Neural regulation of bone remodeling: Identifying novel neural molecules and pathways between brain and bone
Shishu Huang and Zhenxia Li have contributed equally to this study.
Abstract
The metabolism and homeostasis of the skeletal system have historically been considered to be associated with the endocrine system. However, this view has been expanded with the recognition of several neural pathways playing important roles in the regulation of bone metabolism via central relays. In particular, bone metabolism and homeostasis have been reported to be precisely modulated by the central neural signaling. Initiated by the finding of leptin, the axis of neural regulation on bone expands rapidly. The semaphorin–plexin system plays an important role in the cross-talk between osteoclasts and osteoblasts; a complex system has also been identified and includes neuropeptide Y and cannabinoids. These findings facilitate our understanding of the central neuropeptides and neural factors in the modulation of bone metabolism and homeostasis, and these neuronal pathways also represent an area of research scenario that identifies the novel regulation between brain and bone. These regulatory mechanisms correlate with other homeostatic networks and demonstrate a more intricate and synergetic bone biology than previously envisioned. As such, this review summarizes the current knowledge of the neural regulation of bone metabolism and homeostasis, as well as its role in skeletal diseases and discusses the emerging challenges presented in this field.
1 INTRODUCTION
The skeletal system is a living and dynamic system, which coordinately generates new bone and removes old bone by two types of dedicated cells: osteoblasts and osteoclasts, respectively. Osteoblasts are the main cells that are accountable for bone formation. They develop from mesenchymal stem cells and are influenced by numerous local and systemic factors, such as insulin-like growth factor (IGF) and transforming growth factors-β (TGF-β). In contrast, osteoclasts are large multinucleated cells developed from hematopoietic stem cells and accountable for resorption of bone minerals, which are influenced by macrophage colony-stimulating factor and receptor activator of nuclear factor-κB (NF-κB) ligand (RANKL). Interestingly, the processes of bone formation and resorption are firmly coordinated in time and space, rather than occurring at the same site concurrently. However, the disturbance of this balance commonly results in bony fragility and osteoporotic fractures, which is highly prevalent in the aging population and causes marked morbidity and mortality (Spanswick, Smith, Groppi, Logan, & Ashford, 1997; Wilding et al., 1993).
Bone is closely related to the nervous system, with the observation of bone being richly innervated by both sympathetic and sensory neurons (Mach et al., 2002). Immunocytochemistry studies revealed the existence of innervation and receptors for several neural peptides in bone cells (Elefteriou, 2005). In addition, with the retrograde trans-synaptic tracing method, neural tracts from the femoral bone marrow have been identified to link the central nervous system (CNS; Dénes et al., 2005). Given the dynamic nature of bone homeostasis and the precise control required to coordinate bone remodeling, the nervous system is believed to play a critical role in the regulation of bone metabolism and homeostasis. Bone metabolism and homeostasis are attributed to the variations of hormonal factors, which respond to paracrine/autocrine and regional mechanical signals simultaneously (Hao et al., 2015). However, there is increasing evidence for the control of bone metabolism by the CNS, which directly regulate bone tissues via efferent neural connections. To date, it is known that the CNS plays an important role in the maintenance of bone homeostasis and metabolism. For instance, the arcuate nucleus (ARC) is semipermeable to the blood–brain barrier and contacted by circulating factors, including semaphorins and leptin. Changes in those factors altered the secretion of endocrine factors and efferent neural outflow, which feeds back to the bone tissue. The local leptin receptor level in bone has been reported to be responsible for the reduced osteogenesis (Yue, Zhou, Shimada, Zhao, & Morrison, 2016) and leptin is involved in the antiosteogenic process via central control (Sharan & Yadav, 2014), and these effects were mediated through neural circuits, with signals transmitted to bone cells via the sympathetic nervous system (Elefteriou et al., 2005). In addition, semaphorins have been identified in the regulation of bone remodeling, particularly the differentiation and migration of osteoblasts and osteoclasts (Sims & Martin, 2014). Dysregulation of the semaphorin expression has been reported to be the cause of severe bone diseases, such as osteoporosis and osteopenia (Tang, Yin, Lv, Zhang, & Zhang, 2015).
Recent studies have highlighted the notion that the bone tissue is under the dual control of the central and the peripheral neural system, and significant advances have been made in our knowledge of the roles of neural control in bone. As such, this review will focus on the recent evidence for the central peptides arising from the brain and their intracellular signaling in the regulation of bone metabolism, homeostasis, and diseases. We hope that this will shed new light on the understanding of bone biology and its sophisticated mechanisms.
2 SEMAPHORINS
Semaphorins are a family of cell surface and soluble proteins that regulate cell–cell interactions, cell differentiation, and cell functions. They are categorized by a conserved aminoterminal “Sema” domain and are divided into eight subclasses by the similarity of amino acid sequence and C-terminal structure (No Authors, 1999). Vertebrate semaphorins are clustered into Classes 3–7, while Classes 1 and 2 are identified in invertebrates. The Class-8 semaphorins are virus-encoded. Semaphorins are commonly regulated by its receptor plexins. This is a group of nine members that can be divided into four groups, plexins A–D (Worzfeld & Offermanns, 2014).
