Shear stress upregulates regeneration-related immediate early genes in liver progenitors in 3D ECM-like microenvironments
Abstract
The role of fluid stresses in activating the hepatic stem/progenitor cell regenerative response is not well understood. This study hypothesized that immediate early genes (IEGs) with known links to liver regeneration will be upregulated in liver progenitor cells (LPCs) exposed to in vitro shear stresses on the order of those produced from elevated interstitial flow after partial hepatectomy. The objectives were: (1) to develop a shear flow chamber for application of fluid stress to LPCs in 3D culture; and (2) to determine the effects of fluid stress on IEG expression in LPCs. Two hours of shear stress exposure at ∼4 dyn/cm2 was applied to LPCs embedded individually or as 3D spheroids within a hyaluronic acid/collagen I hydrogel. Results were compared against static controls. Quantitative reverse transcriptase polymerase chain reaction was used to evaluate the effect of experimental treatments on gene expression. Twenty-nine genes were analyzed, including IEGs and other genes linked to liver regeneration. Four IEGs (CFOS, IP10, MKP1, ALB) and three other regeneration-related genes (WNT, VEGF, EpCAM) were significantly upregulated in LPCs in response to fluid mechanical stress. LPCs maintained an early to intermediate stage of differentiation in spheroid culture in the absence of the hydrogel, and addition of the gel initiated cholangiocyte differentiation programs which were abrogated by the onset of flow. Collectively the flow-upregulated genes fit the pattern of an LPC-mediated proliferative/regenerative response. These results suggest that fluid stresses are potentially important regulators of the LPC-mediated regeneration response in liver.
Abbreviations
-
- 2-AAF
-
- 2-N-acetylaminofluorene
-
- BMOL
-
- bipotent murine oval liver cell
-
- ECM
-
- extracellular matrix
-
- HA
-
- hyaluronic acid
-
- IEG
-
- immediate early gene
-
- IS
-
- InSight Liver Maintenance medium
-
- LPC
-
- liver progenitor cell
-
- PHx
-
- partial hepatectomy
-
- qRT PCR
-
- quantitative reverse transciptase polymerase chain reaction
-
- WE
-
- Williams E medium
1 INTRODUCTION
The putative hepatic stem cell compartment is located in or near the Canals of Hering of postnatal livers (Lanzoni, Cardinale, & Carpino, 2016; Reid, 2015; Turner et al., 2011). Hepatic stem cells (HSCs) give rise to liver progenitor cells (LPCs), the diploid bipotent cells that give rise to committed hepatocytic and cholangiocytic progenitors (Turner et al., 2011). LPCs make up <0.01% of parenchymal cells in adult livers, but they are known to expand during regenerative processes associated with diseases such as cirrhosis and HBV infection (Feng et al., 2012; Turner et al., 2011).
The regenerative capacity of adult liver has been the subject of intense research, motivated by the prevalence of liver disease and the donor organ shortage. In healthy liver subjected to partial hepatectomy (resection of ∼70% of liver mass), quiescent hepatocytes and other liver cells can re-enter the cell cycle and divide until liver mass is restored (Michalopoulos, 2007; Santoni-Rugiu, Jelnes, Thorgeirsson, & Bisgaard, 2005). Although actively debated, multiple studies suggest that when hepatocyte proliferation is blocked, such as by drugs (Evarts, Hu, Fujio, Marsden, & Thorgeirsson, 1993) or pervasive hepatocyte ablation (Choi, Ninov, Stainier, & Shin, 2014; Lu et al., 2015), the hepatic stem/progenitor cell compartment can undergo transit amplification and contribute extensively to restoring hepatocyte mass and repairing the injured organ (Itoh, 2016; Lanzoni et al., 2016; Reid, 2015). Severely impaired hepatocyte proliferative ability is a common feature of human liver diseases (Lanzoni et al., 2016), due to cellular senescence (Williams, Clouston, & Forbes, 2014) or cell cycle arrest (Nobili et al., 2012). Thus, activation and control of the hepatic stem/progenitor cell regenerative response has attracted attention due to its potential for therapeutic applications to human liver disease (Santoni-Rugiu et al., 2005).
