Bononi, Mazzoni, et al., 2017), computer-assisted analyses enabled us to select two specific BKPyV peptides from the late viral region by comparing the BKPyV VP1 amino acids (a.a.) sequence with that of simian virus 40 (SV40) and JC (JCPyV) polyomaviruses, which are highly homologous to BKPyV as well as with other less homologous, polyomaviruses (http://blast.ncbi.nlm.nih.gov).
Indirect ELISA analyses indicated that the two selected peptides did not cross-react with SV40 and JCV hyperimmune sera used as controls (Pietrobon, Bononi, Lotito, et al., 2017; Pietrobon, Bononi, Mazzoni, et al., 2017).
- VP1 L: NH2-LKLSAENDFSSDSPERK-COOH
- VP1 M: NH2-MLNLHAGSQKVHEHGGGK-COOH
The unrelated human synthetic neuropeptide hNPS, a.a. sequence SFRNGVGTGMKKTSFQRAKS, was used as a negative control (Guerrini, Salvadori, Rizzi, Regoli, & Calo, 2010).
The synthetic peptides, synthesized by standard procedures, were purchased from UFPeptides s.r.l. (Ferrara, Italy).
2.2.2 Peptide coating
Indirect ELISA was developed and standardized to detect specific antibodies against BKPyV in human sera using VP1 L and VP1 M synthetic peptides. Plates were coated with 5 μg of the selected peptide for each well and diluted in 100 μl of Coating Buffer (Candor Bioscience, Wangen, Germany) at 4°C for 16 hr.
2.2.3 Blocking phase
Blocking was performed with 200 μl/well of the Blocking Solution (Candor Bioscience) at 37°C for 90 min. Blocking was performed with 200 μl/well of blocking solution containing casein (Candor Bioscience) at 37°C for 90 min.
2.2.4 Primary antibody
Different wells were covered with 100 μl of serum sample diluted 1:20 in Low Cross-Buffer (Candor Bioscience). The assay contained (i) the BKPyV-positive control represented by a BKPyV-immune rabbit serum; (ii) the negative control represented by immune sera anti-SV40 and anti-JCV; and (iii) three human serum samples, which were found to be BKV negative by the indirect ELISA and HAI assays. Each sample was analyzed three times in duplicate wells. The plate was incubated at 37°C for 90 min.
2.2.5 Secondary antibody addition
The solution contained a goat antihuman or antirabbit IgG heavy and light-chain specific peroxidase conjugate (Calbiochem-Merck, Darmstadt, Germany) diluted 1:10,000 in Low Cross-Buffer (Randhawa et al., 2008).
2.2.5.1 Dye treatment and spectrophotometric reading
Samples were treated with 100 μl of 2,2′-azino-bis2,2′-azino 3-ethylbenzthiazoline-6-sulfonic-bis 3-ethylbenzthiazoline-6-sulfonic acid solution (Sigma-Aldrich, Milan, Italy) for 45 min at room temperature and then read using a spectrophotometer (Thermo Electron Corporation, model Multiskan EX, Vantaa, Finland) at a wavelength (λ) of 405 nm. The color intensity in wells, where the immunocomplexes formed, was determined by the optical density (OD).
2.2.6 Cut-off determination
The cut-off point was determined in each assay by the value of OD reading of three negative controls, added to the standard deviation (SD) multiplied three times (±3 SD). Sera with antibodies against BKPyV were considered positive when reacting to both peptides L and M (Corallini et al., 2012; Martini et al., 2013; Mazzoni et al., 2012; Taronna et al., 2013).
2.3 BKPyV viral working stock
BKPyV working stock was produced in VERO cells infected at a low multiplicity of infection, that is, 10-4 plaque-forming units (PFU)/ml, as reported elsewhere (Corallini et al., 2012; Mazzoni et al., 2015). The viral titer of the BKPyV stock, determined by the hemagglutination (HA) assay, was 0.16 × 103 hemagglutinating units (HAU), corresponding to 1.6 × 106 PFU/ml. BKPyV virions, which act as antigens, were used in both HA and HAI assays.