The functionalities of semaphorins and their receptors have been first validated in the nervous system, although these molecules are frequently expressed outside the nervous system (Perälä, Sariola, & Immonen, 2012). The semaphorin–plexin system has been reported in the involvement of various biological processes, including oncogenesis, angiogenesis, and immunological responses. This also extends to the mechanisms of how signals are transduced by semaphorins through their receptors. In general, Plexin-A1 regulates cytoskeletal organization by modulating the activity of GTPase and cytoplasmic protein kinases (Kruger, Aurandt, & Guan, 2005) and the cytoplasmic domain of plexins has intrinsic R-Ras GAP activity, the effector of which can mediate mitogen-activated protein kinase (MAPK), Ras homolog gene family, member A (RhoA), and Akt signaling pathways. Plexin-B1 binds to its receptor tyrosine kinases ErbB-2 and Met and promotes invasive epithelial growth (Giordano et al., 2002; Swiercz, Worzfeld, & Offermanns, 2008). These findings show the intricate and pleiotropic functions of semaphorin–plexin signaling, which have been found in diverse models including cancer, microvascular, and immune diseases. Recent evidence suggests that the semaphorin–plexin system plays an important role in the cross-talk between osteoclasts and osteoblasts (Table 1; Hayashi et al., 2012). Therefore, comprehensive knowledge of the role of semaphorins in bone metabolism and homeostasis may provide novel insights into the neural regulation of bone metabolism.
| Targets | Validation | Site of expression | References |
|---|---|---|---|
| Sema3A | Osteopenia; intravenous infusion of recombinant SEMA3A increases bone mass | Osteoblasts | No Authors (1999) |
| Sema4D | Sema4d-knockout mice show increased bone mass; anti-SEMA4D antibody reduces bone loss | Osteoclasts | Ahn, Dubern, Lubrano-Berthelie, Clement, and Karsenty (2006) and Allison et al. (2009) |
| Sema6D | Loss of Plexin-A1–Sema6D show decreased osteoclasts | Osteoclasts | Amano, Arai, Goto, and Togari (2007) |
| Sema7A | Recombinant Sema7A increase the migration of osteoblast and the formation of mature osteoclasts | Osteoblasts and osteoclasts | Baldock et al. (2006) |
| Plexin-A1 | Plexin-A1-knockout mice have increased bone mass | Osteoblasts and osteoclasts | Amano et al. (2007) |
| Plexin-B1 | Plexin-A1-knockout mice have increased bone mass | Osteoblasts | Ahn et al. (2006) |
2.1 Semaphorin 3A
Semaphorin 3A (Sema3A) is a secreted semaphorin that is expressed in the olfactory epithelium through the interaction with neuropilin 1 (Nrp-1; Schwarting et al., 2000). Sema3A is involved in the repulsive growth cone guidance in the development of blood vessels and the nerve tissues (Potiron & Roche, 2005). With many nerves and blood vessels infiltrated in bones, the formation of the skeletal system bears many similarities of the cardiovascular and nervous systems, particularly in the context of cell differentiation and migration. Therefore, it is anticipated that Sema3A and its associated ligands are implicated in bone metabolism.
Sema3A-knockout mice have been first identified with abnormalities in the nervous system, the cardiovascular system, and especially in the skeletal system, with the manifestation of misalignment of the rib–sternum junction, partial rib duplications, and fusion of cervical bones (Behar, Golden, Mashimo, Schoen, & Fishman, 1996). Unlike the traditional treatment of humoral factors, which reduced bone synthesis and resorption at the same time, Sema3A was known to be a dual regulator of osteoblasts and osteoclasts, which exert stimulative effects on bone formation and suppressive effects on bone degradation simultaneously (Hayashi et al., 2012; Z. Li et al., 2017). In addition, Sema3A demonstrated a higher expression in the calvaria of the mouse than other members of semaphorins, and its functions mainly involved binding to Plexin-A1 and Nrp-1 (He & Tessier-Lavigne, 1997). The complex of Sema3A and Nrp-1 competed with the Plexin-A1 and Trem-2–DAP12 complex. This has activated a costimulatory pathway in the RANKL-induced osteoclast differentiation and caused the reduced differentiation of osteoclast precursor cells, and the repelling of osteoclast precursors through the Sema3A–Nrp-1 axis nullified RhoA activation (Kular, Tickner, Chim, & Xu, 2012). In Sema3A-deficient and Nrp-1Sema mice, the number of osteoblasts and bone formation was reduced, which suggests that autocrine Sema3A promotes osteoblast differentiation. Notably, Sema3A has been reported in the activation of the small GTPase Rac1 through FARP2, which led to a Wnt3a-induced signaling and stimulated osteoblast formation (Figure 1a; Hayashi et al., 2012). As such, bone regeneration has been enhanced with Sema3A treatment in the cortical bone defect mouse model (Nagashima et al., 2005).