Two rat models of liver regeneration are 70% partial hepatectomy (PHx) alone, and 70% PHx preceded by 2-N-acetylaminoflourene (2-AAF) intoxication. PHx alone prompts hepatocytes and other differentiated cells in the liver remnant to proliferate until mass and function are restored (Michalopoulos, 2007). In contrast, the 2-AAF/PHx model is among the most reliable progenitor cell induction models of liver regeneration, as 2-AAF completely blocks rat hepatocyte proliferation and transit amplification of the progenitor cell compartment is observed (Itoh, 2016; Santoni-Rugiu et al., 2005). In both models, PHx produces profound hemodynamic changes within the liver remnant (Grisham, 2009). It has been theorized that the large increase in portal flow per unit of residual tissue mass may be among the earliest triggers of regeneration (Grisham, 2009; Michalopoulos, 2007), at least in regeneration models involving PHx. It is unknown whether a hemodynamic “triggering” mechanism would be attributable to mechanical factors, hypoxic conditions, changes in growth factor or cytokine concentrations, or other effects associated with the sudden increase in portal flow. However, some evidence suggests that mechanical factors may play a role. For example, increased fluid stresses after PHx were reported to trigger hepatocyte-driven liver regeneration via shear stress-induced signaling cascades involving nitric oxide and prostaglandin (Mei & Thevananther, 2011; Schoen Smith & Lautt, 2005). If portal vein pressure is prevented from rising following PHx, increased hepatocyte apoptosis and deficient activation of HGF are observed (Marubashi et al., 2004; Michalopoulos, 2007). Lastly, a study of rat liver ultrastructure post-PHx revealed sinusoid widening, enlarged sinusoidal fenestrations, and widening of intercellular spaces and spaces of Disse 6 hr after PHx, which were attributed to increased portal pressure as a consequence of PHx (Morsiani, Aleotti, & Ricci, 1998).
The examples cited above raise the possibility that altered fluid dynamics post-PHx may similarly contribute to initiating progenitor-cell driven regenerative processes. In this work, we hypothesized that fluid dynamic changes in the LPC niche play a role in activating the regenerative response of the LPC compartment. Specifically, we hypothesized that immediate early genes (IEGs) with known links to liver regeneration will be upregulated in LPCs exposed to in vitro shear stresses on the order of those produced after PHx. IEGs are a class of genes whose transcription is activated within minutes of an inducing stimulus, through mechanisms independent of protein synthesis (Taub, 1996). Since fluid stresses applied to cells in 2D-monolayer culture are a poor representation of LPCs’ native microenvironment, we developed ECM-like gels coupled with hanging drop spheroid culture methods to create more physiologically realistic environments for flow experiments. Thus, the study objectives were: (1) to develop a shear flow chamber that mimics aspects of LPC niche ECM and permits application of fluid stress to LPCs in 3D culture; and (2) to determine the effects of fluid stress on IEG expression in LPCs.
2 MATERIALS AND METHODS
2.1 Overview
Bipotent murine oval liver (BMOL) cells, a well-established LPC line (Tirnitz-Parker, Tonkin, Knight, Olynyk, & Yeoh, 2007), were used. Cells were studied under four conditions: 2D culture on tissue-culture plastic (2D); 3D spheroid culture using hanging drop methods (3D); 2D cultured cells suspended in ECM (ECM2D); and 3D spheroids suspended in ECM (ECM3D). Quantitative reverse-transcriptase polymerase chain reaction (qRT-PCR) was used to evaluate the effect of culture geometry (2D, 3D, ECM2D, ECM3D) on LPC gene expression. Experiments and technical replicates were done in triplicate.
For mechanosensitivity experiments, interstitial flow was applied to cells in ECM2D and ECM3D conditions using a custom flow chamber. QRT-PCR was used to evaluate the effect of experimental treatments on LPC gene expression. Twenty-nine genes were analyzed, including IEGs linked to hepatic regeneration. Experiments and technical replicates were done in triplicate. In addition to murine LPC experiments, separate experiments were conducted in duplicate on primary human liver progenitor cells exposed to shear stress.
2.2 Cell culture
LPCs were cultured in Williams’ E medium (WE) (Fisher, Waltham, MA), prepared as described (Recker, Bukovec, & Sparks, 2015; Tirnitz-Parker et al., 2007), in 25 cm2 vented culture flasks at 37 °C and 5% CO2 for 2 weeks prior to experiments. Media was changed every 2 days and cells were passaged every 4 days. Plate-cultured (2D) cells were used at 90% confluency.
LPC spheroids (3D) were prepared in GravityPLUS hanging drop culture plates and GravityTRAP plates (InSphero, Brunswick, ME) using InSight Liver Maintenance medium (IS) (InSphero). 2D-cultured cells were transferred to GravityPlus plates at 20,000 cells/40 µl media. Cells were cultured in GravityPlus plates for 4 days to form spheroids and transferred into GravityTRAP plates. Spheroids were cultured for 2 days before being used for experiments.