2.4 HA assay
The BKPyV titer was determined by the HA assay. HA involves the use of human erythrocytes of group 0, Rh+. These erythrocytes were provided by the Blood Bank, the University Hospital of Ferrara, Italy. Serial two-fold BKPyV dilutions were placed in plates, 96 round wells (Nunc, CelBio, Milan, Italy), in the presence of phosphate buffered saline (PBS) 1×, from 1:10 at a 1:5120 dilution, with a final volume of 100 μl. Then, 50 μl of 0.5% erythrocytes were added to each viral dilution. HA titer was read in plates incubated at +4°C for 4 hr when control erythrocytes in PBS had completely sedimented. The highest dilution of the viral antigen yielding complete haemagglutination was considered equivalent to 1 HAU (Corallini et al., 2012; Pietrobon, Bononi, Lotito, et al., 2017; Pietrobon, Bononi, Mazzoni, et al., 2017).
2.5 HAI assay
The HAI assay is an immunoassay based on the ability of BKPyV to agglutinate human erythrocytes of group 0, Rh, whereas IgG antibodies against the virus present in the serum sample inhibit the agglutination operated by BKPyV.
The assay includes serial two-fold dilutions (1:16, 1:32, 1:64, and 1:128) of the serum under analysis for the determination of the antibody titer. Serum (30 μl), heated at 56°C for 30 min, is treated with NaIO4 (15 μl, 0.1 M) to remove nonspecific inhibitors, and incubated at RT for 30 min. Then, as a final step, 15 μl of glycerine at 5% was added to each serum sample.
Serial two-fold dilutions of serum, from 1:16 into 1:128, were in PBS 1×. Then, one volume of PBS containing 8 HAU of antigen, represented by BKPyV virions, was added to each serum dilution. Mixtures were kept at room temperature for 1 hr. Two volumes of 0.5% human erythrocytes, group 0, Rh+, were added and plates were incubated at 4°C. After 4 hr, the HI-antibody and titers were read (Corallini et al., 2012; Pietrobon, Bononi, Lotito, et al., 2017; Pietrobon, Bononi, Mazzoni, et al., 2017).
2.6 Statistical analysis
The different prevalences of serum IgG antibodies against BKPyV in independent groups, that is UM and HS were statistically evaluated. The statistical tests used were (i) the one-way ANOVA test to compare variances among OD and (ii) χ 2 with Yates’ correction or Fisher’s exact test to compare the prevalence of antibodies antiBKPyV in different groups. A p < 0.05 value was considered statistically significant. All statistical analyses were performed using Prism software (GraphPad, San Diego, CA).
3 RESULTS
3.1 Detection of serum IgG antibodies against BKPyV by indirect ELISAs with specific VP1 mimotopes
Human sera from patients with UM and HS were analyzed for IgG antibodies reacting to BKPyV VP1 L and M mimotopes. For this purpose, indirect ELISA with synthetic peptides corresponding to the specific BKPyV VP 1 antigens was used. The unrelated human synthetic neuropeptide hNPS was used as a negative control (Guerrini et al., 2010).
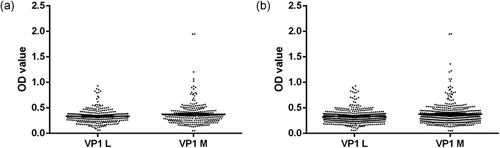
UM serum samples reacting to the mimotope VP1 L reached a prevalence of 85% (51/60), whereas the same sera reacted to the peptide VP1 M with a prevalence of 87% (52/60). HS1 sera yielded a prevalence of 64% (56/87) with the mimotope L and 74% (65/87) with the mimotope M. HS2 sera yielded a prevalence of 67% (80/120) with the mimotope L and 73% (87/120) with the mimotope M.
It is interesting that the antibody prevalence against the BKPyV peptide L and peptide M in samples of each cohort is very close, without a statistical difference.