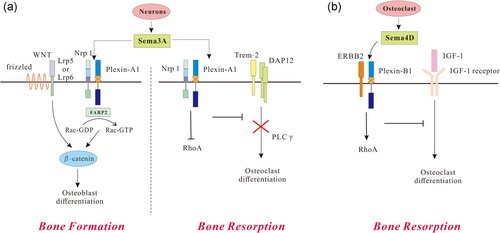
Role of semaphorins and their receptors in bone remodeling. (a) Semaphorin 3A (Sema3A) can induce bone formation by competing with the receptor complex consisting of triggering receptor expressed on myeloid cell 2 (Trem-2) and the adaptor protein DNAX activation protein of 12 kDa (DAP12) in osteoclasts or activating Wnt-induced signaling in osteoblasts. (b) Osteoclast-derived semaphorin 4D (Sema4D) can activate Plexin-B1-mediated and ERBB2-dependent RhoA expression which leads to osteoclast differentiation through insulin-like growth factor-1 (IGF-1). Lrp: low-density lipoprotein receptor-related protein; Nrp-1: neuropilin 1 [Color figure can be viewed at wileyonlinelibrary.com]
Sema3A has also been reported in the regulation of bone remodeling through the sensory nerve development instead of the direct effect on osteoblasts. In fact, the osteoblast-specific mice showed normal bone mass (Rodda & McMahon, 2006). In contrast, neuron-specific Sema3A-deficient mice, like Sema3A-deficient mice, were identified to have low bone mass due to increased bone resorption and decreased bone formation (Fukuda et al., 2013). As such, this neuronal Sema3A accounted for the bone loss in Sema3A-deficient mice (Fukuda et al., 2013). Interestingly, the supplement of Sema3A has been reported in the increase in the callus volume and bone mineral density (BMD) at 4 weeks postfracture, which induced callus ossification and remodeling at 8 weeks postfracture in osteoporotic rats (Y. Li, Yang, He, & Hu, 2015). As such, the effect of Sema3A on bone formation and bone resorption may potentially be useful in the regulation of bone turnover.
2.2 Semaphorin 4D
Semaphorin 4D (Sema4D) is a membrane-bound semaphorin that contains an aminoterminal sequence. It has been reported that Sema4D has been cleaved from the cell surface and is presented in soluble form (Wang et al., 2001). Interestingly, the homodimer of Sema4D showed similarities to that of integrin, a protein commonly expressed in osteoclasts (Janssen et al., 2010), and Sema4D is exclusively expressed by osteoclasts in the context of bone. In the process of bone resorption, Sema4D inhibited the mineralization and formation of osteoblasts and induced osteoclast formation (Dacquin et al., 2011; Negishi-Koga et al., 2011). Sema4D-knockout mice showed an increased bone mass, including accelerated bone formation rates and elevated osteoblast surface (Negishi-Koga et al., 2011). A similar phenotype has been observed in Plexin-B1-deficient mice, suggesting the role of Plexin-B1 as a functional receptor of Sema4D (Dacquin et al., 2011). An in vivo study demonstrated that Plexin-B1 contributed toward the ERBB2-dependent RhoA activation, which led to the inhibition of osteoblast differentiation by the suppression of IGF-1 (Figure 1b; Negishi-Koga et al., 2011). Consistently, Sema4D inhibited the phosphorylation of insulin receptor substrate 1 (IRS-1), a crucial downstream molecule of IGF-1 signaling, which is essential for osteoblast differentiation. The inhibition of Sema4D/Plexin-B1/RhoA/ROCK signaling by fluoride promoted the TGF-β1 expression and osteoblast proliferation. This suppressed the maturation and differentiation of osteoclasts and decreased bone absorption, which eventually caused skeletal fluorosis (Liu et al., 2015). It is noteworthy that the Sema4D-specific antibody has been reported to prevent bone loss by increasing osteoblastic bone formation without osteoclastic bone resorption (Negishi-Koga et al., 2011). Dacquin et al. (2011) have favored an extraosseous mechanism wherein Sema4D indirectly regulates bone resorption and explained the increased bone mass of Sema4D-knockout mice. Therefore, future studies need to clarify the mechanism underlying the effects of Sema4D and Plexin-B1 on bone biology.
2.3 Other semaphorin family members
Although most studies focused on Sema3A and Sema4D in the context of bone research, other semaphorin members, semaphorin 6D (Sema6D), and their receptors have been reported in bone homeostasis. Sema6D, a transmembrane semaphorin, can be activated by its receptor, Plexin-A1, which forms a receptor complex with vascular endothelial growth factor receptor 2 (VEGFR2; Toyofuku et al., 2004). Plexin-A1 binds to Trem-2 that associates with DAP12. These combinations induced MAPK and Akt signaling and PLCγ-calcium-triggered transcriptional regulation. In Trem-2- or DAP12-deficient mice, impaired osteoclast differentiation was observed (Kaifu et al., 2003; Koga et al., 2004). In addition, the phosphorylation of DAP12 was induced by the recombinant Sema6D that RAW264.7 cells expressed Trem-2, DAP12, and Plexin-A1. This Sema6D–Plexin-A1-regulated mediation of osteoclasts was influenced partially by Trem-2 and DAP12 (Takegahara et al., 2006). Plexin-A1-deficient mice showed impaired bone homeostasis and osteoporosis, and the loss of Plexin-A1 caused a decreased number of osteoclasts (Takegahara et al., 2006). Although the activity of osteoclast and bone resorption decreased, the expression of osteoclast differentiation markers showed no difference, suggesting a functional role of Plexin-A1 as the receptor of Sema6D in skeletal tissues.