Human fetal liver cells (hFLCs) were isolated from livers at 18–20 weeks gestation as described (Schmelzer et al., 2007), in accordance with IRB approved protocol (Wake Forest University Health Sciences). Cells were cultured in Kubota media (KM) (Schmelzer et al., 2007) and seeded at 10,000 cells/mm2 on 25 mm coverslips coated with collagen IV (25 µg/cm2) and laminin (5 µg/cm2).
2.3 Extracellular matrix (ECM) gel
ECM-like hydrogels for interstitial flow were prepared from collagen I and thiol-modified hyaluronic acid (HA), as gels composed of these ECM components can sustain hepatic progenitors at the hepatoblast lineage stage (Turner et al., 2008). Collagen I (Life Technologies, Grand Island, NY) was used at a stock concentration of 3 mg/ml (0.3% concentration in media), Hystem HA hydrogel (ESI Bio, Alameda, CA) was dissolved in IS culture media at a concentration of 2.5 mg/ml, and PEGSSDA (ESI Bio) was dissolved in degassed water (ESI Bio) at 10 mg/ml concentration. Prior to an experiment, 10 ml of 10× concentrated PBS was prepared with 80 mg of NaCl, 2 mg of KCl, 14.4 mg of Na2HPO4, and 2.4 mg of KH2PO4 in 8 ml of distilled water, and pH was adjusted to 7.4 with HCl. The 10× PBS was then autoclaved for sterilization. The volume of each component in 1 ml of ECM hydrogel was: 670 µl of Collagen I, 170.4 µl of HA, 42.6 µl of PEGSSDA, and 50 µl of 10× PBS. The pH of the ECM was adjusted to 7.4 by adding about 17 µl of 1 m NaOH. The ECM components were mixed on ice prior to suspending 2D cultured cells or 3D spheroids in the ECM. Individually suspended 2D cultured cells in ECM (ECM2D) and hanging drop cultured spheroids suspended in ECM (ECM3D) were soaked in IS media for 24 hr before application of the experimental treatment (flow). Hyaluronidase/collagenase (STEMCELL Technologies, Vancouver) was used at 1:30 dilution rate in IS media to permit cell extraction from the gel following shear flow exposure.
2.4 Cell viability assay
LIVE/DEAD assay (ThermoScientific, Wilmington, DE) was performed according to manufacturer's protocol. DAPI (200 nm) was used to stain cell nuclei. Microscopy images were taken with a Zeiss AXIO-Vert-A1 microscope. Cell viability was quantified by converting red and green image channels to grayscale, auto-thresholding to produce binary images, and measuring the area ratio of live (green) to dead (red) particles (ImageJ software, U.S. National Institutes of Health).
2.5 Immunofluorescence microscopy
Immunofluorescence microscopy was used to image cells in two conditions: 2D flat plate culture (2DWE) and 3D spheroid culture (3D). Cells were double-stained for co-expression of the biliary marker cytokeratin 19 (CK19) and the mature hepatic marker albumin (ALB), or for co-expression of ALB and hepatocyte nuclear factor 6 (HNF6), a transcription factor important in biliary development (Lucendo-Villarin et al., 2012; Tirnitz-Parker et al., 2007;). Details of the immunofluorescence staining and imaging protocol are provided in the Supplemental Information.
2.6 QRT-PCR
Five target genes (ALB, HNF4α, HNF6, CK19, SOX9) were analyzed in static culture experiments to establish LPC behaviors under different culture geometries in the absence of flow. These genes included albumin (ALB), hepatocyte nuclear factor 4-alpha (HNF4α), hepatocyte nuclear factor 6 (HNF6), cytokeratin 19 (CK19), and SRY-Box 9 (SOX9), since bipotent BMOL cells are known to simultaneously express genes associated with both hepatocytic and cholangiocytic cell fates (Tirnitz-Parker et al., 2007; Turner et al., 2011). For LPC flow experiments, 22 additional target genes were analyzed (Supplementary Tables S1 and S2), representing transcription factors and IEGs upregulated during liver regeneration, morphogens associated with liver development and repair, and growth factors and chemokines linked to the ductular reaction. For hFLC flow experiments, six genes were analyzed (CFOS, LEF1, CDH1, NFKB, β-catenin).