In our study, sera were considered BKPyV positive when reacting with both VP1 L and VP1 M mimotopes or peptides.
Combining the data of BKPyV-positive sera, both for the VP1 L and for the VP1 M mimotopes, the overall prevalence was 85% (51/60) in UM, 62% (54/87) in HS1, and 57% (68/120) in HS2 (Table 1). The difference in the prevalence of BKPyV-positive subjects in the UM group was statistically significant compared with the two groups of control subjects (p < 0.005).
| Number of positive samples (%) | ||||||
|---|---|---|---|---|---|---|
| Human serum | Number of subjects | Median age ± SD | Male % | VP1 L | VP1 M | VPs (L+M) |
| UM | 60 | 66 ± 14 | 58 | 51 (85.0) | 52 (87.5) | 51 (85.0)* |
| HS1 | 87 | 69 ± 13 | 41 | 56 (64.4) | 65 (74.7) | 54 (62.1) |
| HS2 | 120 | 68 ± 11 | 39 | 80 (66.7) | 87 (72.5) | 68 (56.7) |
- Note. Human sera were from UM and HS1–3. The prevalence of BKPyV antibodies in UM sera was statistically higher than that detected in HS1, with the same median age (*p < 0.005). The prevalence of BKPyV antibodies in UM sera was statistically higher than that detected in the HS1 and in HS2 p < 0.005, and p < 0.005, respectively. A statistical analysis was performed using χ2 with Yates’ correction. The p values shown are based on the Tag (A + D)-positive numbers.
- BKpyV, BK polyomavirus; HAI, hemagglutination inhibition; HS, healthy subjects; UM, uveal melanoma.
BKPyV-positive sera tested by indirect ELISA, diluted at 1:20, had a general cut-off, by spectrophotometric reading, in the range of OD 0.17–0.19. This cut-off represents the value that differentiates BKPyV-negative samples, below OD 0.17–0.19, from BKPyV-positive samples, above OD 0.17–0.19.
In our indirect ELISA with BKPyV VP1 mimotopes, the positive control was represented by a rabbit BKPyV hyperimmune serum, which had an OD of up to 1.8, whereas JCPyV and SV40 hyperimmune sera, used as negative controls, had an OD of less than 0.1. Then, additional human sera BKPyV-positive and BKPyV-negative were taken from our serum collection. In previous experiments these samples were analyzed by HAI and indirect ELISA assays, as reported (Pietrobon, Bononi, Lotito, et al., 2017; Pietrobon, Bononi, Mazzoni, et al., 2017).
Indirect ELISAs with two distinct BKPyV VP1 L and M mimotopes yielded overlapping results, thus confirming the presence of antiBKPyV antibodies in human sera from patients affected by UM and in HS (Table 1). The range of ODs was 0.18–1.360.
Combining the data of BKPyV-positive sera, both for the VP1 L and for the VP1 M mimotopes, the overall prevalence was 85% (51/60) in UM, 62% (54/87) in HS1, and 57% (68/120) in HS2 (Table 1). The difference in the prevalence of BKPyV-positive subjects in the UM group was statistically significant compared with both groups of control subjects (p < 0.005; Table 1).
Serologic profiles of serum antibody reactivity to BKPyV mimotopes are presented in Figure 1.

The reproducibility of the serological results was assessed in three replica experiments carried out by independent operators with no data variability. Altogether, these assays allowed us to confirm which serum samples were BKPyV positive.
The interrun and intrarun variability is shown in Figure 2. Intrarun and interrun OD variabilities were not statistically significant (p > 0.05).
3.2 BKPyV antibodies in human sera determined by the HAI assay
Human sera from UM and controls, HS1 sample, were analyzed by the HAI assay. This assay allows the detection of serum antibodies against BKPyV because a BKPyV-immune serum abolishes the agglutination property of this polyomavirus exerted in human red cells of group 0, Rh+ (Pietrobon, Bononi, Lotito, et al., 2017; Pietrobon, Bononi, Mazzoni, et al., 2017).