Semaphorin 7A (Sema7A), also known as CD108, has also been verified in the bone physiology in vitro. The expression of Sema7A was identified in the process of osteoclast and osteoblast differentiation (Delorme, Saltel, Bonnelye, Jurdic, & Machuca-Gayet, 2005). The supplementation of recombinant Sema7A promoted the migration of osteoblast through integrin β1, which also increased the formation of mature osteoclasts (Delorme et al., 2005). This suggests a potential role of Sema7A that induced the migration of osteoclasts and osteoblasts in bone remodeling. In addition, the polymorphisms of Sema7A are associated with increased fracture and decreased BMD in postmenopausal women (Koh et al., 2006). As such, Sema7A is involved in the maintenance of bone homeostasis.
3 NEUROPEPTIDE Y
Neuropeptide Y (NPY) is composed of 36 amino acids and is abundantly expressed in the central and peripheral nervous systems. The important role of NPY in immune responses, endocrine activities, and energy homeostasis has been well established. In addition, the NPY-immunoreactive fibers are adjacent to bone marrow cells and bone lining (Malmström, 2001), which indicates the possibility of neural actions on skeletal tissues. The deletion of neuropeptide Y2 receptors caused elevated bone volume and accelerated bone formation rate (Baldock et al., 2002). The mutual effects of NPY and leptin on the regulation of energy homeostasis suggest the possible interaction in the control of bone formation (Ducy et al., 2000), and considerable progress has been made in the elucidation of NPY as a neural mediator in bone remodeling and homeostasis.
It has been reported that the overexpression of hypothalamic NPY showed antiosteogenic effects in the neural tissue of ARC (Allison et al., 2009; Baldock et al., 2005). In NPY-deficient mice, the bone volume of both trabecular and cortical bone increased with improved osteoblast activity (Baldock et al., 2009). Although the injection of NPY into the ARC showed decreased bone mass, the original phenotype was not restored completely, which implies other peripheral roles of NPY in the regulation of bone homeostasis (Baldock et al., 2009). The direct injection of NPY into the hippocampus demonstrated no change in bone mass, suggesting that NPY arise centrally from ARC and regulated bone homeostasis with site-specific effects (Baldock et al., 2005). As such, the hypothalamic NPY exerts catabolic effects on bone homeostasis by the inhibition of osteoblasts.
3.1 NPY receptors
NPY receptors, Y receptors, are a family of G-protein-coupled receptors and include five subtypes: Y1, Y2, Y4, Y5, and Y6 (Lin, Boey, & Herzog, 2004). Each receptor has specific distributions across central and peripheral tissues (Table 2). Y1 receptors are mainly distributed in the brain and are particularly expressed in the paraventricular nucleus (PVN; Parker & Herzog, 1999). Y2 receptors are predominantly expressed in ARC and Y4 is expressed both in the brain and in peripheral tissues (Blomqvist & Herzog, 1997). Y5 is identified in the CNS, and consistently colocalizes with Y1 and Y6 receptors (Parker & Herzog, 1999; Weinberg et al., 1996).
| Genes | Phenotype | Site of expression | References |
|---|---|---|---|
| Y1 receptor | Increased bone volume | Osteoblasts | Baldock et al. (2007) |
| Y2 receptor | Increased bone volume; decreased activity of osteoblasts and Y1 receptors | Hypothalamus | Baldock et al. (2005; 2009) |
| Y1 and Y2 receptors | Increased bone volume | Central and peripheral tissues | Baldock et al. (2002) |
| Y2 and Y4 receptors | Increased bone volume | Hypothalamus, adipose tissues | Bartell et al. (2011) |
| NPY | Increased bone volume | Central and peripheral tissues | Behar, Golden, Mashimo, Schoen, and Fishman (1996) |
| PYY | Decreased bone volume | No report | Beltramo et al. (2003) |
- Note. NPY: neuropeptide Y; PYY: peptide YY.
Y2 receptors are highly expressed in the hippocampus, hypothalamus, and brain stem, which represent almost two-thirds of the binding capability of NPY in CNS (Lin et al., 2005). In Y2-knockout mice, trabecular bone volume increased with enhanced osteoblast activity. Circulating hormones remained unchanged in the process of bone formation (Baldock et al., 2002). Although Y2 receptors are expressed mainly in NPY-ergic neurons, bone regulation via non-NPY neurons was also identified. Targeted deletion of Y2 receptors in NPY-ergic neurons induced a moderate increase in trabecular bone volume (Shi et al., 2010). The peripheral Y2 receptors are not involved in bone regulation; no significant changes in bone mass have been identified in mice with conditional deletion of these peripheral Y2 receptors (Shi et al., 2011). These findings confirm that the neuronal mechanism of Y2 receptors plays important roles in the regulation of bone formation.
In the CNS, Y1 receptors are particularly expressed in the PVN. The alterations in NPY of the ARC alter Y1 receptor signaling in the PVN (Figure 2; Parker & Herzog, 1999). The germline deletion of the Y1 receptor showed elevated bone volume with enhanced osteoblast activity; however, the specific deletion of the Y1 receptor in PVN did not affect bone homeostasis. Intriguingly, the loss of both Y1 and Y2 receptors displayed no additive effects on the phenotype of bone tissues, suggesting a common pathway shared by Y1 and Y2 receptors in bone homeostasis (Baldock et al., 2007). In addition, the increased bone mass produced by Y1 receptor deletion has been reported to be independent of hypothalamic signaling (Baldock et al., 2007). Late osteoblast-specific knockout of Y1 receptors exhibited an increase in bone mass (Lee et al., 2011) and the loss of Y1 in early osteoblast differentiation further confirmed the importance of Y1 receptors in the inhibition of mineral deposition and the reinforced negative effects of NPY on bone cells (Lee et al., 2015).