Cells cultured under baseline static conditions (2D flat plate culture in WE media, termed 2DWE) served as the control condition. GAPDH and PPIA were used as reference genes. Primers (Supplementary Table S1) were purchased from Integrated DNA Technologies (Coralville, IA). RNA from each experimental condition was immediately extracted with a Maxwell 16 instrument and Maxwell 16 LEV simplyRNA tissue kit (Promega, Madison, WI). The concentration of mRNA was measured with a NanoDrop 2000c (ThermoScientific), and RNA integrity was assessed with an Agilent 2100 Bioanalyzer and Agilent RNA 6000 Pico kit (Agilent Technologies, Santa Clara, CA). RNA integrity of five was used as a threshold to ensure stable gene quantification results (Fleige & Pfaffl, 2006). QScript cDNA super mix (Quanta Bioscience, Gaithersburg, MD) and a 2720 Thermal Cycler (ThermoScientific) were used to convert mRNA to cDNA. The qScript-mRNA mixture was subjected to 5 min of priming at 25 °C, followed by 20 min of reverse transcription at 42 °C, and reverse transcriptase inactivation for 5 min at 85 °C. Quantitative RT-PCR was conducted using a CFX Connect Real Time PCR Detection system (Bio Rad, Hercules, CA) with GoTaq qPCR Master Mix (Promega). For the qRT-PCR protocol, cDNA-enzyme mix was held for 10 min at 95 °C, then cycles of 15 s at 95 °C and 1 min at 62.1 °C were repeated 35 times for cDNA amplification. Melt curve analysis was performed from 65 °C to 95 °C with increments of 0.5 °C for 5 s in order to confirm primer specificity for each primer combination. Three technical replicates were used for all LPC qRT-PCR studies.
2.7 Statistical methods
Data analysis on LPC qRT-PCR results was performed with qBasePlus (Biogazelle, Zwijnaarde, Belgium). Efficiencies for all targets were assumed to be 100% and expression was normalized to two reference genes (GAPDH and PPIA). For quality control, outliers with Cq value differences >0.5 were excluded from a given set of technical replicates, and Cq values outside of 5–35 were also excluded from the analysis. M values <0.5 and coefficient of variance <0.2 were used as reference target stability criteria. Fold change for each gene was analyzed at the biological replicate level (n = 3) and was standardized by log transformation and mean-centered to correct variation between replicates, as described elsewhere (Willems, Leyns, & Vandesompele, 2008). Ninety-five percent confidence intervals were used to determine significant differences and Bonferroni adjustments were made for multiple comparisons.
For primary hFLC trials, expression of target genes CFOS, LEF1, CDH1, NFKB, PAI-1, and β-catenin and were analyzed by qRT-PCR. GAPDH was the endogenous control and results were analyzed using the 2−ΔΔCt method (Livak & Schmittgen, 2001). Fold changes in gene expression were calculated by normalizing the shear-stress-stimulated cells to static controls, which received no flow.
2.8 Shear flow chamber
Custom flow chamber design was adapted from Ng and Swartz (2003) (Figure 1). When the chamber was fully assembled the ECM gel was firmly affixed by functionalized cover glass on top and bottom (Supplementary Information) and by a porous polyethylene (PE) ring that forms a mechanical entanglement with the ECM. This chamber was used for all murine LPC experiments.
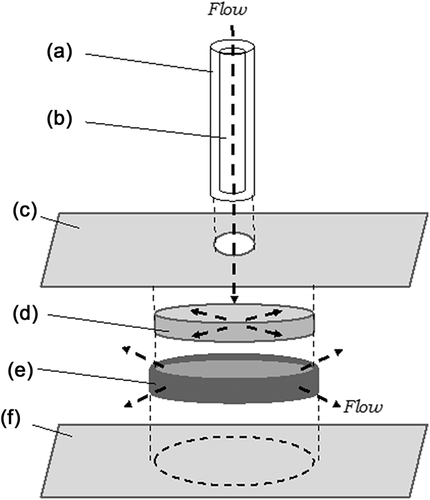
For hFLC experiments, a PFC-1 Shear Flow Chamber (Warner Instruments, Hamden, CT) was used. This chamber can be used with limited cell numbers and is well-validated (Anderson & Knothe Tate, 2007); hence, it was selected for primary cell trials due to limited hFLC availability.
2.9 Shear stress on LPCs after PHx
To select the flow rate for the flow experiments, it was necessary first to estimate shear stresses acting on LPCs and hepatocytes when interstitial flow increases within the space of Disse immediately following PHx (Morsiani et al., 1998). Shear stress acting at the interstitial space was estimated for intact healthy rat liver and for 70% and 90% PHx (Supplementary Tables S3 and S4). From this analysis, we took ∼4 dyn/cm2 as the target shear stress, to approximate interstitial shear stresses associated with 70% PHx.