Serum samples, serially two-fold diluted from 1:16, 1:32, 1:64, to 1:128, were analyzed by the HAI assay to evaluate both the presence of antibodies against BKPyV and their titer.
The seroprevalence of BKPyV-positive samples, diluted 1:128, was 83.3% (50/60) in patients affected by UM. In the control group, represented by the HS sample, the prevalence was 64.4% (56/87), respectively. The seroprevalence of BKPyV-positive samples in patients with UM, determined with two methods, i.e indirect ELISA and HAI assays, was statistically significantly higher than that determined in the control group of HS (p < 0.05).
Human sera from patients with cancer affected by uveal melanoma (UM) and controls represented by HS1 were also analyzed by the HAI assay. The seroprevalence of BKPyV-positive samples, diluted 1:128, was 83.3% (50/60) in patients affected by UM, whereas in the control group represented by HS, the prevalence was 64.4% (56/87) (Table 2).
| Number of positive samples (%) | |||||||
|---|---|---|---|---|---|---|---|
| Human sera | Number of subjects | Median age ± DS | Male % | 1:16 | 1:32 | 1:64 | 1:128 |
| UM | 60 | 66 ± 14 | 58 | 49 (81.2) | 50 (83.3) | 50 (83.3) | 50 (83.3)* |
| HS1 | 87 | 69 ± 13 | 41 | 85 (97.7) | 85 (97.7) | 84 (96.6) | 56 (64.4) |
- Note. Human sera were obtained from UM and HS1. The prevalence of BKPyV antibodies in UM sera was statistically higher than that detected in HS1, with the same mean age (*p < 0.05). A statistical analysis was performed using χ2 with Yates’ correction. BKpyV, BK polyomavirus; HAI, hemagglutination inhibition; HS, healthy subjects; UM, uveal melanoma.
3.3 Dual BKPyV and SV40 antibodies in human sera
In a previous investigation, we showed an association between UM and SV40, revealing a higher prevalence of antibodies against SV40 in patients with UM than in healthy controls (Bononi et al., 2014). Here, a higher prevalence of BKPyV-antibodies was revealed in UM sera compared with HS. The same UM sera were used in the two studies, thus enabling the identification of the double-immune samples. It was found that 15 out of 16 UM sera that tested SV40 positive were also BKPyV positive (Table 3).
| Serum sample tested BKPyV-VP1-positive or samples analyzed (%) | Serum sample tested SV40-VPs-positive or samples analyzed (%) | Serum sample tested BKPyV-VP1-positive among SV40-VPs-positive or samples (%) |
|---|---|---|
| 51/60 (85.00) | 16/48 (33.33) | 15/16 (93.75) |
- Note. BKpyV, BK polyomavirus; SV40, simian virus 40; VP, viral capsid protein.
4 DISCUSSION
In the etiopathogenesis of melanoma, many factors are involved. In the multistep process of oncogenesis, its onset or progression is related to chromosome arrangements, gene mutations, whereas different chemical and physical mutagenic or cancerogenic agents and oncogenic viruses may play a role (Jovanovic et al., 2013).
It has been reported that different viral agents could be involved in the etiology of uveal melanoma, such as Herpes, Papilloma, Toga, and oncogenic RNA viruses (Albert, 1979; Cun et al., 2013). At present, there are no effective treatments when metastasis occurs in UM. An early diagnosis and timely treatment are of paramount importance for a better clinical outcome. It should be kept in mind that the discovery of etiologic factors responsible for the onset of the UM, including viruses with oncogenic potential, can be significant because they could become new biomarkers of malignancy and novel targets of innovative therapies (Albert, 1979; Leyvraz & Keilholz, 2012).
In a previous investigation, we showed an association between UM and SV40, revealing a higher prevalence of antibodies against SV40 in patients with UM than in healthy controls (Bononi et al., 2014).
Here, we investigated the association between UM and BKPyV by analyzing the presence of antibodies against this human polyomavirus with oncogenic potential. In the past, BKPyV was found to be associated with different human cancers, such as brain and bone tumors, insulinoma, Kaposi’s sarcoma, and cancer of the urinary and genital tracts (Tognon et al., 2003).