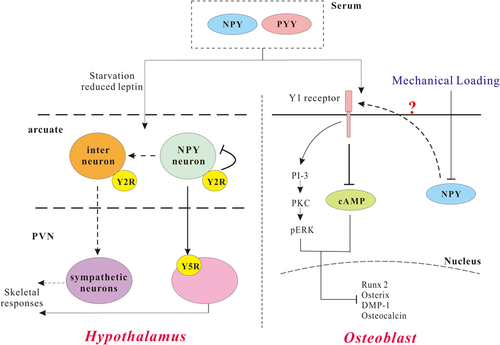
The NPY system as key regulators of bone homeostasis. In the hypothalamus, NPY can regulate skeletal responses through Y5 receptors in the PVN. The NPY neuron can also regulate sympathetic neurons through interneurons to modulate skeletal responses. In the osteoblast, NPY or PYY can activate Y1 receptors. Y1 receptors can inhibit cAMP pathway and activate the PKC and phosphorylation of ERK, both of which will lead to inhibited osteoblastic differentiation. NPY or PYY can activate Y1 receptors to inhibit differentiation. cAMP: cyclic adenosine monophosphate; ERK: extracellular signal-regulated kinase; NPY: neuropeptide Y; PI-3: phosphatidylinositol-3-kinase; PKC: protein kinase C; PVN: paraventricular nucleus; PYY: peptide YY [Color figure can be viewed at wileyonlinelibrary.com]
In addition to Y1 and Y2 receptors, Y4 receptors, which have a high affinity to the pancreatic polypeptide (PP), have also been implicated in the modulation of bone. Germline knockout of Y4 receptors alone did not lead to any alteration in bone mass and the double deletion of both Y2 and Y4 receptors demonstrated a synergistic increase in trabecular bone mass (Sainsbury et al., 2003). Interestingly, this phenotype did not exist in female Y2 and Y4 deletion mice, which may possibly be associated with the decreased leptin levels in male mice rather than the females (Sainsbury et al., 2003), suggesting an additive role of Y2 receptors and leptin in bone.
To date, the role of Y5 and Y6 receptors in bone homeostasis remains unclear. Y5 receptors colocalize with Y1 receptors in the CNS that share a common promoter region. This suggests the role of Y5 receptors in the transduction of NPY-ergic signals from ARC (Herzog et al., 1997). Although not constantly expressed in osteoblasts or bone marrow stromal cells (BMSCs), a significant decrease in Y5 receptors, as well as NPY-induced cell proliferation, was reported in BMSCs from aging rats (Igura, Haider, Ahmed, Sheriff, & Ashraf, 2011; Lee et al., 2010). This suggests the essential role of Y5 in the bone homeostasis in the self-renewal of bone marrow progenitor cells. Although the Y6 receptors are truncated with no significant functions, Y6 receptors share 51% of the sequence with human Y1 receptors (Blomqvist & Herzog, 1997). Further efforts are needed to elucidate the role of Y6 receptors in bone development, homeostasis, and diseases.
3.2 NPY ligands
Although the critical role of Y receptor signaling in bone homeostasis has been verified, it remains elusive why the activity of osteoblasts in NPY-deficient mice cannot be restored by the replenishment of NPY (Baldock et al., 2009). The effect of nonhypothalamic NPY on bone formation is more direct neuroendocrine and independent of the hypothalamic NPY mechanism. For example, NPY in osteoblastic cells prohibited the response of cyclic adenosine monophosphate (cAMP) to noradrenaline and parathyroid hormone (PTH; Igwe et al., 2009). In addition, the injection of NPY in wild-type mice decreased the number of osteoblasts, which cannot be validated in Y1-receptor-deficient mice (Baldock et al., 2007). These findings indicated a role of Y receptors in bone cells and an inhibitive role of peripheral NPY in the periphery. This peripheral NPY may affect bone cells in an autocrine/paracrine manner, rather than Y1 receptors on osteoblasts. In addition to the role of NPY in osteoblastic bone formation, NPY mediated osteoclastic bone resorption as well. NPY has been proven to prohibit activator of RANKL-induced osteoclastogenesis by suppressing PTH and isoprenaline expression through Y1 receptors in mouse BMSCs (Amano, Arai, Goto, & Togari, 2007). NPY tentatively increased the expression of osteoprotegerin, a decoy receptor, which suppresses RANKL transduction (Teixeira et al., 2009). As such, the regulation of NPY on osteoclast activity still remains unclear, although it has been confirmed in an indirect modulation on the behaviors of osteoblasts.