2.10 Shear stress application
Interstitial flow was applied for two hours at 135 µl/min to ECM-embedded LPCs or LPC spheroids using a syringe pump. At this flow rate, maximum fluid velocity in the gel was calculated as 66 µm/s and maximum shear stress was 4.9 dyn/cm2 (Supplementary Information). One ml of cell- or spheroid-suspended ECM gel was prepared for each biological replicate. Five-hundred microliters were transferred into the chamber for shear flow application, and the remainder was transferred into 96-well plates for static culture (control). Samples were incubated for 2 hr then immersed in IS overnight. Flow was applied for 2 hr in the incubator, then the ECM was transferred into collagenase/hyaluronidase (STEMCELL Technologies) at 1:30 dilution in IS. ECM-enzyme solution was incubated for 4 hr to recover cells/spheroids from the ECM. Recovered cells were lysed and mRNA was extracted (section 2.3).
For hFLC experiments, cells were perfused using KM (Schmelzer et al., 2007), or KM supplemented with IGF-1, glycogen-synthase-kinase-3 inhibitor (GSKi), and thiazovivin to test the response to pulsatile flow in combination with growth factors. Shear stress was applied at 4 or 16 dyn/cm2 for 2 hr. Responses to steady versus pulsatile flow at 4 dyn/cm2 were evaluated for 1 hr of exposure. Cells were harvested from coverslips and RNA and DNA were extracted using an AllPrep DNA/RNA MiniKit (Qiagen, Valencia, CA).
3 RESULTS
3.1 Cell viability
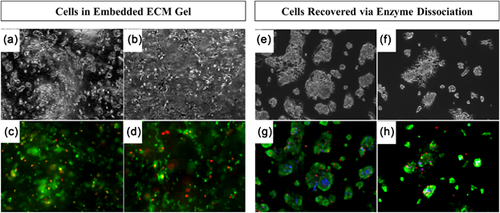
Viability of LPCs in ECM gel and LPCs recovered from gel via enzyme dissociation was examined with LIVE/DEAD stain. Cells were stained before and after shear stress application (Figure 2). Quantification of viability with ImageJ showed 84% viability of LPCs within the ECM hydrogel under static conditions (Figure 2c) and 87% viability after the 2 hr shear flow application (Figure 2d). Cells enzymatically recovered from ECM gel with collagenase/hyaluronidase showed 90% viability for the static condition (Figure 2g) and slightly lower viability at 74% after shear flow application (Figure 2h). Overall, cell survival was confirmed for the combination of ECM gel, enzyme dissociation, and flow rate used for this experiment.
3.2 LPC static culture experiments
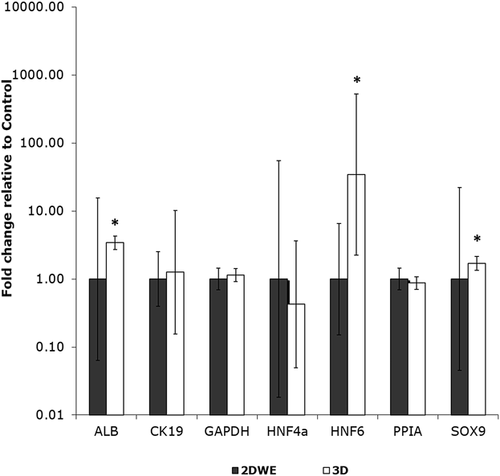
LPC behavior was compared for two culture geometries (2D, 3D) in the absence of flow, via qRT-PCR (Figure 3, Supplementary Table S5) and immunofluorescence (Supplementary Figures S1 and S2). QRT-PCR showed a large increase in HNF6 expression for 3D spheroid cultured cells, with a mean fold change of 34.4 relative to 2D control (2DWE). Expression of ALB and SOX9 increased significantly in 3D relative to 2D control (mean fold changes of 3.43 and 1.70, respectively). Immunofluorescence images showed that LPCs in 2D culture (2DWE) co-expressed hepatocyte marker ALB with biliary markers CK19 and HNF6 (Supplementary Figure S1). Co-expression of ALB/CK19 and ALB/HNF6 was maintained when cells were grown in 3D spheroid culture (Supplementary Figure S2), consistent with retained bipotency of LPCs under 2D and 3D conditions.