Here, the rational of investigating the association between BKPyV and UM is based on previous studies that reported the copresence of BKPyV and SV40 footprints in human neural tumors, such as brain tumors of different histotypes (De Mattei et al., 1995; Martini et al., 1995; Martini et al., 1996; Tognon et al., 1996).
Sera were analyzed by a novel indirect ELISA assay using synthetic peptides as mimotopes of the viral capsid protein 1 (VP1). Then, to verify the specificity of these immunologic data, the same samples were also tested by HAI.
Specifically, samples were analyzed for IgG antibodies reacting, in indirect ELISA, to two BKPyV VP mimotopes: VP1 L and VP1 M. An unrelated human synthetic peptide, used as a negative control, did not react with the sera under investigation, as expected.
The seroprevalence of BKPyV-positive samples in patients with UM obtained by indirect ELISAs is statistically significant higher than that determined in the control groups of HS (Table 1). These ELISA data have been confirmed in serum samples by additional analyses with another highly specific test, such as the HAI assay (Table 2). The high prevalence of BKPyV antibodies in patients affected by UM and normal subjects is due to the widespread prevalence of the BKPyV infection in the general population. Indeed, BKPyV infects the majority of human subjects during childhood, without clinical manifestations in immunocompetent individuals. After the primary infection, BKPyV remains in a latent phase lifelong, with sporadic reactivations or even reinfections (Pietrobon, Bononi, Lotito, et al., 2017; Pietrobon, Bononi, Mazzoni, et al., 2017).
The detection of SV40 and BKPyV antibodies in the same 15 serum samples from UM (Table 3) indicates that these two oncogenic polyomaviruses may act simultaneously or synergistically in transforming the human uveal melanocytes, which are cells of neural origin. In other investigations, SV40 and BKPyV DNA sequences and the coexpression of their Tag oncogenes were detected in the same specimens of human brain tumors, such as glioblastoma multiforme (Tognon et al., 1996), ependimomas, and choroid plexus papillomas (Martini et al., 1996). We may speculate that BKPyV could have a helper function for SV40, which is a monkey virus by origin. It also plausible that patients affected by UM are genetically predisposed to these polyomavirus infections. It is worth noting that patients with UM have a higher prevalence of BKPyV and SV40 antibodies, but their OD values are lower than those in HS.
In conclusion, an association between UM and BKPyV was found in our study by two independent and highly specific assays. At the same time, it is worth recalling that an association does not mean a relation of cause and effect.
ACKNOWLEDGMENTS
This study was supported in part by research grants from the Associazione Italiana per la Ricerca sul Cancro (AIRC; Milan), contract grant number: IG16046 to M. Tognon; Associazione Sammarinese per la Lotta contro le Leucemie e le Emopatie Maligne (ASLEM; San Marino), Contract grant number: CFR 2015 to M. Tognon; University of Ferrara, Contract grant number: FAR Projects 2014 and 2017, to F. Martini and M. Tognon. Dr. Silvia Pietrobon was a Ph.D. student supported in part by a 3-year fellowship from the Fondazione Cassa di Risparmio di Cento, Cento. Dr. Elisa Mazzoni was a fellowship recipient of the Fondazione Umberto Veronesi, Milan.
M. Tognon, F. Martini, I. Bononi, and E. Mazzoni designed research; S. Pietrobon, E. Torreggiani, and M. Rossini carried out the experiments; E. Mazzoni, I. Bononi, E. Torreggiani, M. Rossini, M. Tognon, and F. Martini analyzed data; P. Perri and S. Violanti provided samples and carried out the clinical characterization; and I. Bononi, E. Mazzoni, M. Tognon, and F. Martini wrote the paper.
CONFLICTS OF INTEREST
Data of this study were enclosed, in part, in the Italian patent application number I0167478/BRE-EC/rp, filed on August 9, 2016.