Other NPY-associated peptides contain PP and peptide YY (PYY) that are made up of 36 amino acids and share a common PP-fold, which is key in the mediation of the Y receptors binding (Nordmann, Blommers, Fretz, Arvinte, & Drake, 1999). It has been reported that neither transgenic PP nor PP-deletion mouse models showed the altered phenotype of bone. PYY, in contrast, has been reported to regulate bone metabolism and energy homeostasis as NPY (van der Velde, Delhanty, van der Eerden, van der Lely, & van Leeuwen, 2008). High levels of PYY caused a decrease in BMD of women with anorexia nervosa, which is consistent with the results of NPY- and Y receptor-deficient mice studies (Utz et al., 2008). In addition, the concentration of PYY showed a negative association with the bone mass (Scheid et al., 2011), and the loss of PYY in mouse models demonstrated an osteopenic phenotype with decreased bone mass and strength (Wortley et al., 2007).
4 LEPTIN
Leptin is an adipokine consisting of 167 amino acids, which is involved in the maintenance of energy homeostasis. It is secreted primarily by white adipocytes into the circulation and acts as an adipostat in proportion to adipose mass (Boden, Chen, Mozzoli, & Ryan, 1996). The level of the circulating leptin generally shows the amount of energy in adipose and obesity, and this level has also been correlated with insulin and alcohol intake. Although the receptor of leptin was identified in osteoblasts, the effects of leptin on bone are closely associated with the hypothalamus (Figure 3a).
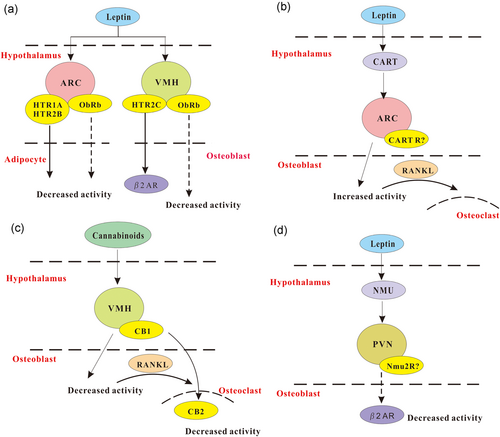
Model of neural peptides in regulating bone remodeling. (a) Leptin and serotonin signaling. Leptin can modulate bone mass through its receptors (ObRb) by stimulating serotonergic neurons. Serotonin can bind to HTR2C receptors of VMH neurons to regulate bone mass, and HTR1A and 2B receptors of ARC neurons to mediate adipocyte activity. (b) CART signaling. CART expression is mediated by serum leptin. Decreased CART can induce an increase in bone resorption via receptor activator of nuclear factor-κB ligand (RANKL), whereas elevated CART expression in the hypothalamus results in increased bone mass. (c) Cannabinoids signaling. The cannabinoid receptor (CB1) is mainly expressed in VMH, and CB2 is distributed in the peripheral tissues like osteoclasts. The mechanism of CB1 and CB2 receptors in regulating the effects of cannabinoid on bone remains elusive. (d) Neuromedin U (NMU) signaling. NMU is a leptin-dependent regulator of bone mass, which acts in the PVN. Efferent sympathetic signaling acts on the molecular clock in the bone to regulate bone mass. ARC: arcuate nucleus; CART: cocaine- and amphetamine-regulated transcript; PVN: paraventricular nucleus; VMH: ventromedial hypothalamus [Color figure can be viewed at wileyonlinelibrary.com]
4.1 Effects of leptin on bone homeostasis: Study from mouse models
Leptin deficiency (ob/ob) and its receptor inactivation (db/db) in the mutant mice showed multiple phenotypes including alterations of the skeletal system, with elevated bone volume in ob/ob, and increased turnover of bone favoring bone formation (Ducy et al., 2000). The loss of leptin exhibited reduced mineralization, shorter femur length, and lower cortical thickness and BMD in the peripheral bone with more adipocytes in the bone marrow (Hamrick, Pennington, Newton, Xie, & Isales, 2004). The intracerebroventricular infusion of leptin-induced bone loss in leptin-null mice, indicating the inhibitive role of leptin in bone formation through CNS (Ducy et al., 2000). Conversely, the destruction of leptin caused the ablation of adrenergic signaling and led to a leptin-resistant high cancellous bone mass (Takeda et al., 2002). The β2-adrenergic agonist-mediated osteoblast proliferation has been reported in the decrease of bone mass in leptin-deficient and wild-type mice and the increase of bone mass in wild-type and ovariectomized mice (Takeda et al., 2002), suggesting a leptin-dependent neural regulation of bone formation. In addition, in agreement with the findings in ob/ob mice, the deletion of the β2-adrenergic receptor increased cancellous bone volume (Elefteriou et al., 2005).
Compared to the effects of central leptin on cancellous and cortical bone in ob/ob mice, the peripheral leptin on cortical bone mass showed opposite results. Increased bone volume, mineralization, cortical thickness, and BMD were observed in the axial bone of leptin-deletion mice with decreased adipocytes in the marrow (Hamrick et al., 2004). More recently, the intracerebroventricular injection of leptin increased bone formation with enhanced expression of pro-osteogenic factors in ob/ob mice (Bartell et al., 2011). The hypothalamic leptin gene therapy increased the perimeter of osteoblast-lined bone in ob/ob mice (Turner et al., 2013). Importantly, db/db mice showed a mild and generalized osteopetrotic-like phenotype and decreased serum markers of bone turnover significantly. This suggests that leptin increases the number and activity of osteoblasts through peripheral pathways (Turner et al., 2013). As such, leptin has been confirmed to play a role in the increase of BMD, bone area, bone mineral content, mineral apposition rate, and marrow adipocytes number in the axial skeleton.