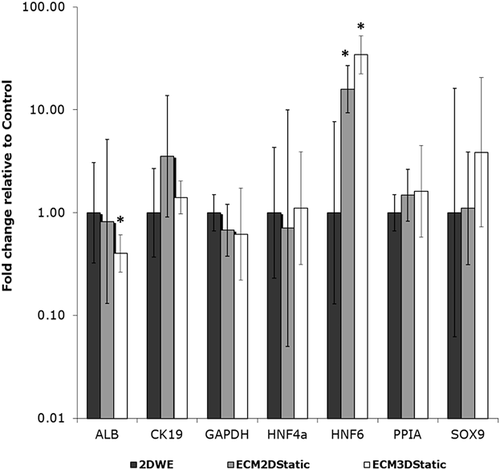
The effect of ECM gel culture environment was examined, in the absence of flow. LPC behaviors in ECM2D and ECM3D were compared to 2DWE (flat plate) control. Static cultured cells and spheroids in ECM gel are shown in Supplementary Figure S3. 2D-cultured cells suspended in ECM gel were evenly spread throughout the gel (Supplementary Figure S3: left panel, a and c), and cells extended projections into the gel connecting to neighboring cells and to the gel (Supplementary Figure S3: left panel, b and d). Cell projections could also be seen in 3D spheroids in ECM gel (Supplementary Figure S3: right panel, b and d). Differences in gene expression between ECM2D, ECM3D, and control (2DWE) conditions are shown in Figure 4 and Supplementary Table S6. Large increases in HNF6 expression were observed in ECM2D and ECM3D relative to control, with a mean fold change of 15.9 (ECM2D) and 34.4 (ECM3D). In addition, ALB expression decreased significantly in ECM3D, with a mean fold change of 0.40.
3.3 Mechanosensitivity experiments
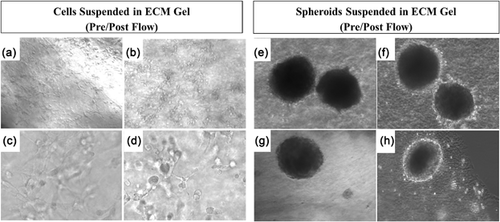
The effects of interstitial flow on LPC behavior were examined via microscopy and qRT-PCR. Flow was applied in ECM2D and ECM3D conditions. Figure 5 shows each condition before and after flow exposure. In ECM2D before flow (Figures 5a and 5c), cells exhibited projections similar to those noted above (Supplementary Figure S3). After flow (Figures 5b and 5d), cells appeared to take on a more rounded appearance with fewer projections and fewer links to neighboring cells. In ECM3D, after flow exposure the cells making up the outer edge of the 3D spheroids formed a bright rim of proliferating cells (Figures 5f and 5h) which was not visible before shear flow application (Figures 5e and 5g). Shape and distribution of spheroids were not affected by flow.
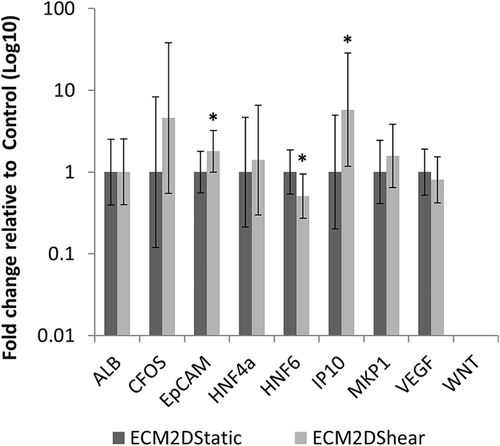
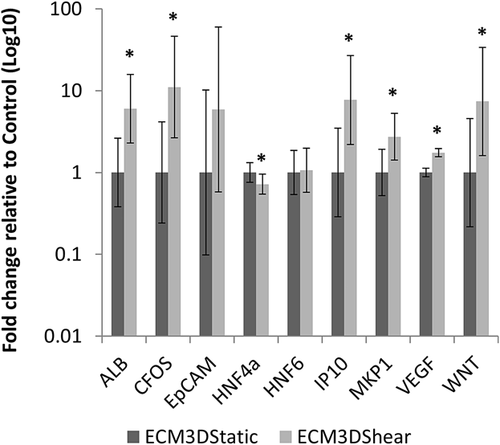
Expression levels of 29 genes (Supplementary Table S2) after shear stress application were compared against static cultured LPCs in ECM (Figures 6 and 7; Supplementary Tables S7 and S8). For ECM2D, three genes showed significant differences between control (ECM2Dstatic) and treatment (ECM2Dshear): EpCAM, HNF6, and IP10 (Figure 6, Supplementary Table S7). IP10 showed the largest increase (mean fold change 5.79), followed by EpCAM (mean fold change 1.80). HNF6 expression decreased with shear flow exposure (mean fold change 0.51). For ECM3D, seven genes showed significant differences between control (ECM3Dstatic) and treatment (ECM3Dshear): ALB, CFOS, HNF4α, IP10, MKP1, VEGF, and WNT (Figure 7, Supplementary Table S8). CFOS showed the largest increase (mean fold change 11.07). IP10, WNT, and ALB also increased significantly after flow exposure (mean fold changes of 7.69, 7.41, and 6.02, respectively). MKP1 and VEGF showed more modest increases (mean fold changes of 2.73 and 1.75), while HNF4α expression decreased significantly (mean fold change 0.72).