Interestingly, central control of bone homeostasis by the hypothalamus has been identified in both leptin and NPY receptor signaling. A close link between leptin and NPY has been reported in the hypothalamus, where NPY served as a downstream effector of leptin-deficient starvation signaling. Notably, the leptin receptor has been observed to be coexpressed in a large proportion of NPY-ergic neurons in ARC (Mercer et al., 1996), and the decrease of leptin led to an increase in NPY expression due to the starvation or in ob/ob mice (Spanswick et al., 1997; Wilding et al., 1993). Double knockout of the Y2 receptor and leptin exerted no additive effect on cancellous bone volume, which suggests that the pathways shared by NPY and leptin are overlapped in the mediation of bone mass (Baldock et al., 2006). Continuous administration of NPY causes an increase in ob/ob and decrease in cancellous bone volume. This indicates that leptin used different pathways together with NPY in the regulation of bone mass (Ducy et al., 2000). Moreover, the inactivation of leptin receptor in NPY-ergic neurons induced an elevated level of NPY in ob/ob mice, and the deletion of NPY in ob/ob mice showed an effect in the correction of the cortical deficit. This confirmed the role of NPY in cortical deficiency and the contribution of the sympathetic nervous system in the increase of cancellous bone volume in ob/ob mice (Erickson, Hollopeter, & Palmiter, 1996).
4.2 Effects of leptin on bone diseases: Study from humans
Individuals with anorexia nervosa showed decreased leptin levels, which are strongly related to low body fat (Grinspoon et al., 1996). Although multiple factors are involved in abnormalities of bone in anorexia nervosa, the levels of leptin have been reported to play a prominent role in bone health, which is positively correlated to bone microarchitecture and structural integrity (Lawson et al., 2010). Notably, low levels of leptin with abnormal microarchitecture showed a higher risk of fracture, even for patients with normal BMD. In addition, bone abnormalities have been identified in obese patients with a state of leptin resistance. Although obesity has been believed to be protective against bone fracture and osteoporosis, recent findings indicated that such benefits might not occur with mild sarcopenia and inflammation (Caffarelli, Alessi, Nuti, & Gonnelli, 2014). At a higher level of adiposity, leptin triggered inflammatory pathways in osteoblasts that reduced bone mass (Yang et al., 2014).
The levels of leptin have also been identified to be markedly lower in women with hypothalamic amenorrhea. This is associated with the reduced BMD and high risk of low-energy bone fractures (Chou & Mantzoros, 2014). The administration of leptin to these patients showed elevated levels of IGF-1, estrogen, and thyroxine, all of which confer beneficial effects on bone. There was also an increase in the expression of bone formation markers including osteocalcin and alkaline phosphatase, which suggests the osteoanabolic role of leptin (Welt et al., 2004). As such, leptin has been confirmed in the re-establishment of the normal state of bone metabolism and functionalities, and further studies are still needed to elucidate the complicated mechanism of leptin in bone metabolism.
5 OTHER NEURAL PEPTIDES AS A REGULATOR OF BONE
5.1 The melanocortin system
Melanocortins are a complex family composed of five melanocortin receptors (MCRs), which are known as G-protein coupled receptors MC1-5, and a number of endogenous ligands (Beltramo et al., 2003). Among the five receptors, the melanocortin 4 receptor (MC4R), which is mainly expressed in the hypothalamus, plays a major role in the regulation of bone homeostasis. MC4R deficiency causes a reduction of bone resorption and high BMD levels (Farooqi et al., 2000). It is noteworthy that the correction of the obesity did not reverse the increased BMD level, which is typical of MC4R deficiency (Farooqi et al., 2000). In addition, the administration of adrenocorticotropic hormone (ACTH) prohibited the glucocorticoid-induced osteonecrosis of the femur and increased the production of VEGF, which promoted the survival and maturation of osteoblasts (Zaidi et al., 2010). In addition, MC2R induced the secretion and expression of ACTH, which was involved in the modulation of osteoblast differentiation and bone mass (Isales, Zaidi, & Blair, 2010).
5.2 Cocaine- and amphetamine-regulated transcript
Cocaine- and amphetamine-regulated transcript (CART) contains two peptides, CART I and CART II. ob/ob Mice showed the effects of leptin on bone resorption through CART, in which decreased CART expression and increased bone resorption was observed, and the supplementation of leptin restored the decreased expression of CART (Figure 3b; Kristensen et al., 1998). Conversely, the loss of CART showed increased bone resorption and enhanced osteopenic phenotype, and elevated expression of RANKL in mice (Elefteriou et al., 2005). Intriguingly, the effect of CART is not cell autonomous and the alteration of central CART was mediated by local RANKL/OPG mechanism (Elefteriou et al., 2005). Consistently, MC4R deficiency also showed increased CART expression and bone mass due to the inhibition of osteoclasts (Ahn, Dubern, Lubrano-Berthelier, Clement, & Karsenty, 2006). The loss of one or two copies of CART showed significantly reduced bone mass in MC4R mutant mice (Elefteriou et al., 2005). Altogether, the CART signaling may confer a central convergent role of leptin and melanocortin signaling, both of which showed an effect in the control of bone homeostasis.
5.3 Cannabinoid system
Endocannabinoids function primarily through CB1 and CB2, which are receptors of cannabinoid and bind to inhibitory G proteins like NPY receptors (Howlett et al., 2002). CB1 is primarily expressed in the CNS and CB2 is expressed predominantly in the periphery (Figure 3c). It has been reported that cannabinoid receptors are abundantly expressed in osteoblasts and osteoclasts, which control bone homeostasis via central regulation. The inhibition of the CB1 receptor showed increased BMD to avoid bone loss induced by ovariectomy (Idris et al., 2005). Interestingly, antagonists of cannabinoid receptor prevented osteoclastic bone resorption, and the deficiency of the CB1 receptor resisted such preventive effects. The inhibitory action of cannabinoid antagonism on osteoclasts is partly induced by the CB1 receptor (Idris et al., 2005).
CB2-deficient mice showed an accelerated cancellous bone loss and cortical expansion with aging, which also exhibited increased bone formation and mineral appositional rate (Ofek et al., 2006). In addition, CB2 receptors are expressed both in osteoclasts and in osteoblasts, and CB2 signaling inhibited monocytes/osteoclasts and stimulated stromal cells/osteoblasts. This contributed to the maintenance of bone mass (Ofek et al., 2006).
5.4 Neuromedin U
Neuromedin U (NMU) is mostly expressed in the hypothalamus and the small intestine. It is considered to be the target of sympathetic activation (Brighton, Szekeres, & Willars, 2004). The deletion of NMU induced the onset of bone formation and an increase of bone mass through a central hypothalamic pathway in mice (Sato et al., 2007). NMU acted in the CNS to modulate bone remodeling, rather than directly on bone cells, in the regulation of bone remodeling (Figure 3d; Sato et al., 2007). It is noteworthy that leptin- or sympathetic nervous system-mediated suppression of bone formation was abrogated in NMU-deficient mice, and the treatment of NMU receptor agonist decreased the bone mass of ob/ob mice. This shows the role of NMU as a leptin-dependent central regulator of bone mass (Sato et al., 2007). Collectively, NMU signaling provides a novel aspect of the transmission of leptin to bone.
5.5 5-Hydroxytryptamine system
Although 5-hydroxytryptamine (5-HT) has primarily been identified in the CNS, the gastrointestinal tract, and the cardiovascular system, it has been identified in the skeletal system (Warden, Robling, Sanders, Bliziotes, & Turner, 2005). 5-HT receptors have been discovered in osteoblasts and periosteal fibroblasts, which contain osteoblast precursor cells (Bliziotes, Gunness, Eshleman, & Wiren, 2002), and 5-HT increased the activity of AP-1 in osteoblasts (Bliziotes, Eshleman, Zhang, & Wiren, 2001). Interestingly, the responses of osteoblasts to mechanical force were mediated by a 5-HT analogue (Westbroek, van der Plas, de Rooij, Klein-Nulend, & Nijweide, 2001), which increased cAMP and PGE2 activity in osteocytes (Bliziotes et al., 2002). The 5-HT transporter was shown to influence osteoclastic differentiation without the activation of osteoclasts (Bliziotes et al., 2002), indicating the indirect effect in bone resorption.
6 CONCLUSION AND FUTURE PERSPECTIVES
The involvement of the CNS in the regulation of basal functions of the body has been appreciated for many years. Recent progress has highlighted the influence of neural signals on bone metabolism, homeostasis, and diseases. The burgeoning field of neural regulation in bone has underlined a novel paradigm emerging in bone and neural research. The identification of these neural pathways has proposed a new role of central regulation in peripheral tissue, which is complementary to the endocrine activity. This demonstrated a sophisticated network in bone mass regulation. Conversely, the dissection of neural receptors on bone cells will affect the bony anabolic and catabolic activities and this could be a novel potential candidate.
Although some efferent neural signals from the brain to bone have been discovered, our knowledge of the feedback control and related mechanism is still limited. The context, in which these neural signaling is prevailing in bone regulation, should be elucidated both physiologically and pathologically. There have been many achievements in mouse models; more integrated studies in humans are needed. In addition, the translation of laboratory findings into clinical applications still remains to be done because of the intricate nature of the neural circuitry and the complexity of multiple endocrine signaling pathways. As such, signaling occurring from the bone to other systems, which is absent from the breadth of those coming from the CNS, may also lead to more beneficial therapeutics.
Despite the many challenges ahead, this field of neural regulation in peripheral bone tissue has advanced our knowledge of the intricacy and sophistication of the bone tissue. The advancement of this understanding will improve the modulation of bone diseases, and facilitate the concept of the bone as a component of the whole-body homeostatic network and potentially contribute toward a broader perspective of bone disorders.
ACKNOWLEDGMENTS
The authors thank the National Natural Science Foundation of China (nos. 81472078, 81471164, 31671116, 31761163005); Key Research Program of Frontier Sciences of CAS (QYZDB-SSW-SMC056); the External Cooperation Program of the Chinese Academy of Sciences (172644KYSB20160057); and Shenzhen Basic Research (grant nos. JCYJ20170307100446585, JSGG20160429190521240, JCYJ20160429190927063, JCYJ20170413164535041).
CONFLICTS OF INTEREST
The authors declare that there are no conflicts of interest.