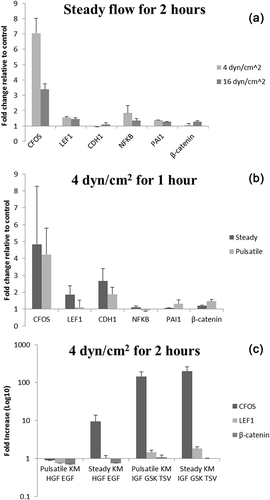
No statistically significant changes were detected for any hFLC experimental condition, but the following trends were observed. CFOS showed the largest increase after flow exposure, with 3–8-fold increases over static controls (Figures 8a and 8b). When media was supplemented with IGF-1, GSKi and thiazovivin, CFOS expression increased over 200 fold in flow-exposed cells compared to static control with the same media (Figure 8c). IGF-1 is a growth factor that supports hepatocyte growth, and GSKi and thiazovivin prevent apoptosis.
4 DISCUSSION
There is emerging evidence that multiple liver cell types exhibit mechanosensitivity to cues such as shear stress (Baptista et al., 2016), hydrostatic pressure (Hsu et al., 2010; Recker et al., 2015), and ECM stiffness (Lozoya et al., 2011). Rashidi, Alhaque, Szkolnicka, Flint, and Hay (2016) reported effects of fluid shear stress on cytochrome P450 drug metabolism and serum protein secretion in hepatocyte-like cells derived from stem cells. Pekor, Gerlach, Nettleship, and Schmelzer (2015) reported increased hepatic differentiation and increased metabolic activity of hFLCs under perfusion conditions. To our knowledge, the present study is the first report to investigate a possible link between fluid mechanical stress and activation of regeneration-related genes in liver progenitor cells. We hypothesized that immediate early genes (IEGs) with known links to liver regeneration will be upregulated in LPCs exposed to in vitro shear stresses on the order of those produced after PHx. The findings suggest that mechanobiological regulatory pathways may participate in LPC-driven regeneration in the setting of severe or chronic liver disease.
More than 100 IEGs have been identified as being activated immediately following PHx (Taub, 2004). IEGs represent several functional classes including transcription factors, growth factors, signal transduction regulators, and other proteins (Taub, 1996). In this work we have demonstrated that four genes (CFOS, IP10, MKP1, ALB), previously identified as IEGs following PHx (Haber, Mohn, Diamond, & Taub, 1993; Taub, 1996), are upregulated in LPCs after 2 hr of interstitial flow exposure at shear stresses of ∼4 dyn/cm2 (Figure 7). Each gene will be considered in turn.
CFOS encodes a 62kDa protein which forms a heterodimer with CJUN, resulting in formation of transcription factor Activator Protein-1. IP10 is a chemokine present in liver injury and activates downstream events via PI3K or MAPK signaling, as well as mechanical events such as actin reorganization (Maire et al., 2008). Both IP10 (Bone-Larson, Hogaboam, Evanhoff, Strieter, & Kunkel, 2001) and CFOS (Schoen, Wang, Minuk, & Lautt, 2001) have been linked to a hepatocyte proliferative response in regenerating livers. Thus, mechanical induction of IP10 and CFOS in LPCs in the present work is consistent with the hypothesis that fluid shear stress can initiate a proliferative/regenerative response in LPCs.
Albumin (ALB) expression is known to increase rapidly following PHx (Taub, 1996); hence, it is considered an IEG in liver regeneration (Taub, 1996). Mitogen-activated protein kinase phosphatase-1 (MKP1) plays a key role in deactivating MAPKs. MKP1 can protect mice against acetaminophen-induced liver injury (Wancket, Frazier, & Liu, 2012) and is induced in mitogen-activated cells including regenerating liver (Chin, Ramirez, Greenbaum, Naji, & Taub, 1995). Thus, mechanical induction of MKP1 observed in the current study is also consistent with the hypothesis of a shear stress-induced proliferative response in LPCs.
In addition to four IEGs discussed above, shear stress was found to upregulate WNT, VEGF, and EpCAM in LPCs (Figures 6 and 7). WNT is a morphogen that regulates liver development and repair, and vascular endothelial growth factor (VEGF) is linked to the ductular reaction in liver (Strazzabosco & Fabris, 2012). Both WNT and VEGF are associated with activation and proliferation of LPCs in liver disease (Strazzabosco & Fabris, 2012; Yang et al., 2008;). Epithelial cell adhesion molecule (EpCAM) expression is known to increase during active proliferation (Maetzel et al., 2009; Oishi & Wang, 2011) and is a direct transcriptional target of the Wnt-β-catenin canonical signaling pathway (Oishi & Wang, 2011). Taken together, rapid upregulation of four IEGs (CFOS, IP10, MKP1, ALB) as well as WNT, VEGF, and EpCAM, is consistent with activation of a proliferative/regenerative response in LPCs by fluid mechanical stress. This interpretation is also supported by the bright rim of proliferative cells observed on the 3D spheroids after shear stress exposure (Figures 5f and 5h). It is further supported indirectly by the slight downregulation of HNF6 and HNF4α induced by flow (Figures 6 and 7), since these transcription factors regulate biliary and hepatocyte fate specification, respectively.
Two trends observed in experiments with primary hFLCs support the findings in LPCs. hFLCS were shear stress sensitive and CFOS was the most activated gene following shear stress exposure (Figure 8). Addition of IGF-1, GSKi, and thiazovivin to the perfusion media dramatically increased shear-induced CFOS expression compared with static culture in the same media. As noted above, IGF-1 is a growth factor that supports hepatocyte growth, and GSKi and thiazovivin prevent apoptosis. GSKi and thiazovivin regulate maintenance and proliferation of pluripotent stem cells (Lu & Atala, 2014). GSKi inhibits glycogen-synthase-kinase-3 (GSK-3), and thiazovivin inhibits Rho kinase (ROCK). Both agents have links to mechanotransduction pathways (Case et al., 2011; Jaalouk & Lammerding, 2009;). Our observations in hFLCs highlight the important interplay between biochemical and biomechanical signaling within the cell.
In static culture experiments without ECM gel, LPCs grown in spheroids showed upregulation of HNF6, SOX9, and ALB compared to LPCs in 2D culture (Figure 3). This gene expression profile suggests that LPCs maintained bipotent status in spheroid culture since they expressed hepatocytic (ALB) as well as cholangiocytic (HNF6, SOX9) markers (Supplementay Figure S2). Yap et al. (2013) also found that LPCs maintained bipotential capacity when cultured as spheroids.
In static culture experiments with ECM gel, HNF6 expression strongly increased for both ECM2D and ECM3D conditions, and ALB expression decreased for the ECM3D condition (Figure 4). These results suggest that plate- and spheroid-cultured LPCs were being driven toward cholangiocyte cell fate in the HA/collagen I gel culture environment, in the absence of flow (Turner et al., 2008). Interestingly, when cells cultured in gel for 24 hr were exposed to 2 hr of flow, HNF6 expression significantly decreased (Figure 6) and ALB expression significantly increased (Figure 7), suggesting that addition of flow may have triggered activation of a different cellular response.
Shear stresses and fluid velocities calculated in our study are subject to limitations (Supplemental Information) and may be regarded as conservative estimates. While previous studies have examined liver interstitial flow (Moran, Baptista, Evans, Soker, & Sparks, 2012; Siggers, Leungchavaphongse, Ho, & Repetto, 2013), these did not consider flow changes after PHx. It would be beneficial to explore interstitial fluid dynamics post-PHx in future work.
In conclusion, this study demonstrated that seven genes with known links to liver regeneration were upregulated in LPCs in response to short-term fluid mechanical stress exposure of ∼4 dyn/cm2. Collectively the flow-upregulated genes fit the pattern of an LPC-mediated proliferative/regenerative response. Trials in hFLCs also suggested shear stress sensitivity. Two physiologically relevant cell culture environments were developed, permitting controlled interstitial flow at a desired rate for LPCs embedded either individually or as 3D spheroids within a HA/collagen I hydrogel. LPCs maintained an early to intermediate stage of differentiation in spheroid culture in the absence of the hydrogel, and addition of the gel initiated cholangiocyte differentiation programs which were then abrogated by the onset of flow. These results provide evidence that mechanical cues are potentially important regulators of the LPC-mediated regeneration response in liver. The results could be applied to develop organotypic models for tissue engineering research. In future, better understanding of the mechanisms controlling regeneration in chronic liver disease could aid in identifying pathways for liver regeneration that could be therapeutically targeted.
ACKNOWLEDGMENTS
The authors acknowledge Miami University's Center for Bioinformatics and Functional Genomics, the Statistical Consulting Center, Dr. Andor Kiss, Dr. Agustin Madrigal, Dr. Jason Berberich, and Dr. Janina Tirnitz-Parker. Funding provided by Miami University.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to declare.
AUTHORS’ CONTRIBUTIONS
KN performed experiments, analysis, and writing. EB, TR, and RW data collection, data processing. EM and SS conducted hFLC trials. JLS contributed in aims/design, analysis, writing, and editing.